Abstract
Activation of signal transducer and activator of transcription 1 (STAT1) is a hallmark of IFN-γ receptor signal transduction but is also part of the signalling pathway of other cytokines/growth factor receptors. In ulcerative colitis, high levels of activation and expression of STAT1 have been observed in comparison with both Crohn's Disease and normal controls. Pouchitis develops in some patients after Ileal-Pouch-Anal-Anastomosis (IPAA). The pathophysiology and aetiology of pouchitis is still unclear. Recent studies have shown an increased production of proinflammatory cytokines including IFN-γ. To investigate the expression and activation of STAT1 in pouchitis and the influence of treatment, patients were followed longitudinally from pouch operation. Diagnosis of pouchitis was made by clinical, endoscopic and histological criteria. Biopsies were obtained during routine endoscopy and snap frozen in liquid nitrogen. Nuclear and cytosolic extracts were prepared and the expression and activation of specific transcription factors were assessed by Western blot, electrophoretic mobility shift assay and immunofluorescence. Patients who develop pouchitis show highly increased levels of STAT1α as well as STAT1β expression and activation in comparison with both normal pouch and normal ileal mucosa. Improvement of pouchitis during antibiotic therapy relates to a normalization of STAT1 expression and activation. We conclude that activation of STAT1 correlates to clinical disease activity and therefore STAT1 could play an important role in the pathophysiology of pouchitis. Similarities in the pattern of activation of STAT1 in pouchitis and ulcerative colitis may suggest a common pathway in the immunopathophysiology of both diseases.
Keywords: Inflammation, ulcerative colitis, signal transduction, intestinal mucosa, pelvic ileal pouch
INTRODUCTION
Restorative proctocolectomy with ileal-pouch anal anastomosis is a well-established surgical procedure for the treatment of ulcerative colitis and familial adenomatous polyposis (FAP) [1–3]. However a major clinical complication after pouch-operation is the development of pouch inflammation (pouchitis) [4, 5] with 15–46% of patients developing pouchitis within 5 years after operation [6, 7]. A chronic relapsing form of pouchitis can be distinguished from a chronic active form [8]. The aetiology of pouchitis is still unknown. Clinical and histopathological similarities between UC and pouchitis, coupled with the fact that pouchitis is more frequently observed in patients with ulcerative colitis than with familial adenomatous polyposis, has lead to the suggestion that pathophysiological similarities exist between both diseases [9–11]. Fecal stasis and bacterial overgrowth may play an important role [4, 12] and might explain the efficiacy of antibiotic treatment in pouchitis [13, 14]. Recent studies investigating the activation of cytokine transcription factors in inflammatory bowel disease (IBD) have shown an increased expression and activation of nuclear factor kappa B (NFκB) as well as members of the STAT(signal transducer and activator of transcription) family [15–17]. An increased expression and activation of STAT1 can be found in UC [18] whereas increased NFκB activation is seen more predominantly in Crohn's disease than in ulcerative colitis [15].
STAT proteins are dormant cytoplasmic transcription factors which consist of a 91-kD and a 84-kD domain, called STAT1 α and STAT1 β. STAT proteins are phosphorylated by janus kinases (JAK) in response to activation of cytokine and growth factor receptors. The process is particularly well characterized in the interferon receptor family: Binding of ligands to the IFNγ or IFNα receptor results in dimerization of the receptor. A complex between the receptor dimer and the kinases JAK1 and JAK2 (IFNγ) or JAK1 and Tyk2 (IFNα), respectively, induces phosphorylation of STAT1 or STAT2 [19, 20]. The mechanisms of STAT1 activation by other cytokine receptors (i.e. growth hormone, EGF and IL-2) is not as well characterized as for the interferons [21]. Activated STAT1 homo- or hetero-dimers translocate into the nucleus and can augment transcription by binding to specific DNA sequences in gene promoter regions of specific genes [22].
Mucosal concentrations of proinflammatory cytokines like interleukin-1ß, interleukin-6 and interleukin-8 are increased in pouchitis in a similiar fashion to ulcerative colitis [23, 24]. In patients with UC and pouchitis an increase in IFNγ producing cells can be found [25]. Therefore it appears interesting to elucidate the role of STAT1 activation in pouchitis.
