Abstract
Recent studies in vitro and in animals have suggested that ribavirin may potentiate the antihepatitis C virus (HCV) activity of interferon-α (IFN-α) by up-modulating the production of T cell-derived cytokines, such as interleukin (IL)-2 and IFN-γ, which play a key role in the cellular immune response against HCV. To study the immune-modulatory mechanisms of ribavirin further, cytokine production by activated T cells and circulating cytokine levels were studied by FACS analysis and ELISA testing in 25 patients with chronic hepatitis C unresponsive to IFN-α, before and after treatment with either ribavirin plus IFN-α or IFN-α alone. After 16 weeks of treatment, both the expression of IFN-γ by activated T cells and the blood levels of IFN-γ, were significantly reduced with respect to pretreatment values in patients treated with ribavirin and IFN-α but not in those undergoing treatment with IFN-α alone. The expression of IFN-γ was significantly lower in patients that gained normal ALT levels with respect to those that did not. No modification of the expression of IL-2, IL-4 and IL-10 was found before and after treatment in either group of patients. In conclusion, the results of this study do not support up-modulation of IFN-γ and IL-2 production as the mechanism by which ribavirin potentiates IFN-α anti HCV activity. In addition, our findings suggest that ribavirin may exert an anti-inflammatory effect and may help reducing IFN-γ-driven T cell activation and liver damage.
Keywords: chronic hepatitis C, ribavirin, IFN-α, IFN-γ
INTRODUCTION
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a synthetic guanosine nucleoside analogue which possesses in vitro antiviral activity against a range of RNA and DNA viruses [1, 2]. Ribavirin treatment of chronic hepatitis C has very little effect on the virus load despite the fact that most patients will normalize ALT levels transiently during treatment [3–7]. However, it has been recently shown that ribavirin may potentiate, in vivo, the capacity of interferon-α (IFN-α) to suppress, in vivo, the replication of hepatitis C virus (HCV) [8–11]. These data suggest that the beneficial effect of ribavirin in combination with IFN-α may be not directly antiviral. Along this line, recent studies in vitro and in animal models have shown that ribavirin may modulate the production of T cell-derived cytokines such as interleukin (IL)-2 and interferon-γ (IFN-γ) [12–14], which have been suggested to play a key role in the pathogenesis of chronic HCV infection [15, 16].
To study the immune-modulatory mechanisms of ribavirin further, we analysed cytokine production by polyclonally activated T cells and circulating cytokine levels in patients with chronic hepatitis C undergoing treatment with ribavirin and IFN-α.
PATIENTS AND METHODS
Patients and treatment
The study included 25 patients with serologically (anti-HCV antibodies and HCV RNA positive) and histologically demonstrated chronic hepatitis C who did not show both biochemical and virological response to a previous treatment with IFN-α alone at a dose of 6 mU thrice weekly, for at least 24 weeks (serum alanine aminotransferase (ALT) levels at least twice the upper limit of the normal range on two separate occasions (at least 1 month apart) and detectable HCV-RNA). A total of 6, age and sex matched, healthy volunteers were taken as controls. Informed consent was obtained from each subject included in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institution's human research committee. The patients who entered the study were assigned to receive recombinant IFN-α (3 mU thrice weekly) and ribavirin at a dose of 1000 mg/day for patients weighing < 75 kg and 1200 mg/day for patients weighing > 75 kg, in two divided doses orally. Nine patients refused to take ribavirin and were re-treated with IFN-α alone (6 mU thrice weekly). Further characteristics of the patients are reported in Table 1. The samples for cytokine analysis were obtained just before the beginning of treatment and four months after. After sampling, the blood was immediately processed for peripheral blood mononuclear cell (PBMC) separation (as described below) and plasma was frozen for subsequent determination of the levels of circulating cytokines.
Table 1.
Base-line characteristics of the patients
| Group | ||
|---|---|---|
| Variable | Ribavirin + IFN-α | IFN-α |
| N° of patients | 16 | 9 |
| Mean age (years) | 50 | 57 |
| Sex (M/F) | 8/8 | 5/4 |
| Genotype (no. of patients) | ||
| 1b | 12 | 7 |
| not 1b | 4 | 2 |
| ALT (mU/ml) | 127 ± 88 | 142 ± 74 |
| HCV RNA levels (copies/ml × 103) | 796 ± 674 | 897 ± 741 |
| Years from the first diagnosis of | 5·4 | 6·6 |
| HCV-related liver disease | ||
| Histological grading (no. of patients)* | ||
| 1–7 | 9 | 4 |
| 8–15 | 7 | 5 |
| Histological staging (no. of patients) | ||
| 1 | 2 | 1 |
| 2 | 4 | 3 |
| 3 | 8 | 3 |
| 4 | 0 | 1 |
| 5 | 2 | 1 |
Note. Data are expressed as mean ± SD ALT, alanine aminotransferase (normal range < 40 mU/ml).
