Abstract
The aim of this study was to investigate the effect of prematurity, neonatal sepsis, respiratory distress syndrome (RDS) and perinatal asphyxia on monocyte HLA-DR expression of neonates using a flow cytometric method based on monocyte negative selection. The subjects were one hundred and thirty-one neonates (59 healthy, 44 septicaemic, 20 with RDS and eight with perinatal asphyxia) and 20 healthy adults. Monocyte HLA-DR expression was measured using one-colour HLA-DR labelling in a gate for monocytes obtained using the combination of CD3-CD19–PE/CD15–FITC MoAbs. In addition, the common dual staining method using MoAbs against two CD14 epitopes (TUK4, MO2) was evaluated. With the one-colour HLA-DR labelling higher purity and recovery values of monocytes were achieved than with the dual labelling method. Healthy neonates had significantly lower percentages of HLA-DR+ monocytes than adults (69 ± 13% versus 91·5 ± 2·5%) and comparable mean fluorescence intensity (MFI) (119 ± 25 versus 131 ± 26). Values did not differ significantly between healthy term and preterm neonates. Preterm neonates with RDS had a significantly lower percentage of HLA-DR+ monocytes than the healthy preterm neonates. In neonates with asphyxia both parameters were comparable to those of the healthy ones. Septicaemic neonates presented significantly lower values of both parameters than the healthy, RDS and asphyxiated neonates. Monocyte negative selection provides a reliable estimation of HLA-DR expression on monocytes. Expression of monocyte HLA-DR is lower in healthy neonates in comparison with adults and is further decreased in neonates with sepsis and RDS, but it is not influenced by prematurity and perinatal asphyxia.
Keywords: neonatal sepsis, perinatal asphyxia, respiratory distress syndrome, monocyte HLA-DR
INTRODUCTION
The monocyte/macrophage system is involved in acute and chronic inflammatory reactions, playing a major role in host defence. The main functions of monocytes, namely phagocytosis, antigen presentation and production of cytokines, are mediated by certain surface molecules, such as Fc receptors for IgG, complement receptor CR1, β2-integrins, CD14, IL-2 receptor and MHC class II HLA-DR molecules [1]. Surface expression of the above molecules reflects the activation state of these cells. Of the molecules expressed on monocytes, HLA-DR allows antigen presentation to T cells and is crucial for the initiation of the immune cascade during sepsis [2]. Monocyte expression of HLA-DR has been found to be decreased in adult sepsis [3–6] and has been proposed as an indicator of septic complications after major surgery or trauma [5].
Diagnosis of sepsis in neonates, especially preterm and the sick neonates receiving intensive care, is difficult because the symptoms are often subtle and non-specific and the haematological parameters currently used have only suggestive value [7]. For this reason, certain immunological parameters have been evaluated as early indicators of neonatal sepsis demonstrating varied accuracy [7–10]. So far, monocyte HLA-DR expression has only been studied in healthy term neonates [11–15], and data from preterm and septic neonates are very limited [15, 16]. This immunological marker is easily assessed using flow cytometry, which gives results within 2 h and requires only a small volume of blood, and thus, measurement of monocyte HLA-DR expression could be a valuable tool for early diagnosis of neonatal sepsis. However, there are certain non-infective neonatal pathological conditions, such as respiratory distress syndrome (RDS) [17], bronchopulmonary dysplasia (BPD) [18, 19] and perinatal asphyxia with consequent brain damage [20–22], in which an acute inflammatory reaction involving activated monocytes/macrophages plays an important pathogenic role. The effect of these conditions on monocyte HLA-DR expression should therefore be more extensively investigated before it can be safely used as an additional marker of sepsis in neonates.
Monocyte HLA-DR expression is currently measured by flow cytometric methods using specific MoAbs against monocyte surface molecules. In most studies anti-CD14 MoAbs have been used to identify monocytes [1, 3, 4, 14, 15, 23–25]. However, it is well known that anti-CD14 MoAbs identify only a subset of peripheral blood monocytes [26–30], the proportion of which varies in sepsis [31], so that CD14 positivity alone cannot accurately identify the entire monocyte population in a blood sample.
