Abstract
The high variability among strains and isolates of Trypanosoma cruzi and the existence of shared antigenic determinants with other pathogens, particularly with members of the Leishmania genus make difficult the specific diagnosis of Chagas' disease. The data reported in this paper show that the T. cruzi KMP11 protein is an immunodominant antigen highly recognized by the sera from chagasic and leishmaniasis patients. By the use of amino- and carboxyl-terminal truncated KMP11 recombinant proteins and synthetic peptides, evidence is provided that while the sera from chagasic patients recognize linear peptides the sera from patients with visceral leishmaniasis must be predominantly directed against conformational epitopes. We found that a particular linear determinant, located in the carboxyl-terminal region of the protein, is recognized with high specificity and sensitivity only by sera from Chagas' disease patients, suggesting it could be a good candidate for differential serodiagnosis of Chagas' disease.
Keywords: KMP11, antigenic determinants, Trypanosoma cruzi, Leishmania, Chagas disease
INTRODUCTION
The Trypanosomatidae family is formed by flagellated protozoan parasites responsible for serious diseases occurring in human such as Chagas and leishmaniasis. These diseases have a world wide distribution. Trypanosoma cruzi and various species belonging to the Leishmania genus are the agents responsible for these infectious diseases. To complete their digenic life cycle both parasites require to infect vertebrates and invertebrates hosts. Chagas disease affects 18 million people and a further 100 million live in endemic areas where there is risk of infection. The annual death rate due to Chagas disease amount to 50 000 [1]. This disease involves an acute asymptomatic phase with high parasitemia which develops rapidly into a chronic phase characterized by the appearance of a wide variety of lesions (heart, digestive tract, nervous system, etc.) depending on the strain of infection [2]. Chagas disease represents a serious health problem for which no effective immunoprophylaxis exists. The drugs used for treatment are rather toxic and not very effective. Most of the methods used for the indirect diagnosis of the disease (chronic phase) are based on the detection of antibodies against total T. cruzi proteins, subcellular fractions and, more recently, against specific recombinant proteins [3–5]. The high variability, however, among T. cruzi strains and even among isolates makes difficult the use of isolated proteins for diagnosis. This difficulty increases since T. cruzi shares antigenic determinants with other pathogens and particularly with members of the Leishmania genus. This means that the routine diagnosis of the Chagas' disease is faced with numerous problems due to cross-reactivity between antigenic proteins.
The kinetoplastids KMP11 protein was first described in Leishmania donovani [6] associated to the lipophosphoglycan (LPG) molecule and located throughout the parasite surface. Specific antibodies against this complex LPG protein revealed the presence of KMP11 in total extracts of a high number of species belonging to Leishmania and Trypanosoma. In recent years the genes coding for KMP11 in L. donovani [7], Leishmania infantum [8], Trypanosoma brucei [9] and Trypanosoma cruzi [10] have been isolated. The wide distribution of the KMP11 protein in kinetoplastids together with their high degree of conservation suggested that the protein may play important functions in the biology of these parasites. This suggestion has been reinforced by the finding that KMP11 is associated with microtubules [10]. Initial studies also showed that immunization with LPG conferred immunoprotection against Leishmania infection in mice [11] but that the ability to stimulate T cells and to induce protection in mice was not a property of LPG but of the associated KMP11 protein [12]. It has been also shown that the L. donovani KMP11 and peptide fractions of the protein act as B-cell and T-cell immunogens during visceral leishmaniasis (VL) [13] and that the inoculation of hamsters with the UR6 avirulent strain of Leishmania expressing at a high level the KMP11 gene confers high protection against a virulent strain [14].
In the present study we have analysed the characteristics of the humoral response to KMP11 during natural infections in the sera from chagasic and leishmaniasis patients. In agreement with previous data we show that the protein is an antigen highly recognized by the sera from patients affected from these diseases but that only the sera from chagasic patients recognize linear peptides. The leishmaniasis sera do not recognize any one of the single peptides. The study of the humoral response using the sera from chagasic and leishmaniasis patients against different peptides and truncated forms of the KMP11 protein together with competition assays enabled us to define the carboxyl-terminal domain of the KMP11 protein from T. cruzi as an antigenic determinant. In addition, we have defined that a single peptide located in that region has high sensitivity and specificity when used for the diagnosis of sera from chagasic patients. Thus, it may be used for differential serodiagnosis of Chagas disease and visceral leishmaniasis.