Preparation PBMC and activation in vitro
PBMC were isolated from patients by gradient centrifugation over Ficoll Hypaque. PBMC (1 × 106 cells in a volume of 500 µl) were maintained in 24 well plates in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 20% heat-inactivated fetal calf serum, 2 mm l-glutamine, 50 units/ml penicillin and 50 µg/ml streptomycin (complete medium), at 37°C in a humidified atmosphere of 5% CO2 in air. PBMC were activated by the addition of 20 ng phorbol myristate acetate (PMA) plus 0·5 µg ionomycin (IO), both from Calbiochem, La Jolla, CA, USA. For FACS analysis, activated PBMC were cultured for 18 h in the presence of 0·5 µg/ml brefeldin A (Sigma Chimica, Milan, Italy). For the measurement of cytokine production in culture supernatants, activated PBMC were cultured for 48 h in the absence of brefeldin A, then supernatants were collected and stored at − 80°C. Cell derived cytokine production was measured by ELISA.
ELISA
Commercially available sandwich ELISA kits (R & D Systems Minneapolis, MN) were used to determine the concentration of IFN-γ, IL-2, IL-4 and IL-10 in either supernatants of PMA-IO-stimulated cultures or plasma samples. The detection limit of these ELISAs are, respectively, 7, 3, 4·1 and 7·8 pg/ml. All the samples were tested in duplicate, in a single analytical set.
HCV-RNA
The quantification of HCV-RNA in serum samples was performed using a commercially available kit: Amplicor HCV monitorTM test (Roche Diagnostic Systems, Branchburg, NJ). The lowest level of detection of this test is less than 200 HCV-RNA copies/ml of sample.
FACS analysis
FACS analysis was performed using a previously described method [17]. Briefly, after activation, PBMC were incubated for 15 min with one of the following mouse antihuman monoclonal antibodies (mAb: Cy-chromeTM-conjugated anti-CD3 (PharMingen, San Diego CA), phycoerytrin (PE)-conjugated anti-CD45RA and PE-conjugated anti-CD45RO. The cells were then fixed in 4% paraformaldehyde and stained with 0·5 µg/million cells of the following mAb: fluorescein isothiocyanate (FITC)-conjugated mouse antihuman IFN-γ, IL-2, or PE-conjugated mouse anti-IL-4 and anti-IL-10 (all from PharMingen). Paired isotype-specific control antibodies (PharMingen) were run with each sample. Cells were finally analysed by a FACScan flow cytometer (Becton Dickinson, Mountain View, USA). Lymphocytes were differentiated from dead cells on the basis of forward angle and 90° scatter. Five thousand cells were gated and analysed using the FACScan research software (Becton Dickinson).
Statistic
Data analysis was performed using unpaired Student's t-test. P-values ≤ 0·05 were considered statistically significant.
RESULTS
Biochemical and virological responses
After 4 months of treatment, 3 of 16 patients (19%) in the ribavirin and IFN-α group and none in the IFN-α group, cleared their HCV RNA. At the same time point, 9 of 16 patients (56%) in the ribavirin and IFN-α group and 3 of 9 (33%) patients in the IFN-α group, gained normal levels of serum ALT.
Cytokine expression by in vitro activated T cells
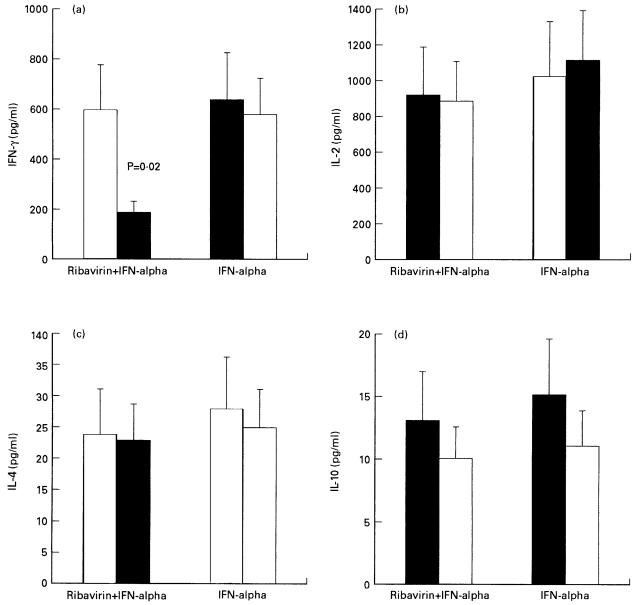
As shown in Table 2, FACS analysis demonstrated that the expression of IFN-γ, but not that of IL-2, IL-4 and IL-10, by activated T cells was significantly reduced with respect to pretreatment values in patients treated with ribavirin and IFN-α but not in those undergoing treatment with IFN-α alone. Similar results were obtained by quantifying cytokine production in supernatants of unfractionated, activated PBMC (Fig. 1)
Table 2.
