Abstract
Secondary amyloidosis (AA amyloidosis) is a systemic disease characterized by the extracellular tissue deposition of insoluble amyloid A (AA) protein. Aberrant metabolism of serum amyloid A (SAA) by macrophages is only one of many putative mechanisms which may be important in AA amyloidogenesis. In this study, we investigated the effects of cytokines on human monocyte-mediated SAA proteolysis. Human peripheral blood mononuclear cells (PBMC) or CD14+ monocytes were cultured with SAA, and the culture supernatants were then subjected to anti-SAA immunoblot. CD14+ monocytes degraded SAA completely. Whereas, when CD14+ monocytes were pretreated with IL-1β or IFN-γ, increasing amounts of SAA-related derivatives were detected in culture supernatants. These findings suggest that activation of monocytes by IL-1β or IFN-γ hampers the proteolysis of a precursor protein and leads to a partial degradation of SAA. This down-regulated proteolysis of SAA protein by cytokine-stimulated monocytes may play a role in the mechanism of AA amyloid formation as well as its removal.
Keywords: cytokines, monocytes, secondary amyloidosis, serum amyloid A
INTRODUCTION
Secondary amyloidosis is a progressive fatal condition that is the occasional consequence of chronic inflammatory diseases [1]. Serum amyloid A (SAA) protein is the circulating precursor of amyloid A (AA), the component of amyloid fibril deposited in organs in the course of this disease. SAA is synthesized by hepatocytes in response to inflammatory stimuli such as IL-1β or TNF-α [2, 3]. However there is no correlation between the serum levels of SAA and the development of amyloidosis [4]. Therefore, overproduction of SAA alone is not responsible for this condition. AA protein is believed to be derived from SAA by proteolytic cleavage of its carboxyl terminus [5]. This proteolytic process appears to play an important role in amyloidogenesis, and a series of studies indicate that SAA is subject to degradation by peripheral blood monocytes [6]. This evidence suggests that the deposition of AA might be attributable to the abnormality in the SAA degradative processes. Although inflammatory cytokines such as IL-1β or TNF-α are known to be the inducers of SAA from hepatocytes [7–9], the precise role of these cytokines in the pathological process of secondary amyloidosis is as yet incompletely understood. In this study, in order to investigate the roles of cytokines on amyloidogenesis, we examined the effects of cytokines on the monocyte-mediated proteolytic processing of SAA.
MATERIALS AND METHODS
Reagents
Human SAA and anti-SAA polyclonal antibodies were kindly provided from Dr N. Kubota (Eiken Chemical Ltd, Tochigi, Japan). In brief, SAA was purified from pooled sera that had high concentrations of C-reactive protein (CRP) by sequential gel filtration as described previously [10]. Purity was assessed by 12% SDS-polyacrylamide gel electrophoresis. IL-1 β nor IFN-γ was detected (<0·1 pg/ml) in SAA containing media under the present experimental conditions as confirmed by the specific ELISA system (Genzyme, Cambrige, MA). Also, this purified SAA did not affect the IL-1β secretion from PBMC (without SAA 20·1 ± 1·2 pg/ml, with SAA 22·3 ± 2·6 pg/ml) and did not induce IFN-γ production from PBMC.
One milligram of SAA in PBS was mixed with an equal amount of Freund's complete adjuvant and injected subcutaneously into New Zealand white rabbits every week for 5 months. The animals were bled and antiserum was purified by sedimentation with 40% ammonium sulphate, and by a DEAE-cellulose column.
Degradation of SAA by PBL
Peripheral blood was obtained from healthy subjects. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Conray (Daiichi Pharmaceutical Co, Tokyo, Japan) gradient centrifugation. PBMC were plated in flat bottomed 96-well plates. For each well, 5 × 105 PBMC were preincubated with various cytokines for 24 h with 200 µl of RPMI medium containing 5% FCS. After 24 h of stimulation, the plates were then added with exogenous SAA (10 µg/ml) for 3–12 h. Samples of the cultures were collected after 3, 6 and 12 h of incubation and centrifuged. Supernatants were frozen at − 30°C. In some experiments, purified T cells, B cells and monocytes were analysed for SAA degradation. For this purpose, PBMC were incubated with anti-CD3, anti-CD20 or anti-CD14 antibodies-coated magnetic beads (Dynabeads M450, Dynal Oslo, Norway) at 10 µg/105 cells for 30 min on ice. Magnetically coated cells were isolated using a magnetically activated cell sorter (MACS, Miltenyi Biotec, Meilzfeld, Germany).
