Abstract
The aim of this study was to investigate the recognition pattern of bovine serum albumin (BSA), a major dietary protein by serum IgG and IgA antibodies. Anti-BSA IgG and IgA antibodies were measured by ELISA technique in 3 different cohorts: 578 unselected persons, 84 new-onset insulin-dependent diabetes mellitus (IDDM) patients and 103 atopic persons. In order to characterize the recognition pattern of the different BSA domains, recombinant BSA and recombinant fragments covering the 3 BSA domains were produced. BSA digestion was monitored in simulated gastric fluid experiments by means of domain specific monoclonal antibodies.
IgG and IgA antibody titres to native BSA were highest in IDDM patients. The three major BSA domains were equally well recognized by IgG antibodies of the three cohorts. Interestingly all three study groups showed a dissociation of their IgG and IgA antibody response to the first BSA domain. The ratio of IgG to IgA antibodies recognizing this domain was 93%/42% in controls, 92%/37% in IDDM patients and 80%/47% in atopic persons. In simulated gastric fluid experiments, the first BSA domain was the first to become undetectable to specific monoclonal antibodies during digestion.
In conclusion humoral IgG and IgA antibodies recognize the major BSA domains with different frequencies. The N-terminal domain of BSA, the first to be degraded during simulated gastric digestion is less well recognized by IgA antibodies. This suggests that early digestion is negatively correlated to the IgA antibody response and that the IgA response associated to the gut associated lymphoid tissue (GALT) and the systemic IgG antibody responses are independent.
Keywords: IgG, IgA, bovine serum albumin, determinants, simulated gastric fluid digestion
INTRODUCTION
The normal fate of dietary proteins is digestion. However small amounts of dietary proteins are taken up in an undigested form from the gastrointestinal tract in healthy persons. The quantity of protein absorbed intact varies widely and can account for up to 1% of the ingested amount [1]. Circulating antibodies to dietary proteins have been demonstrated in many human sera, suggesting that immunization to proteins ingested as food is of frequent and natural occurrence [2]. Antibodies to milk and egg proteins have been extensively investigated. In an early report, the antibovine serum albumin (anti-BSA) response was evaluated in the sera from 900 children and adults [3]. Antibodies to BSA were detected more frequently in children than in older age groups.
Until now the impact of digestion on the antibody response to dietary proteins has hardly been adressed. The aim of this study was to characterize the IgG and IgA antibody response to BSA, a common food antigen. Firstly we measured IgG and IgA serum antibodies to BSA in an unselected population, in insulin-dependent diabetes mellitus (IDDM) patients and in atopic patients. Secondly, recombinant BSA and BSA fragments were expressed in E. coli. As BSA comprises three homologous domains that assemble to form a heart-shaped molecule [4], recombinant BSA fragments were designed so as to conserve the structural integrity of these domains. The IgG and IgA antibody response of persons found to be positive in ELISA with native BSA was further analysed using recombinant BSA and recombinant BSA fragments. Thirdly, we tried to gain insight into the speed and chronology of molecular degradation of BSA by the digestive process in the stomach. This process determines the molecular and antigenic configuration of BSA to be seen by the gut associated lymphoid tissue (GALT) in the small bowel. For this purpose BSA was digested in vitro in a simulated gastric fluid assay and digestion was monitored by means of domain specific monoclonal antibodies.
METHODS
Study population
Serum samples obtained from 578 subjects presenting at the Hospital for blood analysis were used to define the anti-BSA antibody distribution according to age groups (mean age 40·5 years, range 3 months – 93 years). A subgroup of this population (n = 126, mean age 9·6 years, range 3 months – 20 years) was used as age matched control group for a group of IDDM patients and a group of atopic patients.
