Abstract
T-helper cell responses to HIV have been associated with protection against maternal-infant HIV transmission in the absence of antiretroviral treatment, but the effects of antiretroviral treatment, now widely used for prevention, on development of these cell-mediated responses is unknown. We tested whether development of T-helper cell responses to HIV and other antigens would be affected by exposure to short-course regimens of zidovudine-lamivudine (ZDV-3TC) given to prevent maternal-infant HIV transmission. Cord blood samples were collected from 41 infants of HIV-infected mothers enrolled in a clinical trial in which they were treated with regimens of ZDV-3TC and from 29 infants whose HIV-infected mothers were not treated with any antiretroviral drugs. T-helper cell reactivity to HIV envelope peptides and other antigens was measured in vitro using a sensitive culture supernatant titration assay based on IL-2-dependent proliferation. Infants in the clinical trial were followed to 18 months to determine their HIV infection status, and venous blood samples were re-tested at 4·5 and 9 months for T-cell reactivity to HIV. HIV-stimulated T-helper cell reactivity in cord blood was detected 10-fold less frequently among those exposed to antiretroviral prophylaxis (2·4%) than among those unexposed (24·1%) (P = 0·007). Reductions in HIV-stimulated responses in cord blood occurred despite detectable HIV RNA (mean 3·38 standard deviation 0·76 log10 copies per ml) at delivery among treated women and occurred independent of treatment duration. Our results suggest that short-course antiretroviral treatment given to prevent maternal-infant HIV transmission may attenuate HIV-stimulated T-cell memory responses in the neonate.
Keywords: Human immunodeficiency virus, cellular immunity, interleukin-2, antiretroviral treatment, maternal-infant transmission
INTRODUCTION
There is accumulating evidence to support the importance of HIV-specific cell-mediated immune responses in protection against sexual and maternal-infant HIV transmission [1,2]. T-helper cell and cytotoxic T-lymphocyte (CTL) responses to HIV are frequently detected in individuals exposed to HIV, often repeatedly and over extended periods, but who remain apparently protected against infection [3–10]. It has been inferred, but has proved difficult to demonstrate, that these cellular responses constitute a component of acquired immunity to HIV. Uninfected infants of HIV-infected mothers are a special case of an ‘exposed-uninfected’ population. HIV-specific CTL responses have been documented in some uninfected infants of untreated, HIV-infected mothers [11–15], but not all studies have been able to elicit CTL responses in exposed uninfected neonates [16]. In contrast, T-helper cell responses to HIV have been consistently observed among 20–40% of uninfected infants and correlate with reduced risk of maternal-infant HIV transmission [17,18] even when the child is re-exposed to HIV via breast-feeding [19].
Use of antiretroviral treatment is now widespread to prevent maternal-infant HIV transmission in richer countries, with international research efforts devoted to testing shorter and simpler regimens more applicable to low resource settings where the HIV epidemic predominates. Even short antiretroviral regimens can substantially reduce transmission [20–22] although the mechanisms which account for observed benefits are still poorly understood. Anti-retroviral-drug-induced suppression of maternal viral load appears to account for only a small proportion of the reduction in transmission [23]. Effects of antiretroviral treatment on newborn immune function, particularly on development of cell-mediated immune responses to HIV thought to be important in protection against infection in the absence of antiretroviral treatment, have not been investigated. To address this gap, we tested whether exposure to short-course regimens of zidovudine-lamivudine (ZDV-3TC) given to prevent maternal-infant HIV transmission would affect development of T-helper cell responses to HIV and other antigens among uninfected infants of HIV-infected mothers.
METHODS
The study population included HIV-seropositive women and their infants enrolled at the Soweto, South Africa, site of the UNAIDS-sponsored clinical trial of short-course ZDV-3TC [24,25]. As part of the clinical trial, women had been randomized to one of four groups. The first group of women received 300 mg ZDV and 150 mg 3TC twice daily from 36 weeks. From the onset of labour, they received 300–600 mg ZDV then 300 mg every 3 h and 150 mg 3TC every 12 h until delivery, and for one week postpartum. Neonates were given 4 mg ZDV and 2 mg 3TC per kg twice daily for one week. The second group received only the intrapartum and postpartum components of the regimen; the third group only the intrapartum component; and the fourth group placebo. The inclusion criteria for the clinical trial were availability for enrolment at less than 38 weeks gestation, haemoglobin > 8 g/dl, and no prior antiretroviral drug treatment. Written, informed consent was obtained from all study participants.
Over a specified period of time, cord blood was collected from all possible clinical trial participants delivering at the site. Cord blood was collected by cordocentesis, to avoid maternal contamination, immediately after delivery of the placenta. Samples from 47 deliveries were collected (Table 1). A venous blood sample was collected from these infants, if possible, at 4·5 and 9 months. All samples were drawn into EDTA tubes.