The aims of this study were, first, to evaluate a monocyte gating policy based on monocyte negative selection compared with the conventional dual staining method and, second, to investigate the effect of prematurity, neonatal sepsis, RDS and perinatal asphyxia on the monocyte activation state. For this purpose monocyte HLA-DR expression in 20 healthy adults was measured using both the conventional dual staining and the monocyte negative selection methods. Subsequently monocyte negative selection was applied to measure HLA-DR expression in healthy term and preterm neonates and in neonates suffering from sepsis, RDS or asphyxia.
PATIENTS AND METHODS
Studied population
Patients
Seventy-two neonates suffering from sepsis, perinatal asphyxia or RDS were classified into four groups: the group of term septicaemic neonates (n = 11), the group of preterm septicaemic neonates (n = 33), the group of asphyxiated neonates (n = 8) and the group of neonates with RDS (n = 20) (Table 1). Of the 44 septicaemic neonates, 37 had proven sepsis (positive blood cultures) and seven had probable sepsis (clinical and laboratory findings suggestive of sepsis, but negative blood cultures). None of the neonates with asphyxia or RDS had any evidence of sepsis at the time of blood sampling. Exclusion criteria included evidence of congenital infection, major congenital malformations, immunotherapy received prior to blood sampling and absence of parental consent.
Table 1.
Mean (± s.d.) gestational age (GA), birth weight (BW), age at blood sampling and sex distribution of the groups of neonates studied
| n | GA (weeks) | BW (g) | Age (days) | Sex (M/F) | |
|---|---|---|---|---|---|
| Septicaemic term | 11 | 39 ± 1 | 3184 ± 794 | 6 ± 2 | 3/8 |
| Control group | 23 | 39 ± 1 | 3143 ± 734 | 7 ± 1 | 13/10 |
| P | NS | NS | NS | NS | |
| Septicaemic preterm | 33 | 31 ± 2 | 1491 ± 540 | 9 ± 7 | 18/15 |
| Control group | 36 | 32 ± 2 | 1710 ± 443 | 9 ± 12 | 23/16 |
| P | NS | NS | NS | NS | |
| Asphyxiated | 8 | 39 ± 1 | 3016 ± 425 | 1 ± 0 | 3/5 |
| Control group | 15 | 39 ± 1 | 3152 ± 905 | 1 ± 0 | 7/8 |
| P | NS | NS | NS | NS | |
| Neonates with RDS | 20 | 31 ± 4 | 1548 ± 550 | 1 ± 0 | 13/7 |
| Control group | 28 | 32 ± 2 | 1646 ± 315 | 1 ± 0 | 17/11 |
| P | NS | NS | NS | NS |
RDS, Respiratory distress syndrome; NS, not significant.
Controls
Fifty-nine healthy neonates who served as control subjects were categorized into four control groups, each one matching with a group of sick neonates with respect to gestational age, birth weight and age at sampling (Table 1). The protocol was approved by the ethical committee of the study Institution and informed consent was obtained from all parents prior to blood sampling.
Peripheral blood samples (0·4 ml) were collected within 24 h of birth from neonates with RDS and asphyxia. In septicaemic neonates sampling was performed at the time of suspicion of sepsis, which ranged from 4 to 30 days of life (Table 1). The age of the healthy neonates who constituted the corresponding control group ranged from 1 to 32 days (Table 1).
Blood samples obtained from 20 healthy adult volunteers (10 males and 10 females, aged 25–50 years) were used to evaluate the monocyte negative selection method against the dual staining method.
Monocyte HLA-DR measurement
Repetitive measurements were performed on blood samples obtained from the 20 healthy adults by flow cytometry using both the conventional dual staining method described by Hershman et al. [4] and a monocyte negative selection method. Both methods were performed in whole blood samples collected in test tubes containing K3EDTA. After staining with MoAbs, hypotonic erythrocyte lysis was achieved using the Ortho Mune Lysis Reagent (Ortho Diagnostics, Westwood, CA). Two flow cytometers were used for immunophenotyping of all samples tested: the Coulter Epics XL (Coulter Electronics, Hialeah, FL) and the Ortho Cytoron Absolute (Ortho Diagnostics). Isotype-matched fluorochrome-conjugated MoAbs were used as controls to assess the non-specific binding and to set the threshold between positively and negatively stained cell populations.