Cytokine expression by in vitro activated T cell in patients before and after treatment
| Percentage of positive cells | |||
|---|---|---|---|
| Treatment | Cytokine | Baseline | 4 months |
| Ribavirin + IFN-α | IFN-γ | 38 ± 16 | 22 ± 8 (P < 0·01) |
| IL-2 | 50 ± 17 | 45 ± 13 | |
| IL-4 | 3 ± 2 | 4 ± 3 | |
| IL-10 | 2 ± 1 | 2 ± 1 | |
| IFN-α | IFN-γ | 35 ± 13 | 40 ± 10 |
| IL-2 | 49 ± 9 | 46 ± 8 | |
| IL-4 | 3 ± 2 | 3 ± 3 | |
| IL-10 | 2 ± 1 | 2 ± 2 | |
| None | IFN-γ | 24 ± 11 | n.a. |
| IL-2 | 31 ± 20 | n.a. | |
| IL-4 | 4 ± 3 | n.a. | |
| IL-10 | 2 ± 0·5 | n.a. | |
Note. Mitogen-stimulated peripheral blood mononuclear cells were stained with Cy-chromeTM-conjugated anti-CD3, for the determination of their surphace phenotype. Intracellular cytokines were detected by staining with FITC-conjugated anti-IFN-γ and anti- IL-2 and PE-conjugated anti- IL-4 and anti IL-10 monoclonal antibodies. CD3 + gated lymphocytes were analysed by FACS as bivariate dot plots. The threshold of positivity for cytokine production was determined by setting the quadrants of the dot plots according to the negative controls (less than 1% of the isotype control cells appeared positive). The percentage of positive cells was calculated by straight channel integration. A subgroup of nine patients was analysed twice, both before and after treatment, intraseries variation coefficient was < 15%. If not indicated, P Î 0·05. n.a. = not applicable. Data are means ± SD
Fig. 1.
Cytokine production by activated PBMC from patients before (□) and after 4 months of treatment (▪) with ribavirin and IFN-α or IFN-α alone. (a) IFN-γ; (b) IL-2; (c) IL-4; (d) IL-10. Cytokine production was determined by ELISA testing of supernatants from unfractionated PBMC. Bars represent S.D.
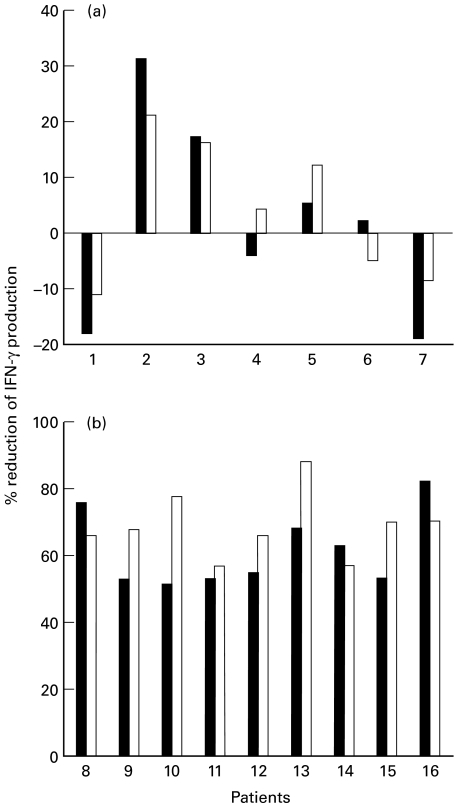
In the ribavirin plus IFN-α group, but not in the group of patients treated with IFN-α alone, both the percentage of IFN-γ producing cells and the production of IFN-γ in supernatants were significantly lower in patients that gained normal ALT levels with respect to those that did not (12 ± 6% vs. 32 ± 13% and 186 ± 44 pg/ml vs. 519 ± 112 pg/ml, P < 0·05, ribavirin plus IFN-α group, 38 ± 9% vs. 42 ± 10 and 658 ± 222 pg/ml vs. 583 ± 187 pg/ml, P > 0·05, IFN-α alone group). On a single patient basis, in 2 of 7 patients (28%) that did not gain normal ALT values IFN-γ expression increased, in 2 (28%) there was no modification and in 3 (43%) a decrease of IFN-γ expression took place, which was less than 50% the values found before treatment (Fig. 2a). As opposed, all the 9 patients with normal ALT values showed a decrease of IFN-γ expression more than 50% with respect to pretreatment values (Fig. 2b). The expression of IL-2, IL-4 and IL-10 in the ribavirin plus IFN-α group, and that of IFN-γ, IL-2, IL-4 and IL-10 in the IFN-α group was not different whether the patients gained or not normal ALT levels (data not shown).