MATERIALS AND METHODS
Patients
Thirty-six sequential patients who suffered from ulcerative colitis and who underwent restorative proctocolectomy with an ileal pouch anastomosis were included in our study. Patients were followed longitudinally from pouch operation. Pouchitis was diagnosed in 12 patients by histological and endoscopic criteria using the pouchitis disease activity index [26]. Stool culture and microscopic examination for parasites were negative.
Mucosal biopsies were obtained during routine endoscopy and immediately snap frozen in liquid nitrogen. All patients were treated with antibiotics (Rifaximin 2 g/d and Ciprofloxacin 1 g/d) as part of a protocol [14] which included biopsies at week 0 and 2 weeks after therapy. Longitudinal follow up was completed in 10 patients.
Nuclear extracts
Biopsies were snap frozen during endoscopy and later homogenized under liquid nitrogen. Nuclear and cytosolic extracts were prepared [27, 28] and solubilized in an aqueous buffer containing 20 mm Hepes(pH 7·9), 25% (v/v) glycerol, 0·1 m NaCl, 1·5 mm MgCl2, 0·2 mm EDTA, 0·5 mm phenylmethylsulphonate, 0·5 mm dithiothreitol, 1 µg/ml aprotinin, 1 µg/ml pepstatine, 1 µg/ml leupeptine, 1 mm benzamidine, 1 mm sodium vanadate, 1 mm NaF, 5 mm β-glycerolphosphate and NaCl.
Total cell lysates
Total cell lysates were prepared using an extraction buffer which was heated to 100°C, containing 1% sodiumdodecylsulphate, 10 mm Tris (pH 7·9) and 1 mm sodiumvanadate. Protein concentrations were assessed using a modified Bradford protein assay (Biorad, Hercules, CA). All samples were adjusted to a similar protein content before analysis and colloidal gold staining was performed on all membranes as a control for equal loading. It has been previously shown that colloidal gold staining relates closely to antihistone staining in nuclear extracts [15].
Western blot analysis
10 µl of cell lysate containing 5–10 µg protein were separated on 10% and 12% denaturing polyacrylamide gels. The separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane by electroblotting (Biorad), for 75 min at 20 V. The membrane was blocked in a buffer which contained 5% non fat milk, 10 mm Tris, pH 7·5, 100 mm NaCl and 0·1% Tween20 for 60 min at room temperature. Blocking buffer was decanted and the membranes were incubated with primary antibody diluted in blocking buffer on a shaker for 60 min at room temperature. After incubation membrane was washed four times with a TBST (Tris Buffered Saline Tween) buffer (10 mm Tris, pH 7·5, 100 mm NaCl and 0·1% Tween20). The secondary antibody (enzyme conjugated human antimouse IgG (horseradish peroxidase)) was diluted in blocking buffer and membranes were incubated for 60 min at room temperature (gently shaking). After a final washing step with TBST-buffer, protein bands were detected by chemiluminescence (Boehringer Mannheim) digitized and analysed by optical densitometry (Image Quant).
Electrophoretic mobility shift assay
The electrophoretic mobility shift assay (EMSA) was used to detect actived STAT1 [29, 30]. Nuclear protein-oligonucleotide binding studies were carried out for 30 min at 24°C in a 12·5-µl reaction volume containing 20 mm Hepes, pH 7·9, 10% glycerol, 1 mm MgCl2, 0·1 mm EGTA, 0·5 mm DTT, 0·25 µg/µl Poly d(I-C), 1 µg 32P-labelled oligonucleotide probe and 10 µg nuclear protein. Protein-DNA complexes were separated on 6% polyacrylamide gels [31]. The used oligonucleotide sequence for the STAT1 detection was the Fcγ R1-GAS (gamma interferon activation site) (5′tcgagtatttcccagaaaaggaac).
The specific retarded protein-bands were detected by autoradiography. The binding specificity was confirmed by incubating the samples with relevant as well as irrelevant oligonucleotides (β-casein GAS (5′ctgaagatttctaggaattcaaatc), ISG15-ISRE (interferon stimulated response element) (5′tcgagggaaaccgaaactg) and unlabelled FcγR1-GAS in 10-fold molar excess to compete binding. Incubation with anti-STAT1 antibody was used to block STAT1 signal.