In the conventional method, samples were concomitantly stained with both anti-CD14 and anti-HLA-DR MoAbs. Anti-CD14 MoAbs directed against various different epitopes of the molecule and conjugated with different fluorescent stains (TUK4–FITC, TUK4–PE (Dako, Glostrup, Denmark), MO2–FITC, MO2–PE (Coulter Immunology, Hialeah, FL) were used in combination with the appropriate (FITC or PE) anti-HLA-DR MoAb (Immuno Quality Products, Gröningen, The Netherlands). Cell surface antigen expression on monocytes was determined by incubating 100 μl of whole blood with 10 μl of the appropriate MoAb at room temperature for 15 min. The antibodies used in the conventional method were conjugated alternatively with FITC and PE in order to assess the possible effect of steric hindrance or quenching phenomena. In the dual staining method (synchronous two-colour fluorescence analysis) parameter settings of forward light scatter and right-angled side scatter were selected to separate the monocyte populations. The percentage of monocytes co-expressing CD14 and HLA-DR was considered to represent HLA-DR monocytes. The monocyte recovery value was determined by dividing the CD14+ cells of the monocyte scatter gate by the CD14+ cells of a larger monocyte scatter gate. The quotient was then multiplied by 100.
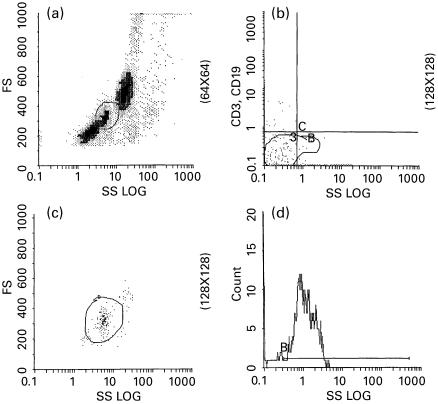
In the negative selection method, monocytes were isolated for the HLA-DR measurements, not only from their scatter characteristics but also from the application of the combination of CD3-CD19–PE/CD15–FITC MoAbs (Dako). In this way, cells with variable HLA-DR expression, such as B and T lymphocytes (stained for CD19–PE and CD3–PE, respectively) and granulocytes (CD15–FITC) were excluded. In this context 100-μl samples were stained for CD3–PE, CD19–PE and CD15–FITC in the monocyte identification tube, and a further 100-μl sample was stained for HLA-DR–PE in a separate tube. In the first tube (monocyte identification tube) monocytes were gated on the basis of rough forward/side scatter pattern (Fig. 1a) with some spillover of lymphocytes and granulocytes. Cells obtained from this gate dimly expressing CD15 but not CD3 and CD19 were considered to be monocytes (Fig. 1b). Only this ‘purified’ monocyte population was gated again in a bitmap by the parameters of forward and side scatter (Fig. 1c) and was saved for the following HLA-DR determinations. Thus, the percentage of monocytes expressing HLA-DR from the latter saved bitmap was calculated in the second tube by one-colour flow cytometric analysis (Fig. 1c).
Fig. 1.
Illustration of the gating strategy followed in the monocyte negative selection method. (a) Gating based on monocyte forward/side scatter properties. (b) A monocyte gate resulting from CD15dim, CD3−, CD19− cells. (c) Gating the previously identified monocytes by their scatter characteristics (a, b and c obtained from the monocyte identification tube). (d) Measurement of HLA-DR monocytes in the previous gate (HLA-DR staining tube).
In preliminary experiments, CD15–FITC was added to the HLA-DR staining tube as an intrapanel control marker, in order to test the consistency of the pure monocyte gate between this latter tube and the monocyte identification tube. In addition, 20 repeat preparations and measurements were performed with this method in three samples with low, three with medium and three with high HLA-DR monocyte count, in order to assess repeatability.
In the neonates, monocyte HLA-DR expression was measured using the monocyte negative selection method.
Statistical analysis
Results are presented as means and s.d. anova and paired t-test analyses were used to compare values obtained by the different flow cytometric approaches. The unpaired t-test was used to evaluate the significance of the differences between the groups of subjects compared.
RESULTS
Clinical characteristics of the neonates studied
The gestational age, birth weight and age at blood sampling of the four groups of sick neonates and the corresponding control groups of healthy neonates are shown in Table 1. Microorganisms isolated from blood cultures from the septicaemic neonates included Klebsiella pneumoniae (n = 12), Staphylococcus spp. (n = 9), Fungi spp. (n = 9), Acinetobacter (n = 4), Streptococcus spp. (n = 1), Enterococcus (n = 1), and E. coli (n = 1).
