Abstract
Synergism between Mycobacterium tuberculosis (M. tuberculosis) and HIV-1 infections was demonstrated in several in vitro models and clinical studies. Here, we investigated their reciprocal effects on growth in chronically HIV-1-infected promonocytic U1 cells and in acutely infected monocyte-derived macrophages (MDM). Phagocytosis of M. tuberculosis induced HIV-1 expression in U1 cells, together with increased TNF-α production. M. tuberculosis growth, evaluated by competitive PCR, was greater in HIV-1-infected MDM compared to uninfected cells. M. tuberculosis phagocytosis induced greater TNF-α and IL-10 production in HIV-1-infected MDM than in uninfected cells. In uninfected MDM, addition of TNF-α and IFN-γ decreased, whereas IL-10 increased M. tuberculosis growth. On the contrary, in HIV-1-infected MDM, addition of TNF-α and IFN-γ increased, whereas IL-10 has no effect on M. tuberculosis growth. TNF-α seems to play a pivotal role in the enhanced M. tuberculosis growth observed in HIV-1-infected MDM, being unable to exert its physiological antimycobacterial activity. Here, for the first time we demonstrated an enhanced M. tuberculosis growth in HIV-1-infected MDM, in line with the observed clinical synergism between the two infections.
Keywords: Mycobacterium tuberculosis, HIV-1, TNF-α, IL-10
INTRODUCTION
HIV-1 infection has had a major impact on the incidence of tuberculosis and is associated with the resurgence of tuberculosis in Western countries and with the emergence of multidrug resistant forms of Mycobacterium tuberculosis (M. tuberculosis). Globally, M. tuberculosis is one of the most common opportunistic infections seen in HIV-1 positive patients. M. tuberculosis infection of HIV-1 positive patients, occurring before the onset of AIDS, potentially reactivates HIV-1-replication, resulting in increased HIV-1 viremia, and hastens HIV-1 disease [1–4]. On the other hand, HIV-1-infection in itself may impair appropriate immune response to M. tuberculosis [5,6].
Macrophages play a dual role in the pathogenesis of tuberculosis and HIV-1 infection, being both the principal effector cells against M. tuberculosis and reservoirs for intracellular growth of both pathogens [7–9]. In vitro studies have shown that phagocytosis of M. tuberculosis induces macrophage activation and pro-inflammatory cytokine production (TNF-α, IL-1β, IL-6, and IL-8) [10–13], which play a key role in M. tuberculosis killing [14], but, at the same time, enhance HIV-1 replication [15–17]. Several animal models have shown that resistance to M. tuberculosis infection is associated with TNF-α and IFN-γ production [18,19], whereas treatment with anti-TNF-α antibodies enhances the susceptibility to M. tuberculosis infection, also via inhibition of IFN-γ production [20–22]. Regarding HIV-1-infection, several evidence from in vitro and ex-vivo studies demonstrates an immunopathogenetic role for TNF-α and associated pro-inflammatory cytokines [23]. Furthermore, TNF-α is able to up-regulate HIV-1 replication in cells of the monocytic as well as of the lymphocytic lineage [24]. IL-10 is one of the principal cytokines with anti-TNF-α activity [25]. There is growing evidence on its role in regulating HIV-1-infection of monocytic cells. In the acute model of monocyte-derived macrophages (MDM) infection, IL-10 was shown both to up-and down-regulate HIV-1 [26–28], and in chronically HIV-1-infected U1 cells, it induced virus expression alone or in synergy with TNF-α [29,30]. The relationship between IL-10 and mycobacterial growth was investigated in the M. avium infection model only. It has been demonstrated that in murine monocytes, IL-10 inhibited intracellular killing, and treatment with anti-IL-10 enhanced resistance to in vivo infection [31–34]. Likewise, the effects of HIV-1 infection on macrophage antimycobacterial activity were investigated in the M. avium infection model only. In one of these studies it was shown that HIV-1 infection of monocytic cells increased M. avium growth, cell death and pro-inflammatory cytokine secretion [34]. Furthermore, monocytes from HIV-1-positive patients displayed increased IL-10 production in response to M. avium [35]. No studies have addressed the possible effects on M. tuberculosis growth and on inducible cytokine secretion during concurrent infection of monocytic cells with HIV-1 and live M. tuberculosis. Furthermore, there are no studies on the effect of exogenous cytokines on M. tuberculosis growth in HIV-1-infected and uninfected monocytic cells. We investigated the in vitro interrelationship between these two pathogens, with regard to their reciprocal effects on growth, the secretion of pro- and anti-inflammatory cytokines, and the modulation of M. tuberculosis growth by exogenous cytokines in HIV-1-infected and uninfected monocytic cell lines, and MDM.