Tissue processing and immunofluorescence
Biopsies were embedded in cryomatrix and snap-frozen in liquid nitrogen. Cryostat sections (7 µm thickness) were thaw-mounted on Superfrost ® slides, postfixed for 5 min in acetone, airdried and stored at −20°C before staining. Two slides of each biopsy were stained with haematoxylin-eosin for evaluation by routine histopathology. The other slides were permeabilized with 0·1% Triton X-100 in 0·1 m phosphat buffered saline (PBS), washed three times in PBS and blocked with 0·75% bovine serum albumin (BSA) in PBS. Sections were subsequently incubated with the respective antibodies (anti-STAT1 monoclonal antibody, Transduction Laboratories, Lexington, KY) all at 1 : 100 dilution in 0·75% bovine serum albumin in PBS for 1 h. After washing in PBS, tissue bound antibody was detected using biotinylated goat-antimouse IgG antibodies (Vector), followed by an avidin-FITC conjugated antibody (Vector), both diluted at 1 : 100 in 5% human serum. Controls with an irrelevant first antibody as well as secondary antibody and avidin-FITC were performed. Nuclear counterstaining with bisbenzimide was performed.
Fluorescence was detected by an Axiophot microscope (Zeiss, Germany) with the appropriate filter systems and photographs were taken on Provia 1600 colour films (Fuji). The numbers of STAT1 positive cells were counted in each sample per viewfield (magnification × 400).
Expression of data
Results were expressed as mean ± standard deviation, if not indicated otherwise. Statistical significance of the differences was examined with the student t-test for normally distributed data and with the Mann Whitney U-test or the Wilcoxon matched pairs test, respectively, for non-normally distributed data [32,33]. Distribution of data was evaluated by calculating Lilliefors probabilities based on the Kolmogorov-Smirnov test [34].
RESULTS
Expression of STAT1 protein
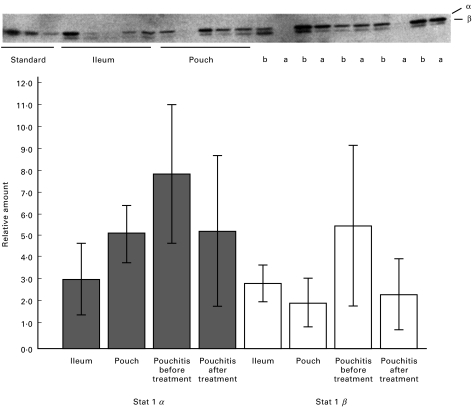
Total cell lysates of pouchitis mucosal biopsy samples were assessed by western blot for protein expression of STAT1. Increased amounts of STAT1 could be detected in non inflamed pouch ileal mucosa in comparison with preoperative normal terminal ileum (Fig. 1). Patients who developed pouchitis showed highly increased levels of STAT1α and β in comparison with both normal ileum and normal pouch mucosa (Fig. 1, P < 0·05 and P < 0·05, respectively).
Fig. 1.
Normal ileum, normal pouch and pouchitis before (b) and after (a) treatment were analysed for STAT1 by Western blot. In pouchitis a marked upregulation of the 91 kD STAT1 α domain (P < 0·05) and the 84 kD β domain (P < 0·05) expression is observed, which returns to levels of normal, uniflamed pouch after successful treatment. Comparison was made against normal controls. The Western blot was calibrated with a set of HT29 derived standards.
Biopsies from patients who had undergone an ileoanal pouch procedure after colectomy for familial adenomatous polyposis (FAP) served as controls. These samples showed low levels of STAT1 which were similiar to non inflamed pouch biopsies (data not shown). In 10 cases mucosal biopsies were taken at the time pouchitis was diagnosed and after antibiotic therapy, which was clinically successful in 9 patients (Table 1). After successful therapy, increased protein levels of STAT1α and β in pouchitis returned to levels similiar to non inflamed pouch (P < 0·5) (Fig. 1).
Table 1.