MATERIALS AND METHODS
Cloning, expression and purification of the T. cruzi KMP11 recombinant protein and the KMP11 truncated proteins
For the cloning the T. cruzi KMP11 complete protein, the cDNA corresponding to the T. cruzi KMP11 gene [10] was digested with MscI and RsaI enzymes, and subcloned in the SmaI digested pQE31 expression vector (Quiagen, Hilden, Germany). The KMP11 protein truncated in the carboxyl-terminal (KMP11-ct) was obtained by cloning in pQE32 vector (Quiagen), BamHI and SalI sites, a PCR amplified fragment which starts in 5′ with the nucleotides corresponding to the ATG initiator of the protein but lacking in 3′ the 51 nucleotides which encode for the last 17 C-terminal amino acids. The amino-terminal truncated KMP11 protein (KMP11-at) was also constructed by cloning into pQE30 vector (Quiagen), BamHI and SalI sites, a PCR amplified fragment which lacks in the 5′ end the 18 nucleotides which encodes for the first 6 amino acids of the protein and ending in 3′ with the stop codon. The BamHI and SalI sites were introduced in the primers used for the PCR reaction and generated ad hoc in the amplified fragments. The recombinant proteins were overexpressed in E. coli after induction for 3 h at 37°C with 0·1 mm IPTG when the complete KMP11 protein was expressed and with 1 mm IPTG when the truncated KMP11 proteins were expressed. The soluble KMP11 recombinant proteins were purified by Ni2+-NTA-agarose affinity column and eluted with phosphate buffer (50 mm NaHPO4, 300 mm NaCl) at pH 4.
Human sera donors
Blood samples were collected from adult patients from CINTROP, Bucaramanga, Colombia and Instituto de Medicina Tropical, Caracas, Venezuela (chagasic and leishmaniasis sera) and from Instituto de Inmunología, Bogotá, Colombia (tuberculosis and malaria sera). Control sera were collected from healthy adult donors.
Immunoblot analysis
The T. cruzi KMP11 purified recombinant protein was electrophoresed in 20% SDS-PAGE and transferred to PVDF membrane (Millipore, Bedford, MA, USA) using the Miniprotean system (Bio-Rad, Hercules, CA, USA). Western blot analysis were carried out according to the standard methodology [15]. A pool of five chagasic sera was used at a 1 : 200 dilution and the purified anti-KMP11 antibody [10] was used at a 1 : 2000 dilution. The blots were developed, respectively, with antihuman and antirabbit IgG alkaline phosphatase conjugate F(ab′)2 fragment at a dilution of 1/5000. Nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate were used as substrate.
Synthesis of peptides
A library of overlapping peptides covering the T. cruzi KMP11 protein sequence was synthesized by simultaneous multiple-peptide solid-phase synthetic method [16]. The peptides were assembled using the standard t-Boc solid phase peptide synthesis (SPPS) strategy on a p-methylbezhydrilamide (MBHA) resin [17,18]. Purity was checked by high performance liquid chromatography (HPLC).
ELISA measurements
The sensitization of wells was performed for 1 h at 37°C and subsequently overnight at 4°C using 0·5 µg/well each one of the proteins diluted in 100 µl of carbonate buffer pH 9·6. Synthetic peptides were coupled to KLH protein by glutaraldehyde treatment [19] and then used for coating the wells at a peptide concentration of 10 µg/ml (1 µg/well). After sensitization, the wells were washed twice with 200 µl of PBS. Afterwards, the antigen-coated wells were incubated for one hour with blocking solution (5% nonfat dried milk powder in PBS-0·05% Tween-20) followed by incubation with the sera at the dilution indicated in the figure legends for two hours at 37°C with shaking. After exposure to the antibody, the wells were washed as described above. As secondary antibody an affinity isolated goat anti-human IgG γ chain) antibody peroxidase conjugated at dilution 1 : 2000 was used. After 1 h at 37°C and washing four times with PBS-0·05% Tween-20 the reactions were developed using orthophenylenediamine. The optical density was measured at 492 nm. For the competition assays the sera at 1 : 1600 dilution were first incubated for 12 h at 4°C with increasing amounts of the recombinant proteins or peptides immobilized in ELISA wells and subsequently assayed in ELISA plates coated with 100 µl of the KMP11 protein at a concentration of 5 µg/ml. The inhibition molar ratios were calculated as the ratio between the molar concentration of the inhibitor (preincubated with the sera) and the molar concentration of KMP11 (used to coat the wells). Competition experiments were performed using 3 different chagasic sera.