Table 1.
Clinical characteristics of HIV-seropositive mother-child pairs enrolled in the clinical trial of zidovudine-lamivudine for whom cord blood samples were collected
| Randomized to receive antiretroviral treatment | Randomized to placebo | Total | |
|---|---|---|---|
| N | 41 | 6 | 47 |
| Mean (standard deviation) | |||
| CD4 + T-lymphocyte count | 459 ± 322 | 437 ± 246 | 456 ± 311 |
| Ratio CD4:CD8 | 0·61 ± 0·41 | 0·58 ± 0·27 | 0·61 ± 0·40 |
| Age (years) | 27 ± 4·6 | 23 ± 7·8 | 27 ± 5·2 |
| Gestational Age (weeks) | 39 ± 1·6 | 39 ± 0·7 | 39 ± 1·5 |
| Birth weight (grams) | 3020 ± 375 | 3112 ± 319 | 3031 ± 366 |
| Percent (n/N) | |||
| Infant gender male | 61 (25/41) | 50 (3/6) | 60 (28/47) |
| Primiparity | 27 (11/41) | 33 (2/6) | 28 (13/47) |
| HIV-related symptoms | 2.4 (1/41) | 0 (0/6) | 2·1 (1/47) |
| Infant breast-fed | 2·4 (1/41) | 17 (1/6) | 4·3 (2/47) |
| Infant HIV-infected | 5·0 (2/40) | 0 (0/6) | 4·3 (2/46)* |
One infant was lost to follow-up before HIV status could be determined
Since the placebo group was suspended soon after sample collection for the present study began, only 6/47 cord blood samples collected were from the placebo group. We therefore supplemented the study by collecting anonymous cord blood from 23 infants of HIV-seropositive women at the same site who did not receive any antiretroviral treatment. Routine antiretroviral treatment was not available at the time this study was conducted. Cord blood samples from 23 HIV-seronegative women at the same site were collected as controls.
Infant blood sampling at regular intervals to 18 months occurred as part of the larger clinical trial to determine the child's infection status. All 6 week samples were tested with HIV DNA PCR. If this result was positive, the birth sample was PCR tested to establish the timing of infection. All available 12, 15 and 18 month samples were tested for HIV antibodies. A child was defined as infected on the basis of a positive 6 week PCR and was defined as uninfected if the 6 week PCR test was negative and, at 12 months or older, the child seroreverted to become HIV antibody negative. HIV RNA was quantified in maternal plasma collected at the time of delivery using the Chiron bDNA assay in 10 of the women treated with antiretroviral drugs. Only 2/47 infants included in the clinical trial were breast-fed; all the others were fed with milk formula from birth. Identifying information was not collected on mother-child pairs who were not part of the clinical trial (i.e. 23 untreated HIV-seropositive mothers and 23 HIV-seronegative mothers for whom only anonymous cord blood was collected) thus they were not available for follow-up.
Samples were tested within 24 h for in vitro T-helper cell responses to HIV envelope peptides and other antigens using a sensitive culture supernatant titration assay based on IL-2-dependent proliferation in a continuous T lymphocyte cell line (CTLL) as previously described [3–5,7–10,18,26,27]. A cocktail of synthetic envelope peptides (shown in Table 2) were used to measure HIV-specific responses [28–30]. Responses to phytohemaglutinin (PHA), to nonviral cellular antigens using undepleted allogenic peripheral blood leucocytes (ALLO) and allogenic peripheral blood leucocytes depleted of antigen presenting cells (APC) by nylon wool adherence (ALLO-NWD), and to recall antigen using Influenza A (FLU) were assessed. Leukocytes for the ALLO and ALLO-NWD stimuli were prepared and aliquoted at the National Cancer Institute from a pool of irradiated (50 Gy) cells from two healthy adult blood donors and shipped on dry ice to Johannesburg for use.
Table 2.