Fig. 2.
Decrease of IFN-γ expression studied on a single patient basis after 4 months of treatment with ribavirin and IFN-α with respect to pretreatment values. (a) Patients that did not gain normal ALT values after 4 months of treatment. (b) Patients with normal ALT values after 4 month of treatment. The percentage of IFN-γ-producing cells was determined by FACS (▪) as described in the legend of Table 2. IFN-γ production by activated PBMC was determined by ELISA (□) testing of supernatants from unfractionated PBMC. The percenc reduction of IFN-γ production was calculated as follows: 100-(baseline percentage IFN-γ expression/4 months percentage IFN-γ expression).
No significant difference was found among patients with either high or low viremia or different HCV genotypes with respect to IFN-γ expression either before of after treatment, in either groups of patients (Table 3 and data not shown).
Table 3.
IFN-γ expression by in vitro activated T cell with respect to viral load, before and after treatment
| Percentage of positive cells | ||||
|---|---|---|---|---|
| Before treatment | After treatment | |||
| HCV-RNA (copies × 103/ml) | Ribavirin + IFN-α | IFN-α | Ribavirin + IFN-α | IFN-α |
| < 0·2 | n.a. (n = 0) | n.a. (n = 0) | 24 ± 6 (n = 3) | n.a. (n = 0) |
| 0·2–100 | 36 ± 11 (n = 2) | 33 ± 8 (n = 1) | 19 ± 9 (n = 7) | 40 ± 16 (n = 3) |
| 100–500 | 39 ± 14 (n = 6) | 40 ± 15 (n = 4) | 22 ± 11 (n = 4) | 42 ± 7 (n = 3) |
| > 500 | 39 ± 9 (n = 8) | 31 ± 13 (n = 4) | 23 ± 5 (n = 2) | 44 ± 11 (n = 3) |
Note. Mitogen-stimulated peripheral blood mononuclear cells were stained and analysed by FACS as described in Table 2. n.a., not applicable. Data are means ± SD
Cytokine levels in peripheral blood
We finally analysed the levels of circulating IFN-γ, IL-2, IL-4 and IL-10. As shown in Table 4, the blood levels of IFN-γ were significantly downregulated in patients at the end of treatment with ribavirin and IFN-α as compared with pretreatment values. Again, no modification of IFN-γ expression was documented in patients treated with IFN-α alone. In addition, no difference was found in the values of IL-2, IL-4 and IL-10 between the two groups.
Table 4.
levels of circulating cytokines in patients before and after treatment
| Mean cytokine levels (pg/ml) ± SD | |||
|---|---|---|---|
| Treatment | Cytokine | Before treatment | After treatment |
| Ribavirin + IFN-α | IFN-γ | 13·7 ± 25·4 | < 7 (P < 0·05) |
| IL-2 | 8·3 ± 18·4 | 7·6 ± 22·2 | |
| IL-4 | < 4·1 | < 4·1 | |
| IL-10 | 32·1 ± 53·5 | 27·3 ± 41·9 | |
| IFN-α | IFN-γ | 15 ± 23·5 | 12·1 ± 30·7 |
| IL-2 | 9·4 ± 19·8 | 16·6 ± 28·1 | |
| IL-4 | < 4·1 | < 4·1 | |
| IL-10 | 32·9 ± 41·7 | 37·3 ± 52·5 | |
If not indicated, P ≥ 0·05
DISCUSSION
We show here that treatment of patients with chronic hepatitis C with ribavirin and IFN-α markedly decreases the expression of IFN-γ. This reduction is not secondary to the effect of antiviral treatment on HCV replication, since no correlation was found between plasma HCV-RNA levels and IFN-γ-values, and is probably related to ribavirin administration, as no modification of IFN-γ expression was seen in patients treated with IFN-α alone. In addition, no modulation of IL-2, IL-4 and IL-10 expression was found either in patients treated with ribavirin and IFN-α or in those treated with IFN-α alone.