RESULTS
Recognition of the T. cruzi KMP11 protein by sera from Chagas' disease patients
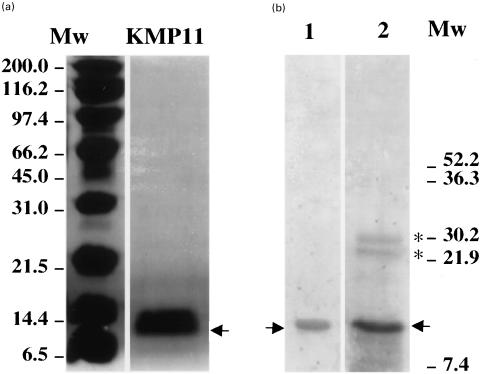
The purified KMP11 recombinant protein was analysed by SDS/PAGE. An intensively stained band of approximately 11 kD was observed which corresponds to the expected size of the protein (Fig. 1a). The western blot shown in Fig. 1b indicates that the recombinant protein is recognized by the antibodies present in the chagasic sera as well as by an affinity-purified anti-KMP11 antibody. Two additional bands corresponding to dimeric and trimeric forms of the KMP11 protein were also labelled with the anti-KMP11 antibody having approximate molecular weights of 22 and 33 kD. The dimeric and trimeric forms represent a minor fraction of the total amount of the purified protein. Similar protein forms have also been observed for the L. infantum and L. donovani KMP11 recombinant proteins [8–12], evidencing that KMP11 has great capacity to aggregate in vitro.
Fig. 1.
SDS-polyacrilamide gel electrophoresis of T. cruzi KMP11 purified recombinant protein. (a) Coomassie blue staining. Lane KMP11, shows the KMP11 recombinant protein purified by Ni2+ -NTA-agarose affinity column (Quiagen). (b) Western blot analysis. Lane 1, filter probed with a pool of five chagasic sera. Lane 2, filter probed with the anti-KMP11 antibody purified by affinity chromatography from a rabbit hyperimmune sera [10]. Mw, molecular weight markers. The arrow indicates the localization of the KMP11 protein. The asterisks show the dimeric and trimeric forms of the KMP11 protein.
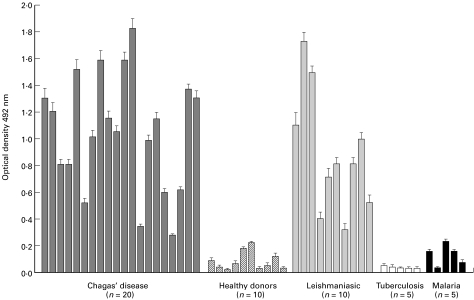
Reactivity of sera from Chagas' disease and leishmaniasis patients against the recombinant T. cruzi KMP11 protein. The reactivity of sera from chagasic patients in the chronic phase of the disease, from patients with visceral leishmaniasis (VL), tuberculosis and malaria were analysed by conventional ELISA for their reactivity against the T. cruzi KMP11 protein. Sera from healthy individuals of Caucasian origin were used as negative controls. The results showed that the sera from patients with Chagas' and VL disease strongly recognize the KMP11 protein (Table 1). The mean reactivity of the chagasic and VL sera was 1·06 (SD ± 0·42) and 0·87 (SD ± 0·44), respectively. Significantly, all the sera from the chagasic (20/20) and leishmaniasis (10/10) patients used in this study recognized the KMP11 protein. The reactivity of the sera from patients with tuberculosis or malaria were negative. We also observed a high variability in the OD values among different sera (Fig. 2), reflecting the heterogeneity in intensity of the humoral response against the protein among individuals. Thus, three different recognition groups (high, medium and low) could be made, each one representing approximately 1/3 of the analysed sera.