| HIV-1 gp160 peptides | Peptide sequences | Amino acid residue |
|---|---|---|
| Constant regions | ||
| T2 | HEDIISLWDQSLK | aa 112–124 |
| T1 | KQIINMWQEVGKAMYA | aa 428–443 |
| TH4·1 | DRVIEVVQGAYRAIR | aa 834–848 |
| Hypervariable loop | ||
| P18 MN | RIHIGPGRAFYTTKN | aa 315–329 |
| P18 IIB | RIQRGPGRAFVTIGK | aa 315–329 |
The HIV stimulus was selected based on previous studies which have identified these peptides to be broadly immunogenic across MHC haplotypes [28–30], and which have documented T-helper cell responses to these peptides in several, independent populations of exposed, uninfected individuals [3,5,7,8,18,27] suggesting some role in protection against primary infection. As positive control antigens, PHA, ALLO and ALLO-NWD were used. PHA was selected to measure helper T-cell responses to mitogen, a powerful stimulus eliciting nonspecific responses independent of priming or APC function. ALLO was selected to measure response to weaker allogenic stimulation, also nonspecific and independent of prior priming, but requiring APC function. Since the ALLO stimulus itself may provide some APC help in culture, we also used a stimulus consisting of allogenic cells depleted of their APC (ALLO-NWD). This was used to confirm APC function of the cord blood leucocytes themselves. As a negative control antigen, we selected the recall antigen FLU [31,32]. A positive response to this antigen would imply helper T-cell response, APC function, and prior antigenic priming to this virus either because of an intrauterine infection or immunization after contact with the virus in utero. One prior study had observed response to this stimulus to be rare in cord blood leucocytes [18].
Within 24 h of sample collection, mononuclear cells were separated on Ficoll-Hypaque, washed twice in PBS, and the number of viable leucocytes determined by trypan blue exclusion. PBMC were re-suspended at 3 × 106/ml in RPMI containing 100 U/ml penicillin and 2 mm glutamine. 3 × 105 PBL were placed in flat bottom wells of a microtitre culture plate in a final volume of 0·2 ml along with the stimuli: synthetic HIV-1 (env) at a final concentration of 2·5 µm, ALLO and ALLO-NWD at 1 × 105 cells per well, FLU at 1 : 500, and PHA at a final concentration of 1 : 100. Three replicates and controls were performed for each stimulus. Human AB serum (5% in medium) was added to each well an hour after sensitization, and, anti-IL-2 receptor antibody (monoclonal anti-Tac) was added to the cell cultures on the first day (concentration 2 mg/ml) to inhibit IL-2 consumption.
The ability of PBMC to produce stimulus-induced IL-2 was determined by culturing the PBMC at 37°C in a moist, 7% CO2 atmosphere in flat-bottomed tissue culture plates for 7 days. Culture supernatants were then harvested, frozen and stored at − 20°C until assayed for IL-2 production.
The IL-2 production assay consisted of culturing 8 × 103 IL-2 dependent CTLL [33,34] per well in the presence of three two-fold dilutions of un-stimulated or stimulated culture supernatants. Twenty-four hours later, the cultures were pulsed with 1uCi of 3H-thymidine and harvested after 18 h using a 96-well cell harvester. 3H determinations were made using a LKB Beta-plate spectrometer.
Results were expressed as stimulation indices (SI) which are the ratios of the mean counts per minute (cpm) in stimulated to un-stimulated cultures. Stimulation indices > 3 were considered as a positive response to a particular antigen. Fisher's exact test (P-value 2-tailed) was used to compare the proportions positive across groups. Mann Whitney U-tests were used to compare the maternal HIV RNA copy numbers.
RESULTS
T-helper cell reactivity to HIV envelope peptides was rare (1/41; 2·4%) among infants whose HIV-seropositive mothers received any antiretroviral treatment. In contrast, reactivity to HIV peptides was 10-fold more frequent (7/29;24·1%) among infants of HIV-seropositive women who received no treatment (P = 0·007) (Table 3). None of the 23 control samples from HIV-seronegative women responded to synthetic HIV envelope peptides. Responses to other stimuli were similar among cord blood from HIV-seropositive and HIV-seronegative women (Table 3).
Table 3.
T-helper cell responses to HIV and other stimuli in cord blood from infants of HIV-seronegative women and HIV-seropositive women treated or untreated with short course zidovudine-lamivudine to prevent transmission
| N (%) infants with positive T-helper cell responses to each stimulus in cord blood | ||||
|---|---|---|---|---|
| HIV-seropositive women | ||||
| Stimulus* | HIV-seronegative women (n = 23) | Anti-retroviral treatment (n = 41) | No treatment (n = 29) | P-value† |
| HIV peptides | 0 (00·0) | 1 (2·4) | 7 (24·1) | 0·007 |
| PHA | 23 (100·0) | 39 (95·1) | 28 (96·6) | > 0·99 |
| ALLO | 10 (50·0)‡ | 21 (51·2) | 18 (62·1) | 0·466 |
| ALLO-NWD | 7 (35·0)‡ | 16 (39·0) | 11 (37·9) | > 0·99 |
| FLU | 0 (00·0) | 3 (7·3) | 6 (20·7) | 0·148 |
HIV peptides were a cocktail of synthetic envelope peptides (T1, T2, TH4·1, P18 MN, P18 IIIB). PHA (phytohemaglutinin) was used as mitogen, ALLO was a pool of undepleted leucocytes from 2 unrelated donors as stimulator cells, ALLO-NWD was a pool of leucocytes from 2 unrelated donors depleted of antigen presenting cells by nylon wool adherence as stimulator cells, and influenza A (FLU).