The pouchitis activity index (26) of all patients was equal or higher than 8 score points before treatment. After successful treatment, the pouchitis activity index decreased to 0 or 1 in 9 of 10 patients. Only 1 patient's pouchitis did not resolve after treatment. The patients did not receive any pretreatment
| Patient No. | Pouchitis activity index before treatment | Pouchitis activity index after treatment | Sex | Age | Duration of ulcerative colitis (years) | Extension of colitis |
|---|---|---|---|---|---|---|
| 1 | 12 | 0 | Female | 25 | 7 | Pancolitis |
| 2 | 11 | 0 | Male | 31 | 5 | Pancolitis |
| 3 | 10 | 0 | Male | 40 | 12 | Pancolitis |
| 4 | 8 | 1 | Female | 39 | 17 | Pancolitis |
| 5 | 9 | 1 | Female | 29 | 4 | Pancolitis |
| 6 | 12 | 0 | Male | 41 | 10 | Pancolitis |
| 7 | 12 | 7 | Female | 32 | 9 | Pancolitis |
| 8 | 11 | 0 | Male | 33 | 18 | Pancolitis |
| 9 | 9 | 1 | Male | 46 | 21 | Pancolitis |
| 10 | 10 | 0 | Female | 24 | 9 | Pancolitis |
Oligonucleotide binding activity of STAT1
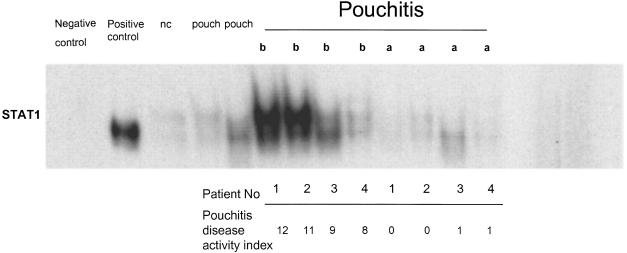
Electrophoretic mobility shift assay analysis demonstrated high levels of specific oligonucleotide binding activity of STAT1 in pouchitis which was greatly reduced by successful treatment. Western Blot confirmed an increased concentration of STAT1 in nuclear extracts from pouch biopsies. Successful treatment greatly reduced the amount of nuclear STAT1 concentrations (Fig. 2). Normal pouch showed no or weak STAT1 signals in oligonucleotide binding studies. The identity of STAT1 as the major part of the complex was confirmed by controls with blocking antibodies, unlabelled specific oligonucleotides and irrelevant oligonucleotides (Fig. 3).
Fig. 2.
Active STAT1 was detected by electrophoretic mobility shift assay.In normal control ileum (nc) no active STAT1 was found, whereas high levels of activation were seen in pouchitis biopsies before anti-inflammatory treatment (b). Normal pouch (pouch) showed only weak signals of STAT1 binding in comparison to pouchitis. Pouchitis after treatment (a) shows no active STAT1. THP1 cells, which were stimulated with γ-interferon served as a positive control, unstimulated THP1 cells as negative control.
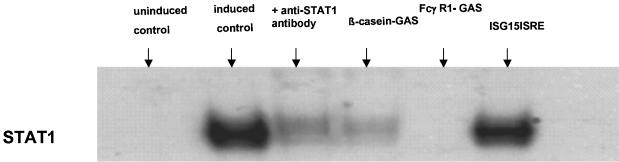
Fig. 3.
γ-interferon induced THP1 and uninduced THP1 cells (controls) were used to define the specificity of the EMSA experiments. Induced THP1 cells showed a strong signal whereas no active STAT1 could be detected in uninduced THP1 cells. Additionally incubation of extracts from γ-interferon induced THP1 cells with anti-STAT1 antibody blocks oligonucleotide binding. Lanes 4 and 5 show an incubation with cold specific oligonucleotides (Fcγ R1 and β-Casein GAS) which compete binding. An irrelevant oligonucleotide (ISG15ISRE) in lane 6 does not affect binding.
Immunofluorescence
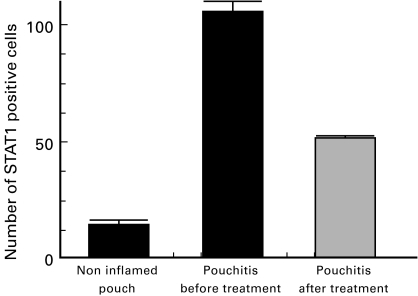
Immunofluorescence studies of pouchitis tissue demonstrated large numbers of STAT1 positive cells in the colonic mucosa of pouchitis biopsies before treatment in comparison with normal ileum and non inflamed pouch. The number of STAT1 positive cells was markedly decreased by successful antibiotic treatment of pouchitis (Fig. 4a–c, Fig. 5). Bisbenzimide staining demonstrated that most of the STAT1 staining was confined to the nucleii (not shown). An isotype control monoclonal antibody was used for controls (Fig. 4d-f).
Fig. 4.