Recent studies in vitro and in murine models have shown that ribavirin may prime human T cell for an increased activation-induced production of cytokines, such as IFN-γ and IL-2, which play a key role in antiviral responses [12–14, 18, 19]. On the basis of these findings it has been suggested that ribavirin may potentiate IFN-α activity against HCV by immune-mediated mechanisms. The findings we presented here do not seem to support this possibility. As opposed, it is possible that reduction of IFN-γ production in patients treated with ribavirin and IFN-α, even in the absence of any modification of other cytokines that inhibit immune cell functions such as IL-4 and IL-10 [20–22], might impair the ability of the host immune system to mount an efficient anti HCV response. Consistently, it has been recently observed that HCV NS3-specific T cells do not proliferate during treatment of chronic HCV infection with ribavirin in combination with IFN-α and proliferated after treatment stopped [23]. The capacity of ribavirin to potentiate the anti-HCV effect of IFN-α is better explained by its ability to increase up to 6·1 fold the intracellular levels of the bioactive enzyme 2',5′-oligoadenylate (2–5 A) synthetase, which mediates most of the antiviral effects of IFN-α, with respect to the levels obtained by IFN-α alone [14].
In humans, the differentiation of uncommitted T cell precursors is regulated by a number of factors including expression of costimulatory molecules on antigen presenting cells, the type and the dose of the antigen and the cytokine milieu in which differentiation takes place [20, 24, 25]. These complex mechanisms of T cell differentiation are similar, but not identical, in animals [20], moreover, they are completely lacking in in vitro systems. Therefore, it is conceivable that, despite its abilty to up-modulate IFN-γ and IL-2 production by T cells in in vitro systems or in animal models, ribavirin may differently affect T cell cytokine profiles in humans.
Several lines of evidence indicate that in chronic hepatitis C patients, liver injury occurs as a consequence of IFN-γ-driven, cell-mediated immune response [15, 16]. Consistently, it has been demonstrated that intrahepatic expression of IFN-γ correlates with serum ALT levels [15]. As we have just demonstrated, the association of ribavirin plus IFN-α is able to down-modulate IFN-γ expression in patients with chronic hepatitis C. More important, the levels of IFN-γ production strictly correlated with ALT values. This suggests that ribavirin may actually exert an anti-inflammatory effect and may help reducing IFN-γ-driven T cell activation and liver damage, resulting in improved liver histology even in the absence of complete control of HCV replication. In line with this, a number of studies have found that treatment with ribavirin and IFN-α of chronic hepatitis C patients reduces ALT levels even in patients in which no virological response can be documented [8–11].
In conclusion, the results of this study do not support up-modulation of IFN-γ and IL-2 production as the mechanism by which ribavirin potentiates IFN-α anti HCV activity. In addition, our findings suggest that ribavirin may exert an anti-inflammatory effect and may help reducing IFN-γ-driven T cell activation and liver damage.
Acknowledgments
This study was supported by grants of the Italian Ministry of the ‘University and Scientific Research’.