Immunoblot analysis
Culture supernatants were subjected to 16% polyacrylamide gels. The fractionated proteins were transferred to nitrocellulose membranes(pore-size: 0·2µ, Bio-Rad, Hercules, CA) and incubated with anti-SAA antibody (1 : 500 dilution) for two hours at room temperature. The filter was rinsed and incubated with donkey anti‐rabbit IgG antibody conjugated with horseradish peroxidase (Amersham, Arlington Heights, IL) and developed using enhanced chemiluminescence (ECL) system (Amersham). The blots were exposed to films.
RESULTS
Degradation of SAA by monocytes
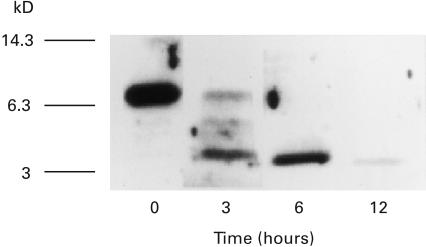
To analyse the cell-mediated proteolytic processing of SAA, we used anti-SAA immunoblots. For this purpose, isolated peripheral blood mononuclear cells (PBMC) were incubated with human SAA for various periods. After incubation, cultures were collected and centrifuged to spin-down cells. Culture supernatants were analysed by anti-SAA immunoblot analysis. Immunoblot analysis of the culture supernatants at 3 h showed the presence of 6Kd and 4Kd SAA derivatives, which were reacted with anti-SAA antibody, in addition to 12 Kd SAA. After 12 h of incubation, these SAA derivatives were abolished, suggesting the complete degradation of SAA and SAA derivatives (Fig. 1).
Fig. 1.
Immunoblot analysis for SAA degradation by peripheral blood mononuclear cells (PBMC). PBMC (5 × 105/well) were incubated with SAA (10 µg/ml) in 96well plates. Culture supernatants were obtained at 0, 3, 6, 12 h and subjected to anti-SAA immunoblot analysis as described in Materials and Methods. A representative example of four independent experiments showing similar results.
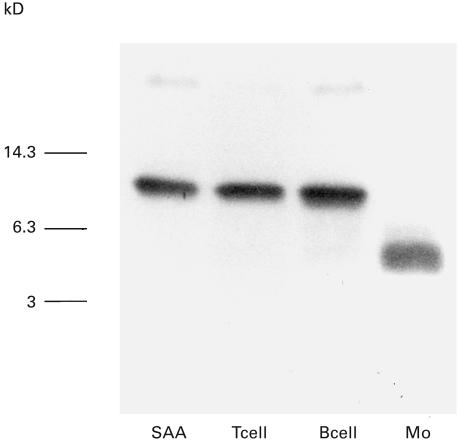
To further determine the origin of PBL that degrades SAA, we purified T lymphocytes, B lymphocytes, and monocytes using magnetic beads. Analysis of culture supernatants from these cells plus SAA indicated that CD3+ T lymphocytes and CD20+ B lymphocytes did not degrade SAA. However, CD14+ monocytes selectively degraded SAA (Fig. 2). These results showed that monocytes contribute to SAA degradation as described previously [6].
Fig. 2.
SAA degradation by peripheral blood monocytes. CD3+ T cells, CD20+ B cells and CD14+ monocytes (Mo) were isolated from peripheral blood using magnetic beads. These isolated cells (1 × 105/well) were incubated with SAA (10 µg/ml) for 3 h. Culture supernatants were subjected to anti-SAA immunoblot analysis. SAA indicated nondegradated SAA standard. A representative example of two independent experiments showing similar results.