Table 1.
ELISA evaluation of the reactivities (mean ± standard deviation of sera from Chagas' disease and leishmaniasis patients and healthy donors against KMP11 recombinant proteins.
| Sera | KMP11 | Truncated KMP11 in amino-terminal | Truncated KMP11 in carboxyl-terminal |
|---|---|---|---|
| Chagasic | 1.06 ± 0.42 | 0.99 ± 0.42 | 0.30 ± 0.19 |
| Leishmaniasis | 0.87 ± 0.44 | 0.80 ± 0.42 | 0.64 ± 0.23 |
| Healthy | 0.09 ± 0.07 | 0.06 ± 0.04 | 0.08 ± 0.06 |
Fig. 2.
ELISA evaluation of the reactivity of 20 sera from Chagas' disease (▪), 10 sera from leishmaniasis ( ), 5 sera from tuberculosis (□), 5 sera from malaria (▪) patients and 10 sera from healthy donors (
), 5 sera from tuberculosis (□), 5 sera from malaria (▪) patients and 10 sera from healthy donors ( ) were assayed against the recombinant KMP11 protein. n, number of sera used in each group. The patient's sera were assayed at 1 : 800 dilution and the healthy donors sera were used at 1 : 200 dilution.
) were assayed against the recombinant KMP11 protein. n, number of sera used in each group. The patient's sera were assayed at 1 : 800 dilution and the healthy donors sera were used at 1 : 200 dilution.
Anti-KMP11 antibodies present in sera from Chagas' disease patients are principally directed against the carboxyl-terminal domain of the protein. To determine the location of the antigenic determinant/s amino- and carboxyl-terminal truncated KMP11 proteins were generated (see in Fig. 3a) and purified to homogeneity (figure not shown). Both proteins were assayed for their reactivity against the sera from chagasic and VL patients. We observed (Table 1) that the mean value of the reactivity of the chagasic sera against the carboxyl-terminal truncated protein (KMP11-ct) was less than 30% of the value obtained against the complete protein. However, the reactivity of the VL sera was close to 75%. The amino-terminal truncated protein (KMP11-at), as shown in Table 1, was highly recognized both by sera from chagasic and VL patients. It seems that the amino-truncated protein contains the main determinants since the value of the reactivity of each one of the sera against the KMP11-at protein is the same as that obtained against the complete protein.
Fig. 3.
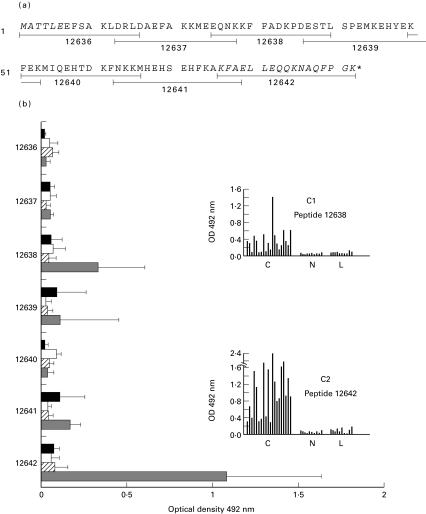
Reactivity of sera from Chagas' disease, leishmaniasis, tuberculosis and malaria patients against the synthetic peptides, 16-mer long overlapped by four residues, covering the entire T. cruzi KMP11 protein. (a) Amino acid sequence of the T. cruzi KMP11 protein. The amino acids absent in the amino (KMP11-at) and carboxyl-terminal (KMP11-ct) truncated KMP11 proteins are indicated in italic. The synthetic peptide sequences are underlined and indicated by consecutive numbers (12636–12642). (b) The horizontal bars represent the absorbance values of the 5 sera from chagasic ( ), VL (
), VL ( ), tuberculosis (▪) and malaria (□) patients, respectively, minus the mean absorbance of 5 healthy donors sera (OD492nm = 0·061) plus three standard deviations (SD = 0·008) for each peptide and sera group. (C1 and C2) Histograms with the values of the reactivities of 20 sera from Chagas' disease patients, 10 sera with visceral leishmaniasis and 10 healthy donors sera used as control, against the synthetic peptides 12638 and 12642 which correspond, respectively, to the central and carboxyl-terminal regions of the T. cruzi KMP11 protein. The sera were assayed at 1 : 200 dilution. None of the used sera recognized the KLH protein.