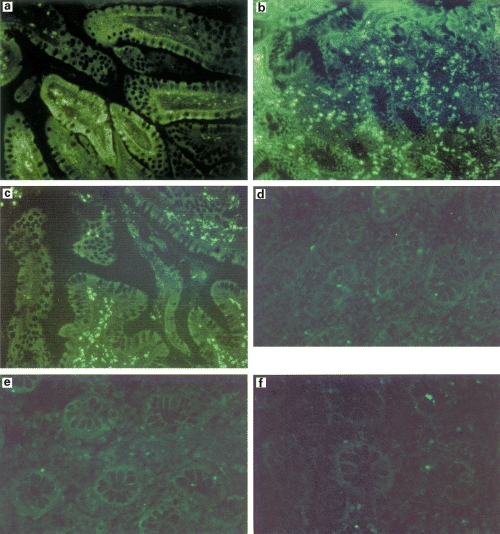
(a-c) Detection of STAT1 by immunofluorescence in (a) non inflamed pouch and in pouchitis (b) before and (c) after treatment. The number of STAT1 positive cells in pouchitis returned to non inflamed levels after therapy. (d-f) An isotype control monoclonal antibody was used for immunofluorescence studies in (d) non inflamed pouch biopsy samples, (e) pouchitis biopsies before treatment and (f) pouchitis biopsy samples after antibiotic treatment. No specific signals could be detected. Representative of five identical eperiments.
Fig. 5.
The number of STAT1 positive cells per viewfield (× 400) was compared between different conditions (5 samples each). Untreated pouchitis samples contain a significantly higher number of STAT1 positive cells in comparison with non inflamed pouch (P < 0·001) and with the samples obtained after treatment (P < 0·001).
DISCUSSION
The immunopathogenesis of pouchitis is unclear. Whether inflammation of the pouch mucosa represents a recurrence of immune mechanisms seen in ulcerative colitis or whether pouchitis represents a new form of inflammatory bowel disease remains a topic of discussion [35]. It appears that the final effector mechanisms are similiar between ulcerative colitis and pouchitis: IgG containing plasma cells and RFD9+ macrophages are increased in the intestinal lamina propria in pouchitis as well as in UC [36–38].
Proinflammatory cytokines, which include interleukin-1ß, TNFα, interleukin-8 and interleukin-12, play an important role in pouch inflammation [39, 40]. Signal transduction of cytokine/growth factor receptors involves the activation of transcription factors as part of the signal transduction pathway. The control of transcriptional events is exerted at specific sites in gene promoter regions.
Our study shows a significant increase of the expression and activation of the proinflammatory transcription factor STAT1 in biopsies from pouchitis patients in comparison with both uninflamed pouch mucosa and normal preoperative ileum. Patterns of STAT1 activation in pouchitis parallel our previous findings in ulcerative colitis [18]. In contrast, only a small degree of STAT1 activation was described in Crohn's disease [22] and was found in mucosal biopsies from patients with FAP.
Recent studies demonstrated an increased number of IFNγ producing cells in biopsies from inflamed pouch mucosa [25]. Our finding of an increased activation of STAT1 in pouchitis are in agreement with these reports.
An increase in the permeability of pouch mucosa, which has been documented in pouchitis [38], would facilitate bacterial invasion into the pouch epithelium. The increased expression and activation of STAT1 in normal pouch mucosa in comparison with normal preoperative ileum may point to the important role of the faecal flora and the adaptation of the ileal pouch mucosa. The exact nature of the interaction between faecal bacteria with pouch mucosa is still debated [41–44].
STAT1 expression and activation paralleled the clinical response to treatment, confirming our view that STAT1 is an important player in the disturbed immune regulation in pouchitis. The data before and after treatment show highly increased STAT1 expression and activation in active pouchitis which revert to normal levels after treatment.
Our data suggest that STAT1 activation may be an important factor in the pathophysiology of pouchitis. Among other candidates, IFNγ may be a major player in activating STAT1 in this setting. The immunological and clinical success of antibiotic therapy, as well as the fact that normal pouch already shows increased levels of STAT1 expression and activation in comparison with normal preoperative ileum, support our interest in exploring the role of fecal bacteria in the pathophysiology of pouch inflammation in further studies.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SCHR 512/1–3 and SFB 415), by the competence network ‘IBD’ (BMBF), by Training and Mobility of Researchers (TMR-II) grant of the European commission, by MFG and by an award from the German Crohn's and Colitis Foundation to TK.
The authors gratefully appreciate the excellent technical help of A.M.Wenner and S.Eidner and are indebted to Prof. Sievers and Prof. Mentlein for their advice on immunofluorescence techniques.