Evaluation of the negative selection monocyte gate and comparison with conventional flow cytometric methods
Recovery of monocytes, as obtained by the dual staining method, was extremely low regardless of the CD14 epitope and fluorescent conjugate used (Table 2). Acceptable recovery (> 85%) was obtained in a maximum of 50% of the measurements only in the case of the HLA-DR–FITC/TUK4–PE combination. A dramatically lower recovery rate was observed in all combinations (from 6·6% to 26·6%, depending on the combination).
Table 2.
Monocyte recovery obtained by the methods studied using the Ortho Cytoron Absolute flow cytometer (Ortho Diagnostics)
| Recovery (%) | |||
|---|---|---|---|
| Method | Mean ± s.d. | Min. | Max. |
| TUK4–FITC/HLA-DR–PE | 67·2 ± 13·4 | 27·2 | 90·0 |
| HLA-DR–FITC/TUK4–PE | 83·3 ± 7·7 | 56·8 | 95·4 |
| MO2–FITC/HLA-DR–PE | 75·1 ± 11·0 | 28·6 | 97·4 |
| HLA-DR–FITC/MO2–PE | 80·4 ± 8·3 | 35·2 | 94·0 |
| HLA-DR–PE of the negatively selected monocyte gate | 96·2 ± 3·0 | 88·9 | 100 |
Significant differences were observed in the percentages of HLA-DR+ monocytes (Table 3) obtained by the use of the above MoAb combinations in the blood samples examined (anova, F = 2·76 and F = 2·18, respectively; P < 0·001), as well as in the mean fluorescence intensity (MFI) (data not shown). It is of note that comparison (paired t-test) of the HLA-DR monocyte counts obtained by the different fluorescence stains (FITC or PE) labelling MoAb against the same CD14 epitope revealed statistically significant differences (P < 0·01).
Table 3.
Percentage of the HLA-DR+ monocytes (mean ± s.d.) in the healthy adult samples as obtained by the methods studied using the Ortho Cytoron Absolute flow cytometer (Ortho Diagnostics)
| Method | HLA-DR monocytes (%) |
|---|---|
| TUK4–FITC/HLA-DR–PE | 99·2 ± 0·87 |
| HLA-DR–FITC/TUK4–PE | 68·7 ± 10·5 |
| MO2–FITC/HLA-DR–PE | 88·8 ± 4·6 |
| HLA-DR–FITC/MO2–PE | 93·6 ± 2·4 |
| HLA-DR–PE of the negatively selected monocyte gate | 91·5 ± 2·5 |
In contrast, the recovery of monocytes as obtained by the monocyte negative selection method was > 85% for all processed samples (> 90% for 93·3% of the samples, Table 3). Furthermore, purity values were also highly acceptable (93·3 ± 5·3%). (Reporting purity in the case of the dual staining method would be meaningless, since CD14 which is strongly expressed only on monocytes was used as a positive marker.)
CD15 expression in the pure monocyte gate was found to be equivalent in the monocyte identification tube and the HLA-DR staining tube. The intramethod coefficient variation in multiple measurements of the same samples was found to be < 5%.
No significant difference (paired t-test, P > 0·05) was observed between HLA-DR monocyte counts obtained from the two flow cytometers.
Monocyte HLA-DR expression in healthy neonates (Table 4)
Table 4.
Mean values (± s.d.) of the percentage of HLA-DR+ monocytes and the mean fluorescence intensity (MFI) in adults and the groups of neonates studied measured by monocyte negative selection
| n | % HLA-DR+ monocytes | MFI | |
|---|---|---|---|
| Adults | 20 | 92 ± 3 | 131 ± 26 |
| Septicaemic term | 11 | 40 ± 15 | 76 ± 29 |
| Control group | 23 | 69 ± 13 | 119 ± 25 |
| P | <; 0·0001 | <; 0·0001 | |
| Septicaemic preterm | 33 | 40 ± 15 | 77 ± 27 |
| Control group | 36 | 69 ± 12 | 126 ± 28 |
| P | <; 0·0001 | <; 0·0001 | |
| Asphyxiated | 8 | 76 ± 8 | 138 ± 28 |
| Control group | 15 | 67 ± 15 | 118 ± 31 |
| P | <; 0·0001 | NS | |
| Neonates with RDS | 20 | 58 ± 17 | 112 ± 40 |
| Control group | 28 | 69 ± 13 | 126 ± 30 |
| P | <; 0·05 | NS |
Additional statistically significant differences: (i) % HLA-DR+ monocytes: adults versus healthy terms, P < 0·0001, adults versus healthy preterms, P < 0·0001, septicaemic preterms versus respiratory distress syndrome (RDS), P < 0·001, septicaemic terms versus asphyxiated, P < 0·0001; (ii) MFI: septicaemic terms versus asphyxiated, P < 0·001, septicaemic preterms versus RDS, P < 0·001.