Two-tailed P-value from Fisher's exact test comparing treated and untreated groups.
Responses to ALLO and ALLO-NWD were measured only among 20 control samples.
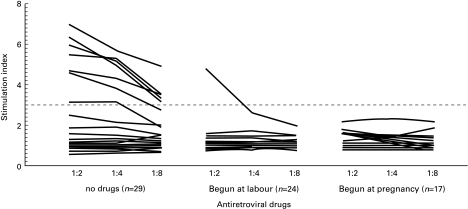
HIV RNA was above the threshold of detection at delivery in all women treated with antiretroviral drugs (mean [standard deviation] 3·38[0·76] log10 copies per ml). If ZDV-3TC was started during pregnancy, RNA copy numbers were lower (although not statistically significantly lower) (mean 3·19[0·66]) than if drugs were only started at the onset of labour (mean 3·51[0·86]). However, the reduction in HIV-stimulated responses among the treated group was observed regardless of the duration of exposure to antiretroviral treatment (Fig. 1).
Fig. 1.
Strength of T-cell responses to HIV envelope peptides in cord blood by exposure to antiretroviral drugs. Dashed line shows stimulation index of 3 above which was considered a positive response.
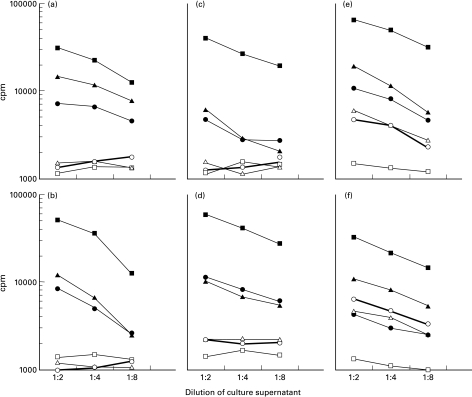
Anti-retroviral treatment was not significantly associated with reduced helper cell responses to other stimuli. Most samples were strongly reactive to PHA, and the magnitude of stimulation indices did not differ between treated and untreated. Percentage of samples reactive to ALLO and to ALLO-NWD were similar among treated and untreated. The percentage responding to FLU was lower among those whose mothers were treated with antiretroviral drugs, but the difference did not reach significance (Table 3). Examples of cord blood responses to each stimulus from 6 subjects is shown in (Figure. 2).
Fig. 2.
Examples of cord blood results from 6 of the 93 subjects: (a-b) HIV-seronegative controls, (c-d) HIV-seropositive mothers treated with antiretroviral drugs, (e-f) HIV-seropositive mothers not treated with antiretroviral drugs. The x-axis indicates the three culture supernatant dilutions, and the y-axis indicates the radioactivity uptake measured in counts per minute. □ Medium; ▪ PHA; ▴ Allo; • ALLO-NWD; ▵ FLU; ○ HIV envelope peptides. Individual points represent the means of triplicate cultures.
A hierarchical trend in responses to the stimuli was observed: a response to PHA occurred among all who responded to ALLO (49/49), and response to ALLO among most (28/35) responding to ALLO-NWD and among most (7/8) responding to HIV peptides. However, response to HIV peptides was independent of response to ALLO-NWD (responses to HIV were observed among 3/35 who responded to ALLO-NWD and among 5/55 who did not) (Table 4). Discordance between HIV and FLU results were common, occurring among 7 of the cord blood samples tested.
Table 4.
Patterns of T-helper cell responses in cord blood from infants of HIV-seronegative and HIV-seropositive women treated and untreated with short course zidovudine-lamivudine
| Number with each pattern of responses: | ||||||||
|---|---|---|---|---|---|---|---|---|
| T-helper responses to: | HIV-negative | HIV-positive | ||||||
| PHA | ALLO | ALLO-NWD | FLU | HIV | Controls | Treated | Untreated | Total |
| − | − | − | − | − | 0 | 2 | 1 | 3 |
| + | − | − | − | − | 9 | 13 | 8 | 30 |
| + | − | − | − | + | 0 | 0 | 1 | 1 |
| + | + | − | − | − | 4 | 10 | 3 | 17 |
| + | + | − | − | + | 0 | 0 | 2 | 2 |
| + | + | + | − | − | 6 | 9 | 7 | 22 |
| + | − | + | − | − | 1 | 4 | 1 | 6 |
| + | − | + | + | − | 0 | 1 | 0 | 1 |
| + | + | + | + | − | 0 | 0 | 1 | 1 |
| + | + | − | + | + | 0 | 0 | 2 | 2 |
| + | + | + | + | − | 0 | 1 | 1 | 2 |
| + | + | + | + | + | 0 | 1 | 2 | 3 |
| 20 | 41 | 29 | 90 | |||||
Among the 41 infants exposed to antiretroviral prophylaxis, the one infant who had had a positive HIV-stimulated T-helper cell response in cord blood was found to be uninfected on follow-up, and had no detectable T-cell responses to HIV when tested again at 4·5 and at 9 months whereas 2/39 (5·1%) who had no detectable T-cell response to HIV in cord blood were found to be HIV-infected on later follow-up. One of the infected infants was PCR positive at birth, the other was PCR negative at birth and PCR positive at 6 weeks. One child was lost to follow-up before HIV status could be determined. The transmission rate is consistent with that observed in the larger clinical trial cohort recruited at this site (∼8%) [24,25].