REFERENCES
- 1.Stahlberg D, Gullberg K, Liljeqvist L, Hellers G, Lofberg R. Pouchitis following pelvic pouch operation for ulcerative colitis: incidence, cumulative risk, and risk factors. Dis Colon Rectum. 1996;39:1012–8. doi: 10.1007/BF02054692. [DOI] [PubMed] [Google Scholar]
- 2.Kock NG, Darle N, Hultén L. Ileostomy. Curr Probl Surg. 1977;14:1–52. doi: 10.1016/s0011-3840(77)80065-8. [DOI] [PubMed] [Google Scholar]
- 3.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy. Brit Med J. 1978;2:85–8. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandborn WJ. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology. 1994;107:1856–60. doi: 10.1016/0016-5085(94)90832-x. [DOI] [PubMed] [Google Scholar]
- 5.Svaninger G, Nordgren S, Oresland T, Hulten L. Incidence and characteristics of pouchitis in the Kock continent ileostomy and the pelvic pouch. Scand J Gastroenterol. 1993;28:695–700. doi: 10.3109/00365529309098275. [DOI] [PubMed] [Google Scholar]
- 6.Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the incidence, timing and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Arch Surg. 1996;131:497–500. doi: 10.1001/archsurg.1996.01430170043007. [DOI] [PubMed] [Google Scholar]
- 7.Rauh SM, Schoetz DJ, Jr, Roberts PL, Murray JJ, Coller JA, Veidenheimer MC. Pouchitis: is it a wastebasket diagnosis? Dis Colon Rectum. 1991;34:685–9. doi: 10.1007/BF02050351. [DOI] [PubMed] [Google Scholar]
- 8.Layer P, Rosien U, Goebell H. Praktische Gatroenterologie. München: Urban und Schwarzenberg-Verlag; [Google Scholar]
- 9.Vecchi M, Gionchetti P, Bianchi MB, et al. p-ANCA and development of pouchitis in ulcerative colitis patients after proctocolectomy and ileoanal pouch anastomosis. Lancet. 1994;344:886–7. doi: 10.1016/s0140-6736(94)92859-2. [DOI] [PubMed] [Google Scholar]
- 10.Binder V. Pouchitis-predictable by immunological or genetic markers? European J Gastroenterol Hepatol. 1996;8:943–5. doi: 10.1097/00042737-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Penna C, Dozois R, Tremaine W, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38:234–9. doi: 10.1136/gut.38.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruseler van Embden JG, Schouten WR, van Lieshout LM. Pouchitis: result of microbial imbalance? Gut. 1994;35:659–64. doi: 10.1136/gut.35.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madden M, McIntyre A, Nicholls RJ. Double-blind cross-over trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci. 1994;39:509–15. doi: 10.1007/BF02093783. [DOI] [PubMed] [Google Scholar]
- 14.Gionchetti P, Rizzello F, Venturi A, Campieri M. Antibiotic combination therapy in patients with chronic, treatment-resistant pouchitis. Aliment Pharmacol Ther. 1999;13:713–8. doi: 10.1046/j.1365-2036.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber S, Nikolaus S, Hampe J. Activation of Nuclear Factor kappa B in inflammatory bowel disease. Gut. 1998;42:477–84. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogler G, Brand K, Vogl D, et al. Nuclear Factor kappa B is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–69. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 17.Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. Increased Secrection of proinflammatory cytokines by polymorphonuclear neutrophils regulation by Interleukin 10 in inflammatory bowel disease. Gut. 1998;42:470–6. doi: 10.1136/gut.42.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber S, Hampe J, Nikolaus S, et al. Activation of Signal Transducer and Activator of Transcription (STAT) 1. Gastroeterology. 1997;112:A1086. doi: 10.1136/gut.51.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darnell JE jr. The Jak-STAT pathway: summary of initial studies and recent advances. Recent Prog Horm Res. 1996;51:391–403. [PubMed] [Google Scholar]
- 20.Sen GC, Lengyel P. The interferon system. J Biol Chem. 1992;267:5017–20. [PubMed] [Google Scholar]