MATERIALS AND METHODS
Reagents
Human recombinant (hr) TNF-α, hrIFN-γ, hrIL-10, were purchased from R & D Systems (Oxford, England, UK); M. tuberculosis H37-Rv virulent strain was provided by National Collection of Type Cultures (Colindale, UK).
Cell lines and culture conditions
Cell lines (the promonocytic U937 and its HIV-1-positive derived U1 cell line) were maintained in complete culture medium (RPMI 1640 supplemented with 10 mm Hepes, 2 mm l-glutamine (Sigma-Aldrich, St. Louis, MO), 10% heat-inactivated FCS (HyClone Laboratories, Logan, UT), penicillin at 100 units/ml and streptomycin at 100 µg/ml (Life Technologies GIBCO BRL, Gaithersburg, MD) in log phase of growth [30]. Before use, cells were washed 3× with PBS to remove exogenous virus. Before phagocytosis cells were plated into 12·5 cm2 flasks (Falcon Labware, Oxnard, CA) at the concentration of 1 × 105/ml (final volume 10 ml) in the presence of vitamin D3 (1 mg/ml) to allow differentiation.
For in vitro studies on PBMC, cells were obtained from blood donor buffy coats by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) density gradient centrifugation and monocytes were isolated by Percoll (Pharmacia) gradient separation. Monocytes were allowed to differentiate into MDM in complete medium (20% FCS in 12·5 cm2 tissue culture flasks at 106 cells/ml) for 7 days. MDM were infected with monotropic HIV-1 BAL strain (1 : 10 dilution) for 48 h, washed and resuspended in complete medium, replaced 3 times/week, for 3 weeks. RT activity and p24 antigen release were measured weekly postinfection. The undiluted viral stocks of HIV-1BAL strain contained 107 infectious Units/ml. The phagocytosis of live and HI-M. tuberculosis (10 micro-organisms/cell) was performed 48 h post HIV-1 infection in complete medium (20% FCS without antibiotics).
The mycobacteria were maintained in culture at 37°C in 5% CO2 humidified atmosphere in solid medium IUTM (International Union Tuberculosis Medium) with 100 mg/ml ampicillin for at least 90 days. Before use in phagocytosis experiments, mycobacteria were sonicated for 4 min to disrupt the clumps, suspended in complete RPMI 1640 medium without antibiotics and then counted at light microscope. To obtain inactive M. tuberculosis the bacilli, suspended in RPMI1640, were heat-killed with an incubation of 15 min at 90°C. Macrophages or cell lines, suspended in 20% FCS-RPMI 1640 without antibiotics, were exposed to live or HI-M. tuberculosis for 24 h in 5% CO2 humidified incubator. Cells were then washed to eliminate the nonphagocytosed mycobacteria. In preliminary experiments phagocytosis of M. tuberculosis was evaluated by light microscopy using a modified Kinyoun staining technique [10]. To evaluate the effect of M. tuberculosis phagocytosis on cell mortality, cells were periodically counted using the trypan blue dye (0·4%, Sigma) exclusion method. M. tuberculosis causes lysis and cell death in 10–14 days. In preliminary experiments, we observed that a 20 : 1 ratio between M. tuberculosis and cells has a high cytopathic effect, a 10 : 1 ratio is better in terms of cell viability (28% in cultures with M. tuberculosis versus 45% in control cultures), while the 5 : 1 ratio is not suitable for competitive PCR experiments. Therefore 10 micro‐organisms/cell was the M. tuberculosis/cell concentration used in all the experiments. The correlation between the PCR and conventional CFU counts has been performed as already described [36]. Supernatants were removed after 48 h for the measurement of cytokine and chemokine secretion, and after 7, 14 and 21 days for the detection of reverse transcriptase (RT) activity and p24 antigen release. To study the growth of M. tuberculosis the cells were harvested 3 weeks after phagocytosis, and M. tuberculosis DNA was extracted for competitive PCR experiments. In MDM cultures the growth of M. tuberculosis was investigated also in the presence of TNF-α (20 ng/ml), IL-10 (20 ng/ml), and IFN-γ (20 ng/ml).
ELISA determinations
Supernatants were assayed for TNF-α, IL-10 (R & D systems), MIP-1α, RANTES, and MCP-1 (Biotrak, Amersham, GB), p24 antigen release (Cellular Products Inc. Buffalo, NY) and Reverse Transcriptase (ELISA Retrosys RT assay, Innovagen, Lund, Sweden), according to the manufacturer's instructions.