REFERENCES
- 1.Hosoya M, Shigheta S, Ishii T, et al. Comparative inhibitory effects of various nucleoside and nonnucleoside analogues on replication of influenza virus types A and B in vitro and in vivo. J Infect Dis. 1993;168:641–6. doi: 10.1093/infdis/168.3.641. [DOI] [PubMed] [Google Scholar]
- 2.Shigeta S, Mori S, Baba M, et al. Antiviral activities of ribavirin, 5-ethynyl-1-beta-Dribofuranosyl-imidazole-4carboxamide, and 6′-(R)-6′-C-methylneoplanocin A against several ortho- and para-myxoviruses. Antimicrobial Agents Chemotherapy. 1992;36:435–9. doi: 10.1128/aac.36.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Bisceglie AM, Shindo M, Fong TL, et al. A pilot study of ribavirin therapy for chronic hepatitis C. Hepatology. 1992;16:649–54. doi: 10.1002/hep.1840160307. [DOI] [PubMed] [Google Scholar]
- 4.Dusheiko G, Main J, Thomas H, et al. Ribavirin treatment for patients with chronic hepatitis C. results of a placebo-controlled study. J Hepatol. 1996;25:591–8. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Conjeevaram HS, Fried MW, et al. Ribavirin as therapy for chronic hepatitis C. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Lau D, Conjeevaram H, et al. Prolonged therapy of chronic hepatitis C with ribavirin. J Viral Hep. 1996;3:247–52. doi: 10.1111/j.1365-2893.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, von Wagner M, Roth WK, et al. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C. J Hepatol. 1998;29:29–35. doi: 10.1016/s0168-8278(98)80175-x. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Marcelin P, Lee SS, et al. Randomised trial of Interferon-α-2b plus ribavirin for 48 weeks or for 24 weeks versus Interferon-α-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–32. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer JT, Hansen BE, Niesters HGM, et al. Early prediction of response in interferon monotherapy and in interferon-ribavirin combination therapy for chronic hepatitis C. HCV RNA at 4 weeks versus ALT. J Hepatol. 1999;30:192–8. doi: 10.1016/s0168-8278(99)80061-0. [DOI] [PubMed] [Google Scholar]
- 10.Bell H, Hellum K, Harthug S, et al. Treatment with interferon-alpha2a alone or interferon-alpha2a plus ribavirin in patients with chronic hepatitis C previously treated with interferon-alpha2a. Scand J Gastroenterol. 1999;2:194–8. doi: 10.1080/00365529950173087. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro G, Di Lorenzo G, Soldini M, et al. Interferon-α-2b and ribavirin in combination for chronic hepatitis C patients not responding to Interferon-α alone: an italian multicenter, randomized, controlled, clinical study. Am J Gastroenterol. 1998;93:2445–51. doi: 10.1111/j.1572-0241.1998.00702.x. [DOI] [PubMed] [Google Scholar]
- 12.Hultgren C, Milich DR, Weiland O, et al. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune response. J Gen Virol. 1998;79:2381–91. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 13.Tam RC, Pai B, Bard J, et al. Ribavirin polarizes human T cell responses toward a type 1 cytokine profile. J Hepatol. 1999;30:376–82. doi: 10.1016/s0168-8278(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Navas S, Quiroga JA, et al. Effects of the ribavirin-interferon α combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine. 1998;10:635–44. doi: 10.1006/cyto.1997.0333. [DOI] [PubMed] [Google Scholar]
- 15.Napoli J, Bishop A, McGuinnes PH, et al. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–65. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 16.Bertoletti A, D'Elios MM, Boni C, et al. Different cytokines profiles of intrahepatic T cells in chronic hepatitis B and hepatitis C virus infection. Gastroenterology. 1997;112:193–9. doi: 10.1016/s0016-5085(97)70235-x. [DOI] [PubMed] [Google Scholar]
- 17.Jung T, Schauer U, HeusSeries C, et al. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 18.de Maeyer E, de Maeyers-Guinard J. Interferons. In: Thomson A, editor. The cytokine handbook. London: Academic; 1991. pp. 215–39. [Google Scholar]
- 19.Male D, Champion B, Cooke A, Owen M. Advanced Immunology. London: Gower Medical; 1991. [Google Scholar]
- 20.Mosmann TR, Sad S. The expanding universe of T cell subset: Th1, Th2 and more. Immunology Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 21.Nagler A, Lanier LL, Phillips JH. The effects of IL-4 on human natural killer cells. α potent regulator of IL-2 activation and proliferation. J Immunol. 1988;141:2349–51. [PubMed] [Google Scholar]
- 22.Martinez OM, Gibbons RS, Garovoy MR, et al. IL-4 inhibits IL-2 receptor expression and IL-2-dependent proliferation of human T cells. J Immunol. 1990;144:2211–5. [PubMed] [Google Scholar]
- 23.Zhang XZ, Milich DR, Peterson DL, et al. Interferon-alpha treatment induces delayed CD4+ proliferative responses to the hepatitis C virus non-structural 3 protein regardless of the outcome of therapy. J Infect Dis. 1997;175:1294–301. doi: 10.1086/516459. [DOI] [PubMed] [Google Scholar]
- 24.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 25.Del Prete G, Maggi E, Romagnani S. Human Th1 and Th2 cells: functional properties, mechanisms of regulation, and role in diseases. Laboratory Invest. 1994;70:299–306. [PubMed] [Google Scholar]