The healthy term and preterm neonates did not differ significantly in either the percentage of HLA-DR+ monocytes (69 ± 13% versus 69 ± 12%) or the MFI (119 ± 25 versus 126 ± 28). Healthy neonates, either term or preterm, had a significantly lower percentage of HLA-DR+ monocytes than adults (P < 0·0001), but comparable MFI (Table 4).
Monocyte HLA-DR expression in septicaemic neonates (Table 4)
Septicaemic term neonates had significantly lower HLA-DR+ monocyte and MFI values than the corresponding control group and the asphyxiated term neonates (Table 4). Values in septicaemic preterm neonates were significantly lower than those in the corresponding control group and the preterm neonates with RDS (Table 4).
Monocyte HLA-DR expression in neonates with RDS or perinatal asphyxia (Table 4)
Preterm neonates with RDS had a significantly lower percentage of HLA-DR+ monocytes than the corresponding control group (58 ± 17% versus 69 ± 13%; P < 0·05), but comparable MFI. Term neonates with perinatal asphyxia presented values of both parameters similar to those obtained from the corresponding control group (Table 4).
DISCUSSION
In flow cytometric monocyte studies, including those involving septicaemic patients [3, 4, 24–26], the bright expression of CD14 is used as a positive monocyte marker in order to exclude cells with dim CD14 expression, which are mainly granulocytes. However, this approach leads to the exclusion of a considerable number of monocytes weakly expressing this receptor. As early as 1989, Passlick et al. [29] identified a novel subset of monocytes with low density expression of CD14 and co-expression of CD16. These CD14dim monocytes, constituting in healthy volunteers about 13% of the common CD14bright monocytes, were of considerably lower size with abundant cytoplasm and multiple cytoplasmic granules. Fingerle et al. [31] reported that the CD14dim monocytes increase in septicaemic patients while the CD14bright monocytes substantially decrease the density of this receptor. It was probably this reduced expression of CD14 antigens on monocytes that was reported to correlate with the development of major infection and sepsis [32,33]. Additionally, the proportion of monocytes detected by CD14 depends on the particular epitope against which the CD14 MoAb is directed [27, 28, 30]. Hence, the unacceptably low and variable recovery values observed by the dual staining method in this study came as no surprise.
Consequently, the results of HLA-DR monocyte studies based on CD14 positivity alone, especially those proposing this parameter as a predictive marker of major infection and sepsis, seem to need some rethinking. The heterogeneity of the expression of HLA class II antigens in the various monocyte subpopulations, being significantly higher on CD14dim than on bright monocytes, renders support to this consideration [29].
Taking into account the metric and biological complexity influencing monocyte studies, values of HLA-DR monocytes, such as the ones obtained in this study by the use of the dual staining method (as low as 68·7 ± 10·5 in healthy volunteers), are far from acceptable. In addition, the within sample variation was inexplicably high not only when using MoAbs against different CD14 epitopes but also when these were labelled with different fluorochromes. In contrast, with the use of the proposed monocyte negative selection method, the HLA-DR monocyte values obtained showed a very low intramethod variation.
These results seem more convincing in view of the high monocyte recovery values achieved as well as the highly satisfactory ability to estimate purity. This was the result of the use of CD15 as a monocyte marker. CD15 is also expressed on granulocytes, but at much higher density [34], allowing the construction of a gate which includes all low CD15-expressing monocytes. Excluding the HLA-DR-expressing T and B lymphocytes from the resulting gate, a ‘purified’ monocyte population can be defined. It is of note that through this flow cytometric approach to identifying monocytes many antigens other than HLA-DR can be reliably measured for the study of various pathological situations. Furthermore, a three-colour experiment would be a more logical way for monocyte negative selection to be achieved, provided that the appropriately stained MoAbs and equipment were available. Such an approach would probably overcome minor problems of the method (i.e. a degree of overcompensation appearing in Fig. 1).