M. tuberculosis DNA extraction
For the extraction of M. tuberculosis DNA cells were incubated for 20 min at 95°C with 200 µl of lysis buffer, followed by the addition of 200 µl phenol-chloroform-isoamyl-alcohol solution and 50 µl 1 m TRIS-HCl pH 7·5. After a second phenol-chloroform-isoamyl-alcohol extraction (v/v) DNA was precipitated with 1 m Na acetate pH 8·4 (1/10 vol) and ethanol 100% (3 vol) at − 80°C for 1 h. Pellets were resuspended in sterile water and then quantified at the spectrophotometer.
Polymerase chain reaction
For competitive PCR, the DNA sample was coamplified with a specific competitor sharing the same primer recognition sites. The competitor entirely shares the nucleotide sequence with the genomic M. tuberculosis target, with exception of an extra 20 bp in the middle, to allow resolution by gel electrophoresis. The target region chosen for amplification was the insertion element IS6110 specific for the M. tuberculosis complex, which has been extensively utilized in literature both for M. tuberculosis detection and typing. One hundred ng of DNA sample and 1 µl of serial dilutions of the competitor, starting from a concentration of 109 molecules/µl, were used (kindly provided by L. Dolzani, Department of Biomedical Sciences, University of Trieste, Italy). The number of competitor molecules was calculated by dividing the DNA concentration by the MW of the competitor, and thereafter by multiplying the molarity by the Avogadro number, according to the following formula: number of competitor molecules/µl = DNA concentration (µg/ml)/MW (n° of bp × 660g/mol) × 6 × 1023 × 10−9. The amplification mixture consisted of 2·5 U Taq DNA Polymerase (Boehringer Mannheim, Indianapolis, IN), 50 pmol of both primers, 200 µm dNTPs (Boehringer Mannheim), and PCR-buffer plus 1·25 mm MgCl2. PCR primers used were sense GAT GCA CCG TCG AAC GGC T and antisense GCG TAG GCG TCG GTG ACA AA. The conditions for the amplification were as follows: 10 min denaturation at 94°C, followed by 35 cycles of 94°C for 50 s, 65°C for 50 s, 72°C for 1 min and 30 s and a final extension at 72°C for 5 min. The primers amplified a fragment of 223 bp and 243 bp for DNA sample and competitor, respectively. PCR products were resolved by electrophoresis through 3·5% agarose gels in ethidium bromide. The number of M. tuberculosis-DNA copies was calculated by dividing the number of competitor molecules at equivalence by 16, considering the copy number of IS6110 inside H37-Rv M. tuberculosis genome.
As control for the DNA quantification a PCR for the housekeeper gene GA3PDH was also performed with the primers sense TGA AGG TCG GAG TCA ACG GAT TTG GT and antisense CAT GTG GGC CAT GAG GTC CAC CAC. The amplification mixture consisted of 1 U Taq DNA Polymerase (Boehringer Mannheim), 50 pmol of both primers, 200 µm dNTPs (Boehringer Mannheim), and PCR-buffer plus 1·25 mm MgCl2. The conditions for the amplification were as follows: 3 min denaturation at 94°C, followed by 25 cycles of 94°C for 45 s, 60°C for 45 s, 72°C for 1 min and a final extension at 72°C for 7 min; the primers amplified a fragment of 983 bp. PCR products were resolved by electrophoresis through 1% agarose gels in ethidium bromide.
Statistical analysis
Results were analysed by two tailed Students' t-test for paired data, after log transformation for non-normally distributed values. A P-value < 0·05 was considered significant.
RESULTS
M. tuberculosis growth in HIV-1 positive and negative MDM and monocytic cell lines
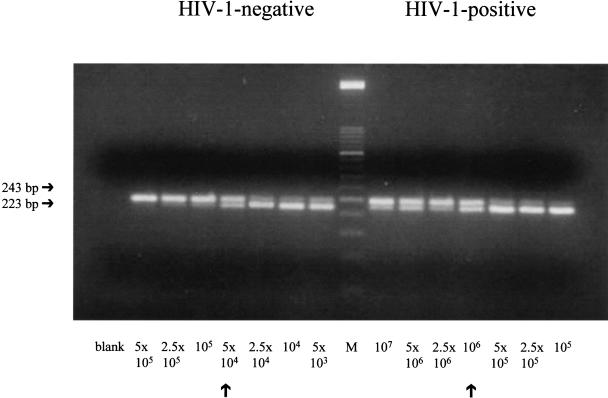
Growth of M. tuberculosis is significantly greater in HIV-1-infected MDM compared to that in HIV-1-negative cells (43532 ± 7596 versus 10500 ± 4164 M. tuberculosis DNA copies/100 ng total DNA, mean ± SE of 7 independent experiments, P = 0·008). A typical competitive PCR experiment is shown in Fig. 1. Conversely, in the pro-monocytic cell line U1, the growth of M. tuberculosis is significantly lower than that found in uninfected parental cells U937 (2345 ± 453 versus 4690 ± 900 M. tuberculosis DNA copies/100 ng total DNA, mean ± SE of 4 independent experiments, P < 0·02).