), tuberculosis (▪) and malaria (□) patients, respectively, minus the mean absorbance of 5 healthy donors sera (OD492nm = 0·061) plus three standard deviations (SD = 0·008) for each peptide and sera group. (C1 and C2) Histograms with the values of the reactivities of 20 sera from Chagas' disease patients, 10 sera with visceral leishmaniasis and 10 healthy donors sera used as control, against the synthetic peptides 12638 and 12642 which correspond, respectively, to the central and carboxyl-terminal regions of the T. cruzi KMP11 protein. The sera were assayed at 1 : 200 dilution. None of the used sera recognized the KLH protein.
Fine mapping of the antigenic determinants of the T. cruzi KMP11 protein
To determine in more detail the region/s of the T. cruzi KMP11 protein involved in the recognition of the protein 7 overlapping peptides of 16 mer covering the complete protein were synthesized (Fig. 3a). The peptide library was screened by ELISA using sera either from chagasic and from VL patients. We also used as controls sera from malaria, tuberculosis patients in addition to sera from healthy individuals. We observed that only peptides 12638 and 12642 were recognized by the sera from chagasic patients while none of the peptides were recognized by the sera from VL patients or the control sera (Fig. 3b). As it is shown in Fig. 3c, panels 1 and 2, the 12638 peptide, located in the central part of the protein, was recognized by 60% of the chagasic sera while peptide 12642 corresponding to the carboxyl-terminal region of the protein was recognized by all the chagasic sera. Thus, at least two main linear antigenic determinants are present in KMP11. In contrast, none of the VL sera recognized the peptides suggesting that the antigenic determinants elicited against KMP11 during natural infection must be conformational.
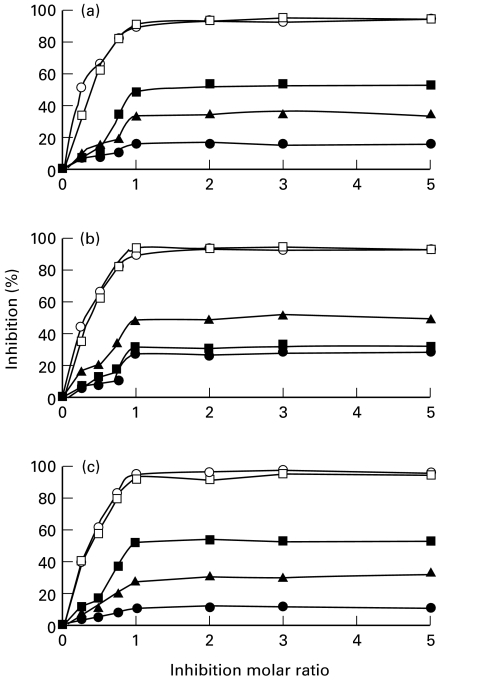
In order to examine whether the antibodies present in the sera from the chagasic patients are directed only against the linear determinants alone, present in peptides 12638 and 12642, or there are other conformational or potential linear determinants not present in the synthesized peptides, competition experiments using as competitors the truncated KMP11 proteins and the synthetic peptides 12638 and 12642 were performed. Three sera that reacted against these peptides were preincubated with known amounts of either the truncated recombinant proteins or the 12638 and 12642 peptides previous to the incubation with the KMP11 protein present in the ELISA wells. The results obtained, shown in Fig. 4, revealed that preincubation of the chagasic sera with the complete KMP11 protein (positive control) and the KMP11-at protein induced total inhibition of the reactivity against the complete KMP11 protein even at a molar ratio of 1 : 1. Thus, we may conclude that the main antigenic determinants of KMP11 are present in KMP11-at. As expected, the level of inhibition obtained when single peptides were used was significantly lower. The peptide 12642 showed a level of competition of 49%, 31% and 51% and the competition level of peptide 12638 was 17%, 28% and 11%, respectively. The results are in agreement with the finding that peptide 12642 is recognized with high values by the chagasic sera. The level of inhibition caused by preincubation with the KMP11-ct protein was 33%, 48% and 28%. The differences observed between the levels of inhibition produced by the KMP11-ct protein and peptide 12638 may be due to the presence in the protein of potential antigenic determinants which are absent in the peptide. Thus, since the sum of the inhibition percentages produced by the 12642 and 12638 peptides is lower than that caused by the complete protein we think that there should be also in the sera from chagasic patients a certain level of antibodies generated against conformational determinants.