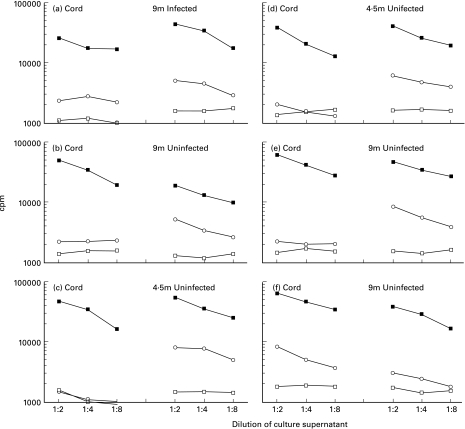
The drug-associated reduction in HIV-stimulated responses appeared to be transient. When re-tested at 4·5 and/or 9 months, 5/30 (16·7%) infants exposed to ZDV-3TC perinatal treatment had HIV-stimulated helper cell responses in venous blood compared to the 1/41 (2·4%) in cord blood (P = 0·03). No postnatal antiretroviral therapy, other than the one week component of the prevention regimen, was given to these infants. Only one of the infants responding to HIV-stimulation during the first year of life was infected; she reacted to HIV at 9 months but the cord blood had not responded. Thus among uninfected infants of HIV-seropositive mothers treated with short-course ZDV-3TC, 4/29 (13·8%) elicited T-helper cell responses to HIV envelope peptides at 4·5 or 9 months compared to 1/38 (2·6%) in cord blood (P = 0·08). None of these responding infants was breast-fed. The results of infants with discordance in HIV-stimulated T-helper cell responses between cord and venous blood samples are shown in Fig. 3. Of the other 6 children in the trial's placebo arm, none was found to be infected (Table 1), and of the 4 available for follow-up T-cell function testing, none had HIV-specific responses.
Fig. 3.
The results of 6 children of HIV-seropositive mothers treated with ZDV-3TC with discordance in HIV-stimulated T-helper cell responses between cord and venous blood samples. (a) An infected child unresponsive to HIV envelope peptides in cord blood but with a detectable response at 9 months. (b–e). Uninfected children unresponsive to HIV envelope peptides in cord blood but with a detectable responses at 4·5 or 9 months. (f) An uninfected child responsive to HIV envelope peptides in cord blood but with no detectable response at 9 months. □ Medium; ▪ PHA; ○ env. Blood sample volumes were insufficient during infancy to test responses to the other antigens. The x-axis indicates the three culture supernatant dilutions, and the y-axis indicates the radioactivity uptake measured in counts per minute. Individual points represent the means of triplicate cultures.
DISCUSSION
This study was designed to investigate fetal cell-mediated immune responses following treatment with short-course antiretroviral therapy to reduce maternal-infant HIV transmission. We observed that whereas T-helper cell responses to HIV peptides were elicited in cord blood from a quarter of infants from untreated HIV-infected mothers, these responses were almost entirely absent in cord blood from infants of mothers treated with ZDV-3TC. Thus it appears unlikely that strengthening of fetal T-helper cell responses to HIV explains benefits of antiretroviral treatment since antiretroviral exposure appears to reduce these responses. Significant reductions in T-helper reactivity was observed only for HIV-specific responses. No differences were observed in responses to PHA, ALLO and ALLO-NWD, and differences in FLU responses were not significant.
Early discontinuation of the placebo group in the clinical trial precluded comparing cord blood responses among those randomly assigned to treatment versus no treatment. We therefore collected an unselected sample of anonymous cord bloods from the same site soon after the end of the clinical trial as untreated controls. Clinical trial participants were representative of the population of women receiving care at the site, since inclusion criteria were broad, hence there is little reason to believe that that the anonymous controls differed appreciably from the trial participants. The frequency of detection of HIV-stimulated T-cell responses among the anonymous control group (24%) is also highly similar to that observed in other populations of untreated, HIV-seropositive deliveries (Table 5). Small variations in maternal clinical parameters between the two groups are unlikely to account for a 10-fold difference in HIV-stimulated responses.