Fig. 1.
M. tuberculosis growth in acutely HIV-1-infected and uninfected MDM. A typical experiment of competitive PCR is shown. DNA sample (100 ng) is amplified in the presence of scalar amounts of competitor molecules. Arrows indicate the equivalence between the competitor and the DNA sample. Number of M. tuberculosis-DNA copies/100 ng of total DNA are calculated as described in materials and methods.
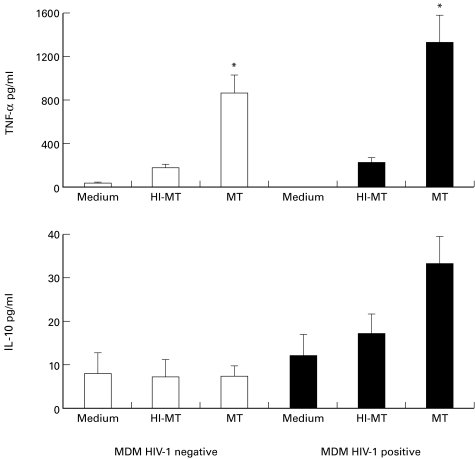
Effect of phagocytosis of live and HI-M. tuberculosis on the production of TNF-α and IL-10 in HIV-1-positive and negative MDM and cell lines
Phagocytosis of live M. tuberculosis induced a thousand-fold greater TNF-α production both in HIV-1-infected and uninfected MDM, compared to resting cells (P < 0·04 for HIV-1-infected, and P < 0·05 for uninfected MDM) (Fig. 2, upper panel). Phagocytosis of HI-M. tuberculosis by HIV-1-infected and uninfected MDM induced little TNF-α production, which was significantly lower compared to live M. tuberculosis (P < 0·05 live versus HI-M. tuberculosis for both HIV-1-positive and negative MDM). Regarding IL-10 (Fig. 2, lower panel), phagocytosis of live M. tuberculosis induced a two-fold greater release over unstimulated cells in HIV-1-infected cells only, whereas no change in cytokine production was observed in HIV-1 negative MDM. Phagocytosis of HI-M. tuberculosis induced intermediate production. It is worth noting that phagocytosis-induced production of TNF-α and IL-10 was greater in HIV-1-infected MDM compared to uninfected cells, with borderline significance (P = 0·06).
Fig. 2.
TNF-α (upper panel) and IL-10 (lower panel) production by HIV-1-infected (▪) or uninfected MDM (□) 4 h after phagocytosis of live M. tuberculosis (MT) and heat inactivated-M. tuberculosis (HI-MT), and in resting conditions. Values are the mean ± SE of four experiments. *denotes statistically significant comparisons for TNF-α production: P < 0·04 M. tuberculosis versus medium for HIV-1-infected, P < 0·05 M. tuberculosis versus medium for uninfected MDM, and P < 0·05 live versus HI-M. tuberculosis for both HIV-1-positive and negative MDM.
Considering HIV-1 positive and negative cell lines, production of TNF-α and IL-10 was very low. Phagocytosis of live M. tuberculosis induced TNF-α release in U1 cells (4·83 ± 1·61 versus 0·67 ± 0·38 pg/ml for unstimulated cells, mean ± SE of 4 independent experiments). Conversely, in U937 cells baseline TNF-α values were unchanged after phagocytic stimulus. Similarly, unstimulated IL-10 production was slightly augmented by phagocytosis of live M. tuberculosis in U1 cells only (13·11 ± 2·62 versus 11·11 ± 2·02 pg/ml, mean ± SE of 4 independent experiments). As observed in MDM cultures, HIV-1-positive cells, compared to uninfected parental cells, produced greater amounts of both TNF-α (4·83 ± 1·61 versus 1·17 ± 0·92 pg/ml, mean ± SE of 4 independent experiments) and IL-10 (13·11 ± 2·62 versus 4 ± 0·8 pg/ml, mean ± SE of 4 independent experiments, P < 0·05).