The use of the monocyte negative selection method on blood samples obtained from healthy neonates showed that both term and preterm neonates had a significantly lower percentage of HLA-DR+ monocytes but similar MFI in comparison with adults. These results agree with those of earlier studies, which showed that the percentage of cord blood monocytes expressing the HLA-DR antigen was lower than that of adult monocytes [11–13], whereas the intensity of the expression was comparable [15] or even higher [1] than that of adult monocytes. Analysis of the results of this study in correlation with gestational age revealed no impact of prematurity on monocyte HLA-DR expression, a finding reported also by Kotirama et al. [16].
Regarding the impact of sepsis on monocyte HLA-DR expression, adult studies showed a significant depression in trauma and surgical patients [3, 4, 5, 6, 26,35,36] which was more severe in those developing septic complications [3,5] and associated with the severity and outcome of sepsis [4, 6, 35, 36]. This study shows that sepsis causes a significant decrease in HLA-DR expression in neonates, similar to that reported in adults. Data on the response of neonatal monocytes to sepsis in terms of HLA-DR expression are very limited [15]. El Mohandes et al. [15] found that in vitro stimulation of neonatal monocytes with lipopolysaccharide resulted in an enhancement of HLA-DR antigen expression [15]. These results seem to contrast with the decreased HLA-DR expression found in the septicaemic neonates of this study. The discrepancy between the in vitro and in vivo response of neonatal monocytes to septic stimuli could be attributed to the release of anti-inflammatory cytokines, such as IL-10, during sepsis [35–38], or more probably to extravasation and migration of the activated monocytes into the inflamed tissues.
Low HLA-DR expression in surgical patients has been proposed as a marker of septic complications [5]. Before such a marker can be safely used for diagnosis of septic complications in sick neonates, the possible impact of certain non-infective neonatal pathological entities, including RDS and perinatal asphyxia, on the activation state of monocytes should be more extensively investigated.
Studies of neonates with RDS demonstrated the major role of the acute inflammatory reaction in the pathogenesis of the syndrome itself and its progression to BPD [17–19]. In bronchoalveolar lavage from neonates with RDS increased numbers of activated macrophages and increased concentrations of the macrophage inflammatory protein-1 alpha, IL-1β, tumour necrosis factor-alpha and IL-8 were found soon after birth and correlated well with subsequent development of BPD [17,19]. In addition, increased expression of HLA-DR was found on pulmonary macrophages from infants dying of BPD [18]. On the basis of these data an increased expression of HLA-DR antigens on circulating monocytes of neonates with RDS would be expected, considering the fact that these cells virtually constitute the tissue-resident macrophage replacement pool. However, in this study the percentage of circulating monocytes expressing the HLA-DR antigen in the group of neonates with RDS was decreased in comparison with healthy preterm neonates. Therefore, it appears that the activation state of peripheral blood monocytes does not reflect the status of the tissue-resident macrophages. In addition, the low HLA-DR expression in neonates with RDS even in the absence of sepsis could limit the value of this parameter in diagnosing septic complications in this group of neonates.
The hypothesis of a relationship between perinatal asphyxia and monocyte activation is supported by experimental animal studies suggesting that brain ischaemia elicits an acute inflammatory reaction in the injured brain involving monocytes, neutrophils and inflammatory mediators, which may be causally related to brain damage [20,21]. Findings from the group of asphyxiated neonates in this study showed no significant effect of perinatal asphyxia on monocyte HLA-DR expression.
In conclusion, all the groups of sick neonates included in this study suffered from pathological conditions in which activated monocytes play a significant pathogenic role. Thus, although an increase of monocyte HLA-DR expression would be expected, no group of sick neonates showed such an increase, whereas in certain conditions a decrease of these molecules on circulating monocytes was found. The most probable explanations for this finding could be a redistribution of monocytes with migration of the HLA-DR+ monocytes to the injured tissues or activation of these cells within the tissues by locally produced inflammatory mediators. The findings of this study suggest that the monocyte negative selection method provides a reliable estimation of HLA-DR expression on circulating monocytes. Using this method it was found that the expression of monocyte HLA-DR in healthy neonates is lower than that in adults and that it is influenced neither by prematurity nor by perinatal asphyxia, but is significantly depressed in neonatal sepsis and RDS.