Table 5.
Comparison of the frequency of HIV-stimulated helper T-cell reactivity in cord blood of HIV-seropositive women in current study vs. two previous studies
| Location of study | Antiretroviral drug use during pregnancy | Mean CD4 count | Mean CD4:CD8 ratio | Mean gestational age | % positive helper T-cell responses to HIV envelope peptides |
|---|---|---|---|---|---|
| Washington, DC [18] | No | 595 | 0·76 | 38 weeks | 35% (8/23) |
| Durban, South Africa [19] | No | 466 | 0·64 | 39 weeks | 38% (33/86) |
| Soweto, South Africa | Yes | 459 | 0·61 | 39 weeks | 2% (1/41) |
Reasons for the reduction in HIV-stimulated T-helper cell responses associated with antiretroviral exposure which we observed are likely to be complex and need to be further investigated. One possible explanation is that the antiretroviral treatment successfully eliminated HIV from maternal compartments, effectively preventing or reducing antigenic exposure (the source of both infection and priming of HIV-specific helper cell responses). Reductions in viral exposure may not, however, be the only explanation for our findings since all women on treatment who we tested had levels of HIV RNA in plasma at delivery above the threshold of detection. Reductions in HIV exposure can also not explain the reductions in HIV-stimulated responses we observed when treatment was started only in the intrapartum period. Previous phase II studies of similar doses of ZDV-3TC observed reductions in HIV RNA copies in plasma ∼1 log10 with a week of treatment, but no changes in viral levels until more than 24 h after the start of treatment [35]. Anti-retroviral treatment started soon after primary HIV infection in adults has been observed to result in weakened HIV-specific cellular and humoral immune responses [36,37] and it has generally been assumed that this is due to reductions in antigenic stimulation. However, it does not necessarily follow that the consequences of antiretroviral treatment for individuals recently infected will be the same as for individuals recently exposed but uninfected. In animal models of exposure but no infection, decreases in infectious doses tend to favour the development of protective type 1 memory immune responses not reduce them [38,39].
ZDV and 3TC both freely cross the placenta, and with maternal dosing, drug concentrations in maternal and cord blood are highly similar [35]. A second explanation, that antiproliferative effects of ZDV account for reduction in HIV-stimulated T-helper cell responses, seems unlikely, since no changes were observed in responses to PHA or ALLO. Primary responses, even to ALLO-NWD which requires antigen-presenting capacity but not prior priming, were unaffected.
Neonatal T-cell have the capacity to develop mature antigen-specific cell-mediated immune responses [38,40] but may be highly sensitive to the conditions required to promote and support the maintenance of these responses. A third explanation is that rather than reducing antigenic exposure, antiretroviral drugs influenced other processes involved in the generation of secondary, antigen-specific memory responses, such as costimulatory signals or APC function. In at least one other study of ‘exposed-uninfected’ individuals (health care workers with occupational exposures to HIV) reductions were observed in HIV-specific CTL responses among those who received ZDV (1/7) compared to those who did not (6/13) [41]. In a non-HIV context, ZDV has been shown to inhibit growth of alloreactive CD8 + cytotoxic T-lymphocyte (CTL) clones in graft-vs.-host disease [42]. If antiretroviral drugs influence processes involved in the support of T-cell memory, response to vaccines given at birth might be compromised and could influence current studies supplementing antiretroviral prevention of perinatal HIV transmission with vaccines.
Recall responses to FLU in fetal cord blood were detected among only 1/23 (4%) HIV-exposed and no unexposed deliveries in the U.S. [18], but responses to FLU in fetal cord blood among deliveries in Africa, where viral exposure may be greater, have not been investigated by others. Although no FLU responses were observed among deliveries of HIV-seronegative women in our study it seems unlikely that the detected FLU responses are simply cross-reactive with HIV antigens since there were many discordant results within the HIV-exposed population. We previously observed that HIV-stimulated, but not FLU-stimulated, T-cell responses were associated with lack of vertical HIV transmission [19] suggesting they indicate different responses. T cell responses to other recall antigens need to be investigated for susceptibility to drug-induced suppression. However, identification of an appropriate positive control recall antigen is difficult in the neonate since exposure to most common recall antigens is thought not to occur in utero.