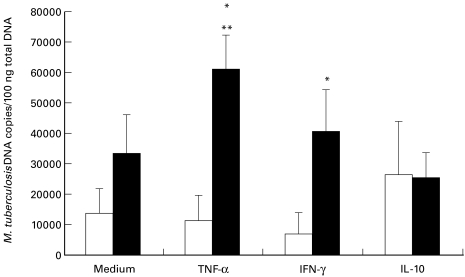
Effect of exogenous cytokines on M. tuberculosis growth, TNF-α production and HIV-1 expression in MDM
Addition of TNF-α to HIV-1-infected MDM induced a significant increase of M. tuberculosis growth, compared to cultures performed in the absence of the cytokine (P = 0·007) (Fig. 3). In uninfected MDM, addition of TNF-α had no effect on M. tuberculosis growth. In presence of TNF-α M. tuberculosis growth was greater in HIV-1-infected cells than in uninfected MDM (P = 0·03). In the latter, addition of IFN-γ induced a slight reduction of M. tuberculosis growth compared to cultures performed in the absence of the cytokine. On the contrary, addition of IFN-γ significantly enhanced M. tuberculosis growth in HIV-1-infected MDM, compared to uninfected MDM (P = 0·01). An opposite effect was observed for IL-10: addition of this cytokine to HIV-1-negative MDM resulted in enhanced M. tuberculosis growth, whereas it had no effect on M. tuberculosis growth in HIV-1-infected MDM.
Fig. 3.
Effect of TNF-α, IFN-γ, and IL-10 (20 ng/ml) on M. tuberculosis growth in HIV-1-infected (▪) and uninfected (□) MDM. Values are expressed as number of M. tuberculosis DNA copies in 100 ng of total DNA, mean ± SE of 5 experiments. Statistically significant comparisons: ** P = 0·007 TNF-α versus medium in HIV-1-infected MDM; * P = 0·03 and P = 0·01 for TNF-α and IFN-γ, respectively, in HIV-1-infected versus uninfected MDM.
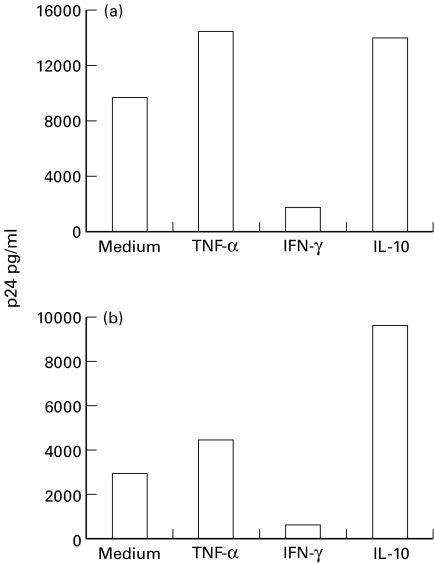
Finally, to investigate whether differences in M. tuberculosis growth were related to different HIV-1 expression induced by the addition of exogenous cytokines, we evaluated p24 and RT values in all the experimental conditions. It was found that TNF-α and IL-10 enhanced, and IFN-γ inhibited HIV-1 expression, with a comparable behaviour in M. tuberculosis-infected and uninfected MDM (Fig. 4).
Fig. 4.
Effect of TNF-α, IFN-γ, and IL-10 on HIV-1 expression in HIV-1-infected-MDM either unstimulated (a) or after phagocytosis of M. tuberculosis (b). A typical experiment is shown.
Effect of phagocytosis of live and HI-M. tuberculosis on HIV-1 expression
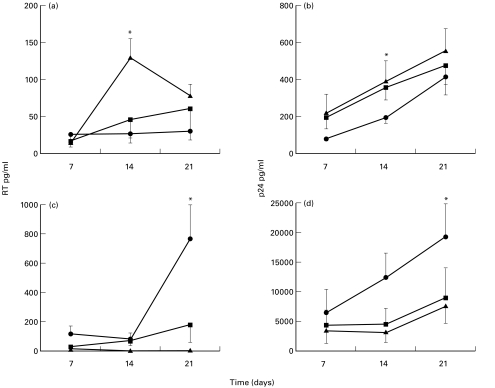
Phagocytosis of M. tuberculosis increased HIV-1 expression in U1 cells, measured by both p24 release and RT activity (Fig. 5). This increase was significant only 14 days after phagocytosis (P < 0·05, M. tuberculosis versus unstimulated cultures, both for p24 and RT). HI compared to live M. tuberculosis increased HIV-1 replication to a lesser degree, particularly when HIV-1 expression was assessed by RT activity (P < 0·05, HI versus live M. tuberculosis at 14 days after phagocytosis). On the contrary, phagocytosis of live M. tuberculosis by MDM significantly inhibited HIV-1 expression at the 21st day post infection, assessed both by p24 release and RT activity (P < 0·05, M. tuberculosis versus unstimulated cultures, both for p24 and RT). The effect on HIV-1 expression was comparable between HI and live M. tuberculosis (P < 0·05, HI-M. tuberculosis versus unstimulated cultures, assessed by RT).
Fig. 5.