REFERENCES
- 1.Marwizt PA, Van Arkel-Vigna E, Rijkers GT, Zegers BJ. Expression and modulation of cell surface determinants on human adult and neonatal monocytes. Clin Exp Immunol. 1988;72:260–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Unanue ER, Beller DI, Lu CY, Allen PM. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984;132:1–5. [PubMed] [Google Scholar]
- 3.Wakefield CH, Carey PD, Monson JRT, Guillou PJ. Changes in major histocompatibility complex class II expression on monocytes and T-cells of patients developing infection after surgery. Br J Surg. 1993;80:205–9. doi: 10.1002/bjs.1800800224. [DOI] [PubMed] [Google Scholar]
- 4.Hershman MJ, Chaedle WG, Wellhausen SR, Davidson PF, Polk HC. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patients. Br J Surg. 1990;77:204–7. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg JM, Oldenburger RH, van den Berg AP, et al. Low HLA-DR expression on monocytes as a prognostic marker for bacterial sepsis after liver transplantation. Transplantation. 1997;63:1846–8. doi: 10.1097/00007890-199706270-00026. [DOI] [PubMed] [Google Scholar]
- 6.Ditschkowski M, Kreusfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–54. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vesikari T. Cytokine determinations and rapid diagnosis of early onset neonatal septicaemia. Acta Paediatr. 1999;88:585–91. doi: 10.1080/08035259950169161. [DOI] [PubMed] [Google Scholar]
- 8.Silveira RC, Procianoy RS. Evaluation of interleukin-6, tumor necrosis factor-aplha and interleukin-1beta for early diagnosis of neonatal sepsis. Acta Paediatr. 1999;88:647–50. doi: 10.1080/08035259950169314. [DOI] [PubMed] [Google Scholar]
- 9.Ng PC, Chenf SH, Chui KM, Fok TF, Wong MY, Wonh W, Wong RPO, Cheung KL. Diagnosis of late onset sepsis with cytokines, adhesion molecules, and C-reactive protein in preterm very low birth weight infants. Arch Dis Child. 1997;77:F221–7. doi: 10.1136/fn.77.3.f221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallman J, Ekholm L, Eriksson M, Malmstrom B, Schollin J. Contribution of interleukin-6 in distinguishing between mild respiratory disease and neonatal sepsis in the newborn infant. Acta Paediatr. 1999;88:880–4. doi: 10.1080/08035259950168829. [DOI] [PubMed] [Google Scholar]
- 11.Zlabinger GJ, Mannhalter JW, Eibl MM. Cord blood macrophages present bacterial antigens to paternal T cells. Clin Immunol Immunopathol. 1983;28:405–12. doi: 10.1016/0090-1229(83)90107-1. [DOI] [PubMed] [Google Scholar]
- 12.Stiehm RE, Stein MB, Steeg PS, Mann D, Nweland C, Blaese RC, Oppenheim JJ. Deficient DR antigen expression on human cord blood monocytes: reversal with lymphokines. Clin Immunol Immunopathol. 1984;30:430–6. doi: 10.1016/0090-1229(84)90028-x. [DOI] [PubMed] [Google Scholar]
- 13.Glover DM, Brownstein D, Burchett S, Larsen A, Wilson CA. Expression of HLA-DR class II antigens and secretion of interleukin-1 by monocytes and macrophages from adults and neonates. Immunology. 1987;61:195–201. [PMC free article] [PubMed] [Google Scholar]
- 14.Kampalath B, Cleveland RP, Kass L. Reduced CD4 and HLA-DR expression on neonatal monocytes. Clin Immunol Immunopathol. 1998;87:93–100. doi: 10.1006/clin.1997.4505. [DOI] [PubMed] [Google Scholar]
- 15.El-Mohandes AA, Rivas RA, Kiang E, Wahl E, Katona IM. Membrane antigen and ligand receptor expression on neonatal monocytes. Biol Neonate. 1995;68:308–17. doi: 10.1159/000244251. [DOI] [PubMed] [Google Scholar]
- 16.Kotiranta-Ainamo A, Apajasalo M, Pohjavuori M, Rautonen N, Rautonen J. Mononuclear cell subpopulation in preterm and full-term neonates: independent effects of gestational age, neonatal infection, maternal preeclampsia, maternal betamethason therapy, and mode of delivery. Clin Exp Immunol. 1999;115:309–14. doi: 10.1046/j.1365-2249.1999.