We observed that short-course ZDV-3TC given to prevent perinatal HIV transmission reduced memory T-cell responses to HIV in the newborn. This raises the concern that antiretroviral exposure may have adverse immunological consequences. In two natural history cohorts, HIV-infected children exposed to ZDV during the pre‐ and peri-natal period were observed to have more rapid disease progression than unexposed children if effective combination therapy was not promptly initiated [43,44]. Of greater concern, loss of T-helper cell responses to HIV may increase vulnerability of uninfected infants to postnatal transmission with continued exposure to HIV through breast-feeding. However, in at least one study, no increase in breast-feeding transmission with short-course ZDV was observed [45]. Effects of antiretroviral exposure on helper cell responses may be transient opening only a narrow window of increased susceptibility. Further investigation of the duration and nature of these apparent attenuations in cellular immune responses in newborns exposed to antiretroviral treatment is needed and may provide insight into mechanisms of infection in the context of antiretroviral exposure. However, until further understanding of the immunological consequences of antiretroviral treatment can be obtained, existing guidelines encouraging use of antiretroviral drugs to prevent maternal-infant HIV transmission should be supported unchanged.
Acknowledgments
We would like to thank Dr Zena Stein for helpful advice throughout the study and Sr Shabalala and Brenda Kilroe for technical assistance.
REFERENCES
- 1.Shearer GM, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–4. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg ES, Walker BD. HIV type 1-specific helper T cells: a critical host defense. AIDS Res Hum Retroviruses. 1998;14(Suppl. 2):S143–S147. [PubMed] [Google Scholar]
- 3.Clerici M, Giorgi JV, Chou CC, et al. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J Infect Dis. 1992;165:1012–9. doi: 10.1093/infdis/165.6.1012. [DOI] [PubMed] [Google Scholar]
- 4.Beretta A, Weiss SH, Rappocciolo G, et al. Human immunodeficiency virus type 1 (HIV-1) -seronegative injection drug users at risk for HIV exposure have antibodies to HLA class I antigens and T cells specific for HIV envelop. J Infect Dis. 1996;173:472–6. doi: 10.1093/infdis/173.2.472. [DOI] [PubMed] [Google Scholar]
- 5.Mazzoli S, Trabattoni D, Lo Caputo S, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nature Med. 1997;3:1250–7. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 6.Rowland-Jones S, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nature Med. 1996;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 7.Kaul R, Trabattoni D, Bwayo J, et al. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–9. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Levin JM, Kessler HA, et al. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA. 1994;271:42–6. [PubMed] [Google Scholar]
- 9.Langlade-Demoyen P, Ngo-Giang-Huong N, Ferchal F, Oksenhendler E. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J Clin Invest. 1994;93:1293–7. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinto LA, Sullivan J, Berzofsky JA, et al. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J Clin Invest. 1995;96:867–76. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–1. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 12.Cheynier R, Langlade-Demoyen P, Marescot MR, et al. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur J Immunol. 1992;22:2211–7. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 13.Aldhous MC, Watret KC, Mok JY, Bird AG, Froebel KS. Cytotoxic T lymphocyte activity and CD8 subpopulations in children at risk of HIV infection. Clin Exp Immunol. 1994;97:61–7. doi: 10.1111/j.1365-2249.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maria A, Cirillo C, Moretta L. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. J Infect Dis. 1994;170:1296–9. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]
- 15.Mcfarland EJ, Harding PA, Luckey D, Conway B, Young RK, Kuritzkes DR. High frequency of Gag- and envelope-specific cytotoxic T lymphocyte precursors in children with vertically acquired human immunodeficiency virus type 1 infection. J Infect Dis. 1994;170:766–74. doi: 10.1093/infdis/170.4.766. [DOI] [PubMed] [Google Scholar]
- 16.Luzuriaga K, Koup RA, Pikora CA, Brettler DB, Sullivan JL. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991;119:230–6. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 17.Wasik TJ, Bratosiewicz J, Wierzbicki A, et al. Protective role of B-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J Immunol. 1999;162:4355–64. [PubMed] [Google Scholar]
- 18.Clerici M, Sison AV, Berzofsky JA, et al. Cellular immune factors associated with mother-to-infant transmission of HIV. AIDS. 1993;7:1427–33. doi: 10.1097/00002030-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn L, Coutsoudis A, Moodley D, et al. T-helper cell responses to HIV envelope peptides in cord blood: Protection against intrapartum and breastfeeding transmission. AIDS. 2001;15:1–9. doi: 10.1097/00002030-200101050-00003. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomized controlled trial. Lancet. 1999;353:773–80. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 21.Wiktor SZ, Ekpini ER, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Côte D'Ivoire: a randomized trial. Lancet. 1999;353:781–5. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 22.Dabis F, Msellati A, Meda N, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breast fed children in Côte d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. Lancet. 1999;353:786–92. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 23.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med. 1996;335:1621–9. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 24.Saba J. Results of the PETRA intervention trial to prevent perinatal transmission in sub-Saharan Africa. 6th Conference on Retroviruses and Opportunistic Infections; January 31–February 4; Chicago, IL. [Google Scholar]