Time kinetics of HIV-1 expression in U1 cells (a,b) and MDM (c,d), assessed by RT activity (a,c) and p24 release (b,d), after phagocytosis of live (▴) and HI-M. tuberculosis (▪) compared to unstimulated cells (•). Values are the mean ± SE of four experiments. * denotes statistically significant comparisons: P < 0·05, RT values of M. tuberculosis versus unstimulated cultures and live versus HI-M. tuberculosis in U1 cells, live and HI-M. tuberculosis versus unstimulated cultures in MDM; P < 0·05, p24 values of M. tuberculosis versus unstimulated cultures in U1 and MDM.
To address whether inhibition of HIV-1 expression in dually infected MDM compared to MDM infected with HIV-1 only, could be due to soluble mediators released after phagocytosis of M. tuberculosis, we investigated the production of chemokines after phagocytosis in HIV-1-infected MDM (and in uninfected MDM, as control). As shown in Table 1, phagocytosis of M. tuberculosis induced a significant increase of both RANTES and MIP-1α production over unstimulated cultures, both in HIV-1-infected and uninfected MDM. As regards MCP-1, a high basal production was found, which was further increased by M. tuberculosis phagocytosis, although not significantly. Thus, the observed inhibition of HIV-1 expression after M. tuberculosis phagocytosis in dually infected MDM, compared to MDM infected with HIV-1 only, could be related to the production of chemokines, which are well known inhibitors of HIV-1 infection.
Table 1.
Chemokine production 48 h after phagocytosis in HIV-1-positive and negative MDM
| HIV-1 positive Medium | M. tuberculosis | P-value | HIV-1 negative Medium | M. tuberculosis | P-value | |
|---|---|---|---|---|---|---|
| RANTES (pg/ml) | 34 ± 13 | 579 ± 174 | 0·07 | 30 ± 6·7 | 351 ± 117·5 | 0·003 |
| MIP-1α (pg/ml) | 75·14 ± 27 | 4613 ± 1125 | 0·05 | 117·4 ± 49·4 | 4017 ± 1445 | 0·05 |
| MCP-1 (pg/ml) | 37542 ± 13188 | 66437 ± 4381 | N.S.* | 60142 ± 11110 | 57356 ± 2300 | N.S. |
Data are the mean ± SE of four independent experiments.
N.S. not significant
DISCUSSION
Our results showed that M. tuberculosis growth was greater in HIV-1-positive MDM, compared to uninfected cells. Since several cytokines are known to regulate M. tuberculosis growth [14,20,37], we investigated whether differences in its growth could be due to different cytokine patterns induced by phagocytosis in HIV-1-infected and uninfected macrophages. Furthermore, we tested whether M. tuberculosis growth could be differently modulated by exogenous cytokines in HIV-1-infected and uninfected cells. HIV-1-infected MDM produce higher concentrations of TNF-α following phagocytosis of M. tuberculosis. This enhanced production was not simply a consequence of phagocytosis itself, because HI-M. tuberculosis induced lower cytokine release, but implies an active role of mycobacterial growth. TNF-α is known as one of the principal cytokines involved in M. tuberculosis killing [14,20,37], in synergy with IFN-γ [20]. As a matter of fact, in our in vitro model of uninfected MDM, addition of IFN-γ reduces, whereas TNF-α has no effect on M. tuberculosis growth, although we do not know the effect of the simultaneous presence of these cytokines. In contrast, addition of TNF-α to HIV-1-positive MDM resulted in enhanced M. tuberculosis growth, suggesting that TNF-α has no antimycobacterial activity in HIV-1-positive MDM. This behaviour, peculiar for HIV-1-positive MDM, might indicate that HIV-1-infection of monocytic cells profoundly affects normal monocyte function, resulting in a positive feedback mechanism, in which enhanced M. tuberculosis growth is maintained by enhanced TNF-α production and viceversa.
HIV-1-induced macrophage alterations could also explain the different effect of other exogenous cytokines on M. tuberculosis growth in uninfected and HIV-1-infected MDM. The different modulation of mycobacterial growth is particularly evident for IL-10 and IFN-γ, which have opposite effects in HIV-1-infected and uninfected macrophages. As expected, IFN-γ reduced and IL-10 increased M. tuberculosis growth in uninfected MDM. This finding is in disagreement with Shiratsuchi et al. [38], who found no effect of IL-10 on M. avium growth in human MDM, but is in line with other reports, showing that anti-IL-10 antibodies decreased in vitro mycobacterial growth in murine MDM and enhanced resistance against M. avium infection in vivo [31–34]. In contrast with uninfected MDM, we found that addition of IL-10 has no effect, whereas IFN-γ enhanced mycobacterial replication in HIV-1-infected MDM.