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murch SH, Costeloe K, Klein NJ, MacDonald TT. Early production of macrophage inflammatory protein-1 alpha occurs in respiratory distress syndrome and is associated with poor outcome. Pediatr Res. 1996;40:490–7. doi: 10.1203/00006450-199609000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson JD, Truog WE, Benjamin DR. Increased expression of human leukocyte antigen-DR on pulmonary macrophages in bronchopulmonary dysplasia. Pediart Res. 1993;34:341–4. doi: 10.1203/00006450-199309000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Jones CA, Cayabyab RG, Knong KY, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA. Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res. 1996;39:966–75. doi: 10.1203/00006450-199606000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimoto T, Nishihira J, Tada M, Houkin K, Abe H. Induction of macrophage inhibitory factor messenger ribonucleic acid in rat forebrain by reperfusion. Neurosurgery. 1997;41:648–53. doi: 10.1097/00006123-199709000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Penkowa M, Moos T, Carrasco J, Hadberg H, Molinero A, Bluethmann H, Hidalgo J. Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia. 1999;25:343–57. [PubMed] [Google Scholar]
- 22.Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann NY Acad Sci. 1997;825:179–93. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- 23.Zapata-Sirvent RL, Hansbrough FJ. Temporal analysis of human leukocyte surface antigen expression and neutrophil respiratory burst after thermal injury. Burns. 1993;19:5–11. doi: 10.1016/0305-4179(93)90093-n. [DOI] [PubMed] [Google Scholar]
- 24.Cheadle WG. The human leukocyte antigens and their relationship to infection. Am J Surg. 1993;165:75s–81s. doi: 10.1016/s0002-9610(05)81210-3. [DOI] [PubMed] [Google Scholar]
- 25.Cheadle WG, Hershman MJ, Wellhausen SR, Polk HC. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am J Surg. 1991;161:639–45. doi: 10.1016/0002-9610(91)91247-g. [DOI] [PubMed] [Google Scholar]
- 26.Lin RY, Astiz ME, Saxon JC, Rackow EC. Altered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, CD11b, CD14, and IL-2R expression. Chest. 1993;104:847–53. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- 27.Griffin JD, Ritz J, Nadler LM, Schlossman SF. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981;68:932–41. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimitriou-Boca A, Burmester GR, Waters SJ, Winchester RJ. Human mononuclear phagocyte differentiation antigens. J Immunol. 1983;130:145–52. [PubMed] [Google Scholar]
- 29.Passlick B, Flieger D, Ziegler-Heitbrock HWL. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 30.Wang SY, Mak KL, Chen LY, Chou MP, Ho CK. Heterogeneity of human blood monocyte: two subpopulations with different sizes, phenotypes and functions. Immunology. 1992;77:298–303. [PMC free article] [PubMed] [Google Scholar]
- 31.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock L. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 32.Heinzelmann M, Mercer-Jones M, Cheadle WG, Polk HC. CD14 expression in injured patients correlates with outcome. Ann Surg. 1996;224:916–21. doi: 10.1097/00000658-199607000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkenmaier C, Hoivg YS, Horn JK. Modulation of the endotoxin receptor (CD14) in septic patients. J Trauma. 1992;32:473–8. [PubMed] [Google Scholar]
- 34.Knapp W, Dorden B, Gilks WR, Rieber EP, Schmidt RE, Stein H, Knapp W. White cell differentiation antigens. Oxford: Oxford University Press; 1989. Leukocyte typing IV; pp. 789–816. [Google Scholar]
- 35.Astiz M, Saha D, Lustbader D, Lin R, Rackow E. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996;126:594–600. doi: 10.1016/s0022-2143(96)90132-8. [DOI] [PubMed] [Google Scholar]
- 36.Lin RY, Astiz ME, Saxon JC, Saha DC, Rackow EC. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit Care Med. 1994;22:1595–602. [PubMed] [Google Scholar]
- 37.Klava A, Windsor AC, Farmery SM, Woodhouse LF, Reynolds JV, Ramsden CW, Boylston AW, Guillou PJ. Interleukin-10. A role in the development of postoperative immunosuppression. Arch Surg. 1997;132:425–9. doi: 10.1001/archsurg.1997.01430280099016. [DOI] [PubMed] [Google Scholar]
- 38.Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin-10 response in pediatric sepsis induced multiple organ failure. Chest. 1998;113:1625–31. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]