Effects of cytokines on SAA degradation by monocytes
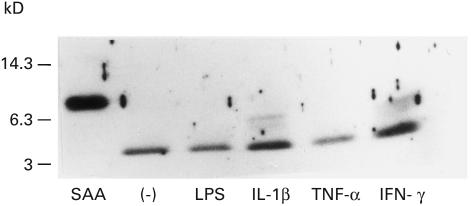
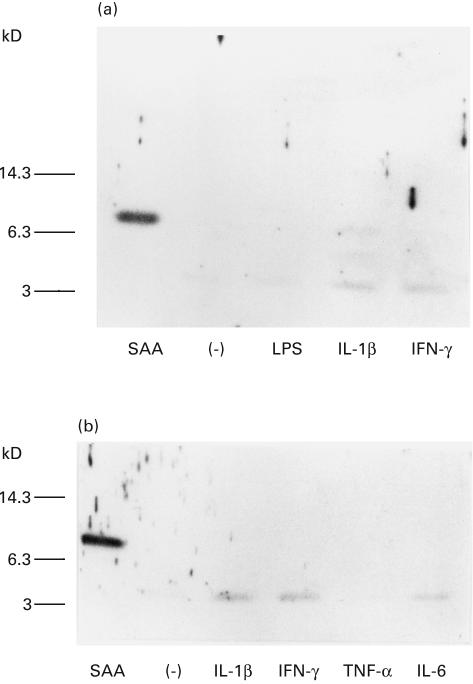
In order to determine the factors which affect monocyte-mediated SAA degradation, we treated PBL with LPS or cytokines. When PBMC were pretreated (24 h) with IL-1β or IFN-γ, and then incubated with SAA for 6 h, 6Kd and 4Kd SAA derivatives were detected in the culture supernatants. In contrast, these 6Kd SAA derivatives were not demonstrated in control (nontreated), LPS or TNF-α-treated PBMC cultures (Fig. 3). After 12 h of incubation, control PBMC or LPS-treated PBMC degraded SAA completely, since SAA-related molecules were not detected in anti-SAA immunoblot. However, 4Kd SAA derivatives were still demonstrated in IL-1β or IFN-γ-treated PBMC cultures (Fig. 4a). We also investigated the effects of TNF-α or IL-6, which are involved in hepatic SAA production, on SAA degradation. Although few amounts of SAA-derivatives were detected in IL-6-treated PBMC cultures, SAA-related molecules were not detected in TNF-α-treated PBMC cultures. On contrast, SAA derivatives were detected in IL-1β or IFN-γ-treated PBMC cultures (Fig. 4b). These results indicated that the rate of PBMC-mediated SAA degradation was partially reduced by these cytokine treatments.
Fig. 3.
In vitro SAA degradation by PBMC pretreated with LPS or cytokines. PBMC were pretreated with LPS (10 µg/ml), TNF-α (200u/ml), IL-1 β (20 u/ml) or IFN-γ (200u/ml) in 96 well plates for 24 h. Then SAA was added and cultured for another 6 h. Culture supernatants were subjected to anti-SAA immunoblot analysis. A representative example of three independent experiments showing similar results.
Fig. 4.
In vitro SAA degradation by PBMC pretreated with LPS or cytokines. A: PBMC were pretreated with LPS (10 µg/ml), IL-1 β (20 u/ml) or IFN-γ (200u/ml) in 96 well plates for 24 h. Then SAA was added and cultured for another 12 h. Culture supernatants were subjected to anti-SAA immunoblot analysis. B: PBMC were pretreated with IL-1 β (20 u/ml), IFN-γ (200u/ml), TNF-α (200u/ml) or IL-6 (200u/ml) in 96 well plates for 24 h. Then SAA was added and cultured for another 12 h. Culture supernatants were subjected to anti-SAA immunoblot analysis. A representative example of three independent experiments showing similar results.
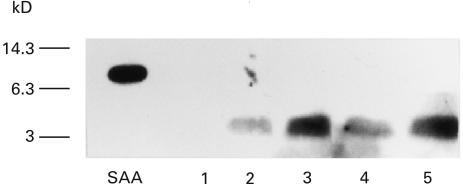
Finally, to further confirm this phenomenon, we examined the effects of these cytokines using purified CD14+ monocytes. CD14+ monocytes isolated by magnetic beads. These CD14+ monocytes were pretreated with IL-1β or IFN-γ for 24 h and then added with SAA and cultured for another 12 h. As shown in Fig. 5, nontreated monocytes completely degraded SAA. In contrast, IL-1β or IFN-γ treatment inhibited monocyte mediated SAA degradation in a dose-dependent manner and 4Kd SAA derivatives were remained in the culture supernatants.
Fig. 5.