- 21.Ihle JN, Witthuhn BA, Quelle FW. Signalling through the hematopoetic cytokine receptors. Ann Rev Immunol. 1995;13:369–98. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- 22.Horvath CM, Darnell JE. The state of STATs: recent development in the study of signal transduction to the nucleus. Curr Opin Cell Biol. 1997;9:233–9. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 23.Gionchetti P, Campieri M, Belluzzi A, et al. Macrophage subpopulations and interleukin-1ß tissue levels in pelvic ileal pouch pouches. European J Gastroenterol Hepatol. 1994;6:217–22. [Google Scholar]
- 24.Reinecker H-C, Steffen M, Witthoeft T, et al. Enhanced secretion of tumor necrosis factor alpha, IL-6 and Il-1ß by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stallmach A, Schäfer F, Hoffmann S, et al. Increased state of activation of CD4 positive T cells and elevated interferon γ production in pouchitis. GUT. 1998;43:499–505. doi: 10.1136/gut.43.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–15. doi: 10.1016/s0025-6196(12)61634-6. [DOI] [PubMed] [Google Scholar]
- 27.Hou J, Schindler U, Henzel WJ. An interleukin-4 induced transcription factor. Il-4 STAT. Science. 1994;265:1701–6. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 28.Osborne L, Kunkel S, Nabel GJ. Tumor necrosis factor and interleukin 1 stimulate the human immunodeficiancy virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2335–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassanain HH, Dai W, Grupta SL. Enhanced gel mobility shift assay for DNA-binding factors. Anal Biochem. 1993;213:162–7. doi: 10.1006/abio.1993.1400. [DOI] [PubMed] [Google Scholar]
- 30.Fried MG. Measurements of protein–DNA interaction parameters by electrophoretic mobility shift assay. Electrophoresis. 1989;10:366–76. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- 32.Sachs L. Heidelberg. New York Tokyo: Springer; 1992. Angewandte Statistik. [Google Scholar]
- 33.Mann HB, Whitney DR. On a test wether one of two random variables is stochastically larger than the other. Ann Mathemat Statistics. 1947;18:50–60. [Google Scholar]
- 34.Lilliefors HW. On the Kolmogorow-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;64:399–402. [Google Scholar]
- 35.Luukkonen P, Järvinen H, Tanskanen M, Kahn A. Pouchitis-recurrence of the inflammatory bowel disease? Gut. 1994;35:243–6. doi: 10.1136/gut.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Silva HJ, Jones M, Prince C, Kettlewell M, Mortensen NJ, Jewell DP. Lymphocyte and macrophage subpopulations in pelvic ileal pouches. Gut. 1991;32(10):11160–5. doi: 10.1136/gut.32.10.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahida JR, Patel S, Gionchetti P, Jewell DP. Macrophage subpopulations in the lamina propria of normal and inflamed colon and terminal leum. GUT. 1989;30:826–34. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel RT, Bain I, Youngs D. Cytokine production in pouchitis is similar to that in ulcerative colitis. Dis Colon Rectum. 1995;38:831–7. doi: 10.1007/BF02049839. [DOI] [PubMed] [Google Scholar]
- 39.Gionchetti P, Campieri M, Belluzzi A. Mucosal concentrations of interleukin-1ß, interleukin-6, interleukin-8 and tumor necrosis factor –α in pelvic ileal pouch. Dig Dis Sci. 1994;39:1525–31. doi: 10.1007/BF02088059. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg PA, Herbst F, Beckett CG. Leukocyte typing, cytokine expression, and epithelial turnover in the ileal pouch in patients with ulcerative colitis and familial adenomatous polyposis. Gut. 1996;38:549–53. doi: 10.1136/gut.38.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onderdonk AB, Dvorak AM, Cisneros RL, et al. Microbiologic assessment of tissue biopsy samples from ileal pouch patients. J Clin Microbiol. 1992;30:312–7. doi: 10.1128/jcm.30.2.312-317.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandborn WJ, Tremaine WJ, Batts KP, et al. Fecal bile acids, short-chain fatty acids, and bacteria after ileal pouch -anal anastomosis do not differ in patients with pouchitis. Dig Dis Sci. 1995;40:1474–83. doi: 10.1007/BF02285195. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg PA, Talbot IC, Nicholls RJ. Ileo-anal‐pouch histology. Int J Colorectal Dis. 1993;8:226. doi: 10.1007/BF00290313. [DOI] [PubMed] [Google Scholar]
- 44.Kühbacher T, Schreiber S, Runkel N. Pouchitis: pathophysiology and treatment. Int J Colorectal Dis. 1998;13:196–207. doi: 10.1007/s003840050162. [DOI] [PubMed] [Google Scholar]