On the other hand, it has long been known that cytokines influence HIV-1-expression. TNF-α up-regulates HIV-1 replication in all in vitro and in vivo models of viral infection, whereas IFN-γ and IL-10 have a less defined behaviour [23–29,39]. In the chronically HIV-1-infected U1 cell line, IFN-γ induces virus replication but decreases PMA-induced HIV-1 expression, via redistribution of membrane-associated versus intracytoplasmic virus [40]. Likewise, IL-10 increases HIV-1 expression via induction of endogenous TNF-α production, and in synergy with exogenous TNF-α [30]. In the acute model of MDM infection, it has been shown that IFN-γ inhibits virus expression [41], while IL-10 both up- and down-regulates HIV-1, depending on the experimental protocol employed [26–28]. Given these complex and sometimes conflicting results, we investigated whether the addition of exogenous cytokines to our HIV-1/M. tuberculosis coinfection model, could modify mycobacterial growth as a consequence of HIV-1 modulation. In dually infected MDM, we found that HIV-1 expression was increased by TNF-α and IL-10, whereas it was reduced by IFN-γ. The findings in TNF-α-supplemented cultures, i.e. the enhanced HIV-1-positivity associated with the increased M. tuberculosis growth, suggest that the effects of TNF-α on mycobacterial growth could be mediated via enhanced HIV-1 expression. However, this was not the fact for IL-10- and IFN-γ-added cultures. In IL-10-supplemented cultures, in spite of a greater HIV-1-positivity, M. tuberculosis growth was comparable to cultures in the absence of the cytokine. Likewise, in IFN-γ-added cultures, M. tuberculosis growth was greater notwithstanding a reduced HIV-1 expression. These data suggest that TNF-α plays a leading role in enhancing both HIV-1 expression and M. tuberculosis growth, whereas the effects of other cytokines are less straightforward. In attempt to speculate on the in vivo situation, in the context of dually infected patients, the inflammatory response may be deleterious for both infections, whereas a prevalent type-1 or type-2 response has a greater effect on M. tuberculosis growth and HIV-1 expression, respectively.
Taken together our results demonstrate that, in latently HIV-1-infected promonocytic cells, phagocytosis of M. tuberculosis induced viral expression, and that, in the acutely HIV-1-infected MDM, M. tuberculosis growth was increased. TNF-α seems to play a pivotal role in the enhanced M. tuberculosis growth, being unable to exert its physiological antimycobacterial activity. Here, for the first time we demonstrated an enhanced M. tuberculosis growth in HIV-1-infected MDM, in line with the observed clinical synergism between the two infections.
Acknowledgments
This work was supported by National Research Program on AIDS, 1998 (grant n. 50B.3), and National Program on Tuberculosis, 1996 (grant n. 96/D/T15), Istituto Superiore di Sanità.
We thank L. Dolzani (Department of Biomedical Sciences, University of Trieste, Italy) and M. Giacca (International Centre for Genetic Engineering and Biotechnology, Trieste) for providing M. tuberculosis PCR primer sequences and competitor.
REFERENCES
- 1.Goletti D, Weissmann D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 2.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. New Engl J Med. 1999;340:367–73. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 3.Nakata K, Rom WN, Honda Y, Condos R, Kanegasaki S, Cao Y, Weiden M. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 4.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–35. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 5.Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR, Jr, Hopewell PC. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphism. N Engl J Med. 1992;326:231–5. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 6.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, Walker AT, Friedland GH. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–50. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 7.Chan J, Kaufmann S. Immune mechanisms of protection. In: Bloom B, editor. Tuberculosis: pathogenesis, protection and control – 1994. Washington DC: American Society for Microbiology; 1994. pp. 389–416. [Google Scholar]
- 8.Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, Haase AT. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–62. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 9.Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 10.Friedland JS, Remick DG, Shattock RJ, Griffin GE. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J Immunol. 1992;22:1373–8. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 11.Friedland JS, Shattock RJ, Griffin GE. Phagocytosis of M. tuberculosis or particulate stimuli by human monocytic cells induces equivalent Monocyte Chemotactic Protein 1 gene expression. Cytokine. 1993;5:150–6. doi: 10.1016/1043-4666(93)90054-9. [DOI] [PubMed] [Google Scholar]