Fig. 4.
Competition assay between the recombinant protein by truncated KMP11 proteins and the 12642 and 12638 synthetic peptides. The serum samples (a) BF (b) RP and (c) PRM that reacted against 12638 and 12642 peptides were preincubated at a 1 : 1600 dilution with increasing amounts of either the KMP11 protein (○), the amino-terminal truncated KMP11 protein (□), the carboxyl-terminal truncated KMP11 protein (▴) or an equimolecular amount of the synthetic peptide 12642 which corresponds to the carboxyl-terminal region of the T. cruzi KMP11 protein (▪) and the synthetic peptide 12638 which corresponds to the central region of the protein (•). The data represent the mean of values obtained in three independent experiments (SD ≤ 5%). The inhibition molar ratios (moles of inhibitor/moles of coating antigen) were calculated considering the amount of KMP11 antigen (40 pmol) used to coat the ELISA wells.
DISCUSSION
In the last years, the cloning and the molecular characterization of the genes coding for the KMP11 protein from some trypanosomatid species have been described. Interestingly, they share similar structural features although some differences may be observed regarding the nucleotide sequence and the genomic arrangement [8–10]. We have recently described that the T. cruzi KMP11 protein is associated with the parasite's cytoskeleton and the existence of a self-regulating mechanism operating at the translational level that controls the steady state level of the protein [10]. There is, however, limited information about the specific antigenic and immunogenic properties of this kinetoplastid-specific protein. Has been reported that the majority of the sera from VL, mucocutaneous (MCL) and even some cutaneous leishmaniasis patients contain detectable IgG antibodies against the Leishmania KMP11 protein [21]. The data presented in this paper demonstrate that the KMP11 protein behaves as a strong immunogen during T. cruzi infection being the target of a strong humoral immune response. Our results indicate that 100% of the analysed sera from human patients in the chronic phase of the disease contain a significant level of anti-KMP11 antibodies. We also show that the sera from VL patients recognize the T. cruzi KMP11 protein. In this context, the lack of specificity would make the protein not to be appropriate for differential serological diagnosis of the Chagas' disease relative to VL. We show, however, that there is not any cross-reactivity between the T. cruzi KMP11 protein and the sera from patients with other infectious diseases such as tuberculosis or malaria. This contrasts with previous reports [13] that detected cross-reactivity to the L. donovani KMP11 protein in Sudanese patients infected with P. falciparum. This discrepancy may be due to the existence of coinfection with Leishmania in these Sudanese patients as it has been previously reported for Gambia malaria patients [22]. In fact, the authors show there is no cross-reactivity of the L. donovani KMP11 protein with the sera from Danish patients infected by P. falciparum [13].