In vitro SAA degradation by CD14+ monocytes pretreated with IL-1β or IFN-γ CD14+ monocytes were pretreated with IL-1 β (2, 20u/ml) or IFN-γ (20, 200u/ml) in 96 well plates for 24 h. Then SAA was added and cultured for another 12 h. Culture supernatants were subjected to anti-SAA immunoblot analysis. SAA indicated nondegradated SAA standard. lane 1: nontreated, lane 2: treated with IL-1β (2u/ml), lane 3: treated with IL‐1β (20u/ml), lane 4: treated with IFN-γ (20 u/ml), lane 5: treated with IFN-γ (200u/ml). A representative example of three independent experiments showing similar results.
DISCUSSION
AA amyloidosis is a condition characterized by the deposition of an extracellular fibrillar protein, amyloid A (AA), which may lead to organ failure [11, 12]. AA protein is derived from the proteolysis of an acute-phase reactant, serum amyloid A (SAA) protein. Thus it has been speculated that this proteolytic process plays an important role in AA amyloidogenesis. Monocytes, which mediate SAA degradation, are believed to play a central role in the development of AA amyloidosis [6]. In this study, we provide a useful method for amyloid research by analysing monocyte-mediated SAA degradation in vitro using anti-SAA immunoblots. We investigated the ability of human peripheral blood mononuclear cells to degrade SAA in vitro. Our data showed that the degradation of SAA was induced and AA-like intermediates were selectively detected in the monocyte culture but not by the lymphocyte cultures. The effects of cytokines on monocyte's ability to degrade SAA were also examined. Treatment of monocytes with either IL-1β or IFN-γ prevented this monocyte-mediated SAA degradation. These data indicate that these cytokines may lead to an impaired SAA catabolism.
Monocytes are believed to play a central role in the development of AA amyloidosis by affecting the proteolytic processing of SAA. For example, kuppfer cells obtained from AA amyloidotic animals showed an impaired ability to degrade SAA, in contrast to normal kupffer cells that degrade SAA completely [13]. These observations suggest that amyloid deposition might be due to a defective SAA degradation by cells of monocyte origin. Interestingly, activated Kupffer cells degrade SAA incompletely [13].
Although the biological effects of cytokines on SAA production has been well documented [7–9], the mechanism through which cytokines contribute to AA amyloid deposition remains to be disclosed. Our data suggest that IL-1β and IFN-γ stimulation might affect monocyte-mediated SAA catabolism and initiate the partial degradation of SAA and lead to amyloid deposition.
Evidence indicates that SAA is degraded by elastase-like protease in association with intracellular membrane structure and in lysosomes [14, 15]. Other proteinases, such as cathepsins or aspartic proteinases, are also involved in extracellular cleavage of SAA [16, 17]. Abnormalities in this proteinases-mediated SAA degradation process may be responsible for the increased AA accumulation. To support this idea, inhibitors of these proteinases lead to an accumulation of SAA and accelerate amyloid fibrill formation in murine experimental amyloid induction models [17].
Our data suggest that cytokines also may affect these processes. Alternatively other factors, such as proteogycans and serum amyloid P (SAP) protein, which block amyloid proteolysis and stabilize amyloid deposition [18, 19], could be involved. Further studies are required to define the mechanism whereby these cytokines affect the monocyte-mediated proteolytic-cleavage of SAA.
Cytokines-mediated impaired SAA degradation observed in our in vitro experiments may reflect in vivo amyloidosis. Wegelius et al. [20] demonstrated that amyloid-A-degrading activity is reduced in RA patients with amyloidosis. Furthermore, this amyloid-A-degrading activity was reduced in acute phase reaction and correlated with serum albumin level [21]. Our data suggest that longstanding inflammation could lead to the impaired monocyte-mediated SAA proteolysis by inducing IL-1β or IFN-γ as well as the SAA elevation in RA patients. Therefore, high SAA levels and cytokine-mediated impaired SAA proteolysis could be induced and may play a role in amyloidogenesis in RA patients.
In conclusion, we examined the molecular kinetics of monocyte-mediated SAA degradation in order to explore the mechanism of SAA proteolysis. Our results clearly indicated that monocytes are capable of degrading SAA, and that IL-1β and INF-γ hampered this monocyte-mediated SAA catabolism. These observations lead to the hypothesis that AA amyloidosis might be attributed to impaired monocyte-mediated SAA proteolysis.