- 12.Friedland JS, Shattock RJ, Johnson JD, Remick DG, Holliman RE, Griffin GE. Differential cytokine gene expression and secretion after phagocytosis by a human cell line of Toxoplasma gondii compared with Mycobacterium tuberculosis. Clin Exp Immunol. 1993;91:282–6. doi: 10.1111/j.1365-2249.1993.tb05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom WN. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Invest. 1995;95:586–92. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munk ME, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Eur Respir J. 1995;S20:668s–75s. [PubMed] [Google Scholar]
- 15.Shattock RJ, Friedland JS, Griffin GE. Modulation of HIV transcription in and release from human monocyte cells following phagocytosis of Mycobacterium tuberculosis. Res Virol. 1993;144:7–12. doi: 10.1016/s0923-2516(06)80005-1. [DOI] [PubMed] [Google Scholar]
- 16.Shattock RJ, Friedland JS, Griffin GE. Phagocytosis of Mycobacterium tuberculosis modulates HIV transcription in human monocytic cells. J Gen Virol. 1994;75:849–56. doi: 10.1099/0022-1317-75-4-849. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Nakata K, Weiden M, Rom WN. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;95:2324–31. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon-gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J Immunol. 1990;145:149–54. [PubMed] [Google Scholar]
- 21.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bacterial granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 22.Denis M. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J Leuk Biol. 1991;50:495–501. doi: 10.1002/jlb.50.5.495. [DOI] [PubMed] [Google Scholar]
- 23.Fauci AS. Multifactorial nature of Human Immunodeficiency Virus disease: implications for therapy. Science. 1993;262:1011–8. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 24.Poli G, Fauci AS. Cytokine modulation of HIV expression. Semin Immunol. 1993;5:165–73. doi: 10.1006/smim.1993.1020. [DOI] [PubMed] [Google Scholar]
- 25.Howard M, O'Garra M. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 26.Saville MW, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses HIV-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994;83:3591–9. [PubMed] [Google Scholar]
- 27.Weissman D, Poli G, Fauci AS. IL-10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J AIDS. 1995;9:442–9. [PubMed] [Google Scholar]
- 28.Weissman D, Poli G, Fauci AS. Interleukin-10 blocks HIV replication in macrophages by inhibiting the autocrine loop of TNF-α and IL-6 induction of virus. AIDS Res Hum Retroviruses. 1994;10:1199–206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 29.Angel JB, Saget MS, Wang M, Wang A, Dinarello CA, Skolnik PR. Interleukin-10 enhances human immunodeficiency virus type 1 expression in a chronically infected promonocytic cell line (U1) by a tumor necrosis factor-α independent mechanism. J Interferon Cytokine Res. 1995;15:575–84. [PubMed] [Google Scholar]
- 30.Barcellini W, Rizzardi GP, Marriott JB, Fain C, Shattock RJ, Meroni PL, Poli G, Dalgleish AG. Interleukin-10-induced HIV-1 expression is mediated by induction of both membrane bound tumor necrosis factor (TNF-α) and TNF receptor type 1 in U1 cell line. AIDS. 1996;10:835–42. doi: 10.1097/00002030-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Bermudez L, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–30. [PubMed] [Google Scholar]
- 33.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–95. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman GW, Kelley TG, Gan H, Kandil O, Newman MJ, Pinkston P, Rose RM, Remold HG. Concurrent infection of human macrophages with HIV-1 and Mycobacterium avium results in decreased cell viability, increased M. avium multiplication and altered cytokine production. J Immunol. 1993;151:2261–72. [PubMed] [Google Scholar]
- 35.Muller F, Aukrust P, Lien E, Haug CJ, Froland SS. Enhanced interleukin-10 production in response to Mycobacterium avium products in mononuclear cells from patients with human immunodeficiency virus infection. J Infect Dis. 1998;177:586–94. doi: 10.1086/514222. [DOI] [PubMed] [Google Scholar]
- 36.Ligozzi M, Pelosi E, Fontana R. Development of a rapid method for quantitative evaluation of Mycobacterium tuberculosis growth based on competitive polymerase chain reaction. J Med Microbiol. 1998;47:933–6. doi: 10.1099/00222615-47-10-933. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa T, Uchida H, Kusumoto Y, Mori Y, Yamamura Y, Hamada S. Increase in tumor necrosis factor-alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991;59:3021–5. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiratsuchi H, Hamilton B, Toossi Z, Ellner JJ. Evidence against a role of Interleukin-10 in the regulation of growth of Mycobacterium avium in human monocytes. J Infect Dis. 1996;173:410–7. doi: 10.1093/infdis/173.2.410. [DOI] [PubMed] [Google Scholar]
- 39.Rizzardi GP, Marriott JB, Cookson S, Lazzarin A, Dalgleish AG, Barcellini W. Tumour necrosis factor (TNF) and TNF-related molecules in HIV-1+ individuals: relationship with in vitro Th1/Th2-type response. Clin Exp Immunol. 1998;114:61–5. doi: 10.1046/j.1365-2249.1998.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas P, Poli G, Kinter AL, et al. Interferon-γ induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J Exp Med. 1992;176:739–50. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–51. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]