It was surprising to detect that the sera from chagasic patients recognize linear determinants while the sera from VL patients do not do it. Two different regions of the protein determined by two peptides located in the protein's central and carboxyl-terminal regions were recognized with high specificity only by the sera from chagasic patients. The peptide which maps in the central region of the protein, recognized by 60% of the chagasic sera, corresponds with the short turn region that interrupts the two highly amphipatic helices forming the amino and carboxyl-terminal structure of the KMP11 protein [23,24]. The peptide belonging to the carboxyl-end is recognized by all of the analysed chagasic sera. ELISA assays carried out with the truncated KMP11 proteins indicate that most of the antibodies produced against the KMP11 protein in T. cruzi natural infection are directed against the antigenic determinant found in the carboxyl terminal region. This was confirmed by competition assays. It was observed that while the amino-terminal truncated protein inhibits to a large extent the reactivity of the chagasic sera against the complete KMP11 protein, the carboxyl-terminal truncated protein does non inhibit this reactivity. Remarkably, the peptide corresponding to the carboxyl-terminal of the KMP11 protein inhibits the reactivity from 31% to 51% against the native KMP11 protein. Being that so and because all the sera recognize peptide 12642 with high sensitivity and specificity we think that it could be a useful tool for the serodiagnosis of Chagas disease and for blood bank sample screening.
The divergence in the type of recognition of the protein between the chagasic and VL sera may be attributed to the specificity of triggering the immune response against the protein in both diseases since the amino acid sequence similarity between the T. cruzi KMP11 protein and of those from different species of Leishmania revealed only a divergence of approximately 14% [7,8,10]. Our suggestion is that since Leishmania infects macrophages and T. cruzi may infects a wide variety of cells including nonphagocyte cells, the processing and presentation mechanisms leading to the immune response against the protein may be different.
Acknowledgments
This work was supported by grants FIS98/0914 and 1FD1997–0630-C02–01 from the Fondo de Investigaciones Sanitarias and Plan Nacional I + D – FEDER (DGESIC), Spain. Dr M.C. Thomas was supported by FIS N°97/4207 Postdoctoral Fellowship, ISCIII. E. Carmelo was supported by the Departamento de Parasitología, Universidad de la Laguna. C. Marañón was partially supported by Novo Nordisk Pharma SA and a MEC Predoctoral Fellowship supported L. Planelles. The authors thank Dr V.M. Angulo from CINTROP, Colombia and Drs O. Noya and A. Maekel from Instituto de Medicina Tropical, Venezuela for providing some of the sera used in this study.
REFERENCES
- 1.World Health Organization. WHO-Tropical Disease Research. Geneva: WHO; 1995. Twelfth Programme Report of the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases; pp. 125–6. [Google Scholar]
- 2.Andrade ZA. Pathogenesis In Chagas Disease. Res Immunol. 1991;142:126–9. doi: 10.1016/0923-2494(91)90021-a. [DOI] [PubMed] [Google Scholar]
- 3.Almeida E, Krieger MA, Carvalho MR, Oeleman W, Goldenberg S. Use of recombinant antigens for the diagnosis of Chagas disease and blood bank screening. Mem Inst Oswaldo Cruz. 1990;85:513–7. doi: 10.1590/s0074-02761990000400023. [DOI] [PubMed] [Google Scholar]
- 4.Solana ME, Katzin AM, Umezawa ES, Sosa-Miatello C. High specificity of Trypanosoma cruzi epimastigote ribonucleoprotein as antigen in serodiagnosis of Chagas' disease. J Clin Microbiol. 1995;33:1456–60. doi: 10.1128/jcm.33.6.1456-1460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruber A, Zingales B. Trypanosoma cruzi: Characterization of two recombinant antigens with potential application in the diagnosis of Chagas' disease. Exp Parasitol. 1993;76:1–12. doi: 10.1006/expr.1993.1001. [DOI] [PubMed] [Google Scholar]