Acknowledgments
We are grateful to N. Kubota for providing anti-SAA polyclonal antibody and purified SAA.
REFERENCES
- 1.Kisilevsky R. Amyloidosis: a familial problem in the light of current pathogenic developments. Labo Invest. 1983;49:381–90. [PubMed] [Google Scholar]
- 2.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–8. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal CJ, Franklin EC, Frangione B, Greenspan J. Isolation and partial characterization of SAA, an amyloid related protein from human serum. J Immunol. 1975;116:1415–8. [PubMed] [Google Scholar]
- 4.Wegelius O, Teppo MM, Maury CPJ. Reduced amyloid degrading activity in serum in amyloidosis associated with rheumatoid arthritis. Br Med J. 1982;284:617–9. doi: 10.1136/bmj.284.6316.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tape C, Tan R, Nesheim M, Kisilevsky R. Direct evidence for circulating apoSAA as the precursor of tissue AA amyloid deposits. Scand J Immunol. 1988;28:317–24. doi: 10.1111/j.1365-3083.1988.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 6.Lavie G, Zucker-Franklin D, Franklin EC. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978;148:1020–31. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selinger MJ, McAdam KPW, Kaplan MM, et al. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980;285:498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- 8.Ganapathi MK, Rzewnicki D, Samois D, Jiang SL, Kushner I. Effects of combinations of cytokines and hormones on synthesis serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991;147:1261–5. [PubMed] [Google Scholar]
- 9.Smith JW, McDonald TL. Production of serum amyloid A and C-reactive protein by HepG2 cells stimulated with combinations of cytokines or monocyte conditioned media. Clin Exp Immunol. 1992;90:293–9. doi: 10.1111/j.1365-2249.1992.tb07945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marhaug G, Husby G. Characterization of human amyloid-related protein SAA as a polymorphic protein, association with albumin and prealbumin in serum. Clin Exp Immunol. 1981;45:89–106. [PMC free article] [PubMed] [Google Scholar]
- 11.Glenner GG. Amyloid deposits and amyloidosis -the β-fibrilloses. I N Engl J Med. 1980;302:1283–92. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 12.Glenner GG. Amyloid deposits and amyloidosis -the β-fibrilloses. II N Engl J Med. 1980;302:1333–43. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- 13.Fuks A, Zucker-Franklin D. Impaired Kupffer cell function precedes development of secondary amyloidosis. J Exp Med. 1985;161:1013–28. doi: 10.1084/jem.161.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavie G, Zucker-Franklin D, Franklin EC. Elastase-type proteases on the surface of human blood monocytes; possible role in amyloid formation. J Immunol. 1980;125:175–80. [PubMed] [Google Scholar]
- 15.Silverman SL, Cathcart ES, Skinner M, Cohen AS. The degradation of serum amyloid A protein by activated polymorphonuclear leucocytes: participation of granulocytes elastase. Immunology. 1982;46:737–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T, Kluve-Beckerman B, Liepnieks JJ, Benson MD. In vitro degradation of serum amyloid A by cathepsin D and other acid proteases: possible protection against amyloid fibril formation. Scand J Immunol. 1995;41:570–4. doi: 10.1111/j.1365-3083.1995.tb03609.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Liepnieks J, Benson MD, Kluve-Beckerman B. Accelerated amyloid deposition in mice treated with the aspartic protease inhibitor, pepstatin. J Immunol. 1996;157:901–7. [PubMed] [Google Scholar]
- 18.Kisilevsky R. Heparan sulfate proteoglycans in amyloidogenesis: an epiphenomenon, a unique factor, or the tip of a more fundamental process ? Lab Invest. 1990;63:589–91. [PubMed] [Google Scholar]
- 19.Pepys MB, Rademacher TW, Amatayakul-Chantler S. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogenous glycostructure. Proc Natl Acad Sci USA. 1994;91:5602–6. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegelius O, Teppo AM, Maury CP. Reduced amyloid-A-degrading activity in serum in amyloidosis associated with rheumatoid arthritis. B M J. 1982;284:617–9. doi: 10.1136/bmj.284.6316.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maury CP, Teppo AM. Mechanism of reduced amyloid-A-degrading activity in serum of patients with secondary amyloidosis. Lancet. 1982;2(3292):234–6. doi: 10.1016/s0140-6736(82)90322-1. [DOI] [PubMed] [Google Scholar]