- 25.Gray G. The PETRA study: early and late efficacy of three short ZDV/3TC combination regimens to prevent mother-to-child transmission of HIV-1. XIII International AIDS Conference; 9–14 July; Durban, South Africa. [Google Scholar]
- 26.Clerici M, Stocks NI, Zajac RA, et al. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature. 1989;339:383–5. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- 27.Clerici M, Berzofsky JA, Shearer GM, Tacket CO. Exposure to human immunodeficiency virus type 1-specific T helper cell responses before detection of infection by polymerase chain reaction and serum antibodies. J Infect Dis. 1991;164:178–82. doi: 10.1093/infdis/164.1.178. [DOI] [PubMed] [Google Scholar]
- 28.Berzofsky JA, Pendleton CD, Clerici M, et al. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and humans of multiple MHC types. J Clin Invest. 1991;88:876–84. doi: 10.1172/JCI115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hale PM, Cease KB, Houghten RA, et al. T cell multideterminant regions in the human immunodeficiency virus envelop: toward overcoming the problem of major histocompatibility complex restriction. Int Immunol. 1989;1:407–15. doi: 10.1093/intimm/1.4.409. [DOI] [PubMed] [Google Scholar]
- 30.Cease KB, Margalit H, Cornette JL, et al. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelop protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc Natl Acad Sci USA. 1987;84:4249–53. doi: 10.1073/pnas.84.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clerici M, Depalma L, Roilides E, Baker R, Shearer GM. Analysis of T helper and antigen-presenting cell functions in cord blood and peripheral blood leukocytes from healthy children of different ages. J Clin Invest. 1993;91:2829–36. doi: 10.1172/JCI116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clerici M, Seminari E, Suter F, et al. Different immunologic profiles characterize HIV infection in highly active antiretroviral therapy-treated and antiretroviral-naive patients with undetectable viraemia. AIDS. 2000;14:109–16. doi: 10.1097/00002030-200001280-00005. [DOI] [PubMed] [Google Scholar]
- 33.Gillis S, Ferm MM, Ou W, Smith KAT. cell growth factor: Parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027–32. [PubMed] [Google Scholar]
- 34.Gillis S, Smith KA. Long-term culture of tumor-specific cytotoxic T cells. Nature. 1977;268:154–6. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 35.Moodley J, Moodley D, Pillay K, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis. 1998;178:1327–33. doi: 10.1086/314431. [DOI] [PubMed] [Google Scholar]
- 36.Markowitz M, Vesanen M, Tenner-Racz K, et al. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J Infect Dis. 1999;179:527–37. doi: 10.1086/314628. [DOI] [PubMed] [Google Scholar]
- 37.Tindall B, Carr A, Goldstein R, Penny R, Cooper DA. Administration of zidovudine during primary HIV-1 infection may be associated with a less vigorous immune response. AIDS. 1993;7:127–8. doi: 10.1097/00002030-199301000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 39.Clerici M, Clark EA, Polacino P, et al. T-cell proliferation to subinfectious SIV correlates with lack of infection after challenge of macaques. AIDS. 1994;8:1391–5. doi: 10.1097/00002030-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Trivedi HN, Hayglass KT, Gangur V, Allardice JG, Embree J, Plummer F. Analysis of neonatal T cell and antigen presenting cell functions. Human Immunol. 1997;57:69–79. doi: 10.1016/s0198-8859(97)00202-4. [DOI] [PubMed] [Google Scholar]
- 41.D'Amico R, Pinto LA, Meyer P, et al. Effect of zidovudine postexposure prophylaxis on the development of HIV-specific cytotoxic T-lymphocyte responses in HIV-exposed healthcare workers. Infect Control Hospital Epidemiol. 1999;20:428–30. doi: 10.1086/501646. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura M, Hidaka N, Akaza T, Tadokoro K, Juji T. Differential effect of azidothymidine on resting and activated cells: Potentiality of this drug for treatment of post-transfusion graft-versus-host disease. Res Comm Mol Pathol Pharmacol. 1998;100:131–8. [PubMed] [Google Scholar]
- 43.Italian Registry for HIV Infection in Children. Rapid disease progression in HIV-1 perinatally infected children born to mothers receiving zidovudine monotherapy during pregnacy. AIDS. 1999;13:927–33. [PubMed] [Google Scholar]
- 44.Kuhn L, Abrams EJ, Weedon J, et al. Disease progression and early viral dynamics in HIV-infected children exposed to zidovudine during the pre- and peri-natal period. J Infect Dis. 2000;182:104–11. doi: 10.1086/315678. [DOI] [PubMed] [Google Scholar]
- 45.Ditrame ANRS 049 Study Group. 15-month efficacy of maternal oral zidovudine to decrease vertical transmission of HIV-1 in breastfed African children. Lancet. 1999;354:2050–1. [PubMed] [Google Scholar]