- 6.Jardim A, Funk V, Caprioli RM, Olafson RW. Isolation and structural characterization of the Leishmania donovani kinetoplastid membrane protein-11, a major immunoreactive membrane glycoprotein. Biochem J. 1995;305:307–13. doi: 10.1042/bj3050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jardim A, Hanson S, Ullman B, McCubbin WD, Kay CM, Olafson RW. Cloning and structure-function analysis of the Leishmania donovani kinetoplastid membrane protein-11. Biochem J. 1995;305:315–20. doi: 10.1042/bj3050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berberich C, Requena JM, Alonso C. Cloning of gene and expression and antigenicity analysis of the Leishmania infantum KMP-11 protein. Exp Parasitol. 1997;85:105–8. doi: 10.1006/expr.1996.4120. [DOI] [PubMed] [Google Scholar]
- 9.Bridge MA, Zhou Q, Koop BF, Pearson TW. Cloning and characterization of the kinetoplastid membrane protein-11 gene locus of Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:359–63. doi: 10.1016/s0166-6851(97)00229-6. [DOI] [PubMed] [Google Scholar]
- 10.Thomas MC, García-Pérez JL, Alonso C, López MC. Molecular characterization of KMP11 from Trypanosoma cruzi: a cytoskeleton-associated protein regulated at the translational level. DNA Cell Biol. 2000;19(1):47–57. doi: 10.1089/104454900314708. [DOI] [PubMed] [Google Scholar]
- 11.McConville MJ, Bacic A, Mitchell GF, Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophos- phoinositol lipid anchor. Proc Natl Acad Sci USA. 1987;84:8941–5. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jardim A, Tolson DL, Turco SJ, Pearson TW, Olafson RW. The Leishmania donovani lipophosphoglycan T lymphocyte-reactive component is a tightly associated protein complex. J Immunol. 1991;147:3538–44. [PubMed] [Google Scholar]
- 13.Jensen ATR, Gasim S, Ismail A, et al. Humoral and cellular immune response to synthetic peptides of the Leishmania donovani kinetoplastid membrane protein-11. Scad J Immunol. 1998;48:103–9. doi: 10.1046/j.1365-3083.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay S, Sen P, Bhattacharyya S, Majumdar S, Roy S. Immunoprophylaxis and immunotherapy against experimental visceral leishmaniasis. Vaccine. 1999;17:291–300. doi: 10.1016/s0264-410x(98)90017-2. [DOI] [PubMed] [Google Scholar]
- 15.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarin VK, Tam JP, Merrifield RB. Quantitative monitoring of solid phase peptide system by the ninhydrin reaction. Ann Biochem. 1981;117:147–57. doi: 10.1016/0003-2697(81)90704-1. [DOI] [PubMed] [Google Scholar]
- 17.Houghten RA. General method for the rapid solid phase synthesis of large numbers of peptides: Specificity of antigen antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82:5131–5. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puentes F, Guzmán F, Marín V, Alonso C, Patarroyo ME, Moreno A. Leishmania. Fine mapping of the leishmanolysin molecule's conserved core domains involved in binding and internalization. Exp Parasitol. 1999;93:7–22. doi: 10.1006/expr.1999.4427. [DOI] [PubMed] [Google Scholar]
- 19.Reichilin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Meth Enzymol. 1990;70:159–65. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- 20.Tolson DL, Jardim A, Schnur LF, et al. The kinetoplastid membrane protein 11 of Leishmania donovani and African trypanosomes is a potent stimulator of T-lymphocyte proliferation. Infect Immun. 1994;62:4893–9. doi: 10.1128/iai.62.11.4893-4899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trujillo C, Ramírez R, Vélez ID, Berberich C. The humoral immune response to the kinetoplastid membrane protein-11 in patients with American leishmaniasis and Chagas' disease: prevalence of IgG subclasses and mapping of epitopes. Immunol Lett. 1999;70:203–9. doi: 10.1016/s0165-2478(99)00146-7. [DOI] [PubMed] [Google Scholar]
- 22.Facer CA. Direct antiglobulin reactions in Gambian children with P. falciparum malaria. III. Expression of IgG subclass determinants and genetic markers and association with anemia. Clin Exp Immunol. 1980;41:81–90. [PMC free article] [PubMed] [Google Scholar]
- 23.Stebeck CE, Baron GS, Beecroft RP, Pearson TW. Molecular characterization of the kinetoplastid membrane protein-11 from African trypanosomes. Mol Biochem Parasitol. 1996;81:81–8. doi: 10.1016/0166-6851(96)02678-3. [DOI] [PubMed] [Google Scholar]
- 24.Fuertes MA, Berberich C, Lozano RM, Gimenez-Gallego G, Alonso C. Folding stability of the kinetoplastid membrane protein-11 (KMP11) from Leishmania infantum. Eur J Biochem. 1999;260:559–67. doi: 10.1046/j.1432-1327.1999.00217.x. [DOI] [PubMed] [Google Scholar]