The serum samples from 84 caucasian IDDM patients (mean age 9·7 years, range 11 months – 19 years) were obtained at onset of insulin therapy. In addition 103 sera were collected from patients presenting at the clinic for allergic workup (mean age 12·9 years, range 9 months – 30 years). Atopy was defined by the presence of at least one positive specific IgE test (Pharmacia CAP‐system, Uppsala, Sweden) ≥ 3·5 kU IgE/l serum.
Molecular cloning
RNA was extracted from bovine liver by acid guanidinium thiocyanate-phenol-chloroform extraction [5]. Reverse transcription and PCR were performed as described for the cloning of the cDNA coding for cat albumin [6].
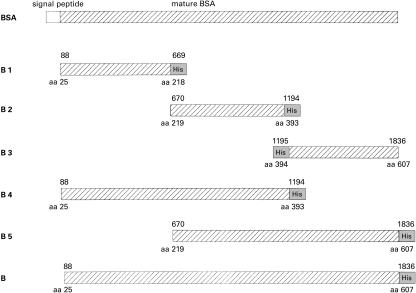
The cDNA coding for BSA was inserted into the BamHI site of the pQE-70 expression vector (Qiagen, Hilden, Germany) creating pQE-B. For expression of albumin fragments, the cDNA was divided into 3 parts and new restriction sites were introduced by PCR. Fragment 1 (nt 88–669) and fragment 2 (nt 670–1194) were cloned into the BamHI site of pQE70 generating the vectors pQalbB1 and pQalbB2. Fragment 3 (nt 1195–1836) was cloned into the pQE40 vector digested with BamHI/HindIII generating pQalbB3. Fragments 4 (nt 88–1194) and 5 (nt 670–1836) were generated to contain the sequences of fragments 1 and 2 and 2 and 3, respectively. They were inserted into the BamHI/HindIII site of pQE70.
Production of recombinant BSA and BSA fragments
pQE expression vectors containing the cDNA coding for BSA were transformed into E. coli ad 494 (Novagen, Madison, USA). Recombinant protein was purified by Ni-NTA resin according to the manufacturers protocol (Qiagen). The protein concentration was measured with Bradford protein assay reagent (BIORAD, California, USA) according to the manufacturers instructions.
Recombinant albB, albB1, albB2, albB4 and albB5 were expressed with C-terminal His-tails, albB3 was expressed with an N-terminal His-tail. Plasmid pQE-16 and pQE-30thioredoxin (kindly supplied by Dr Steinert, Qiagen, Germany) expressing the mouse dihydrofolate reductase and thioredoxin, respectively, were used as controls.
Detection of antibodies by ELISA
BSA (Sigma, St.Louis, Missouri, USA) was coated at a concentration of 5 µg/ml in 0·1 m Carbonate buffer (pH 9·6) to microtiter plates for 2 h at room temperature. Residual binding sites were saturated by incubation with blocking buffer (2% cold water fish gelatine in PBS/0·1% Tween) for 30 min at room temperature. 100 µl of sera diluted 1/40 in blocking buffer were added to each well. In parallel one positive serum was diluted serially and added to each plate to constitue an internal standard for calculation of anti-BSA IgG or IgA units (U). HRP-labelled antihuman IgG or antihuman IgA (DAKO, Glostrup, Denmark) diluted 1/1000 in blocking buffer were added for detection of human antibodies. Optical density was read at 405 nm when the lowest standard dilution reached an OD of approximately 1·8. The levels of antibodies were expressed as arbitrary units calculated from a dilution series of a standard serum pool with a high concentration of antibodies.
Recombinant BSA was coated at a concentration of 5 µg/ml, recombinant BSA fragments at 2·5 µg/ml. Dihydrofolate reductase or thioredoxin were included as negative controls in each assay to correct for antibodies reacting with bacterial proteins possibly contaminating the protein preparations. The assay procedure was the same as above.
Incubation in simulated gastric fluid
BSA was incubated in simulated gastric fluid (SGF) according to Astwood et al. (7) with some modifications. SGF contained 0·32% (w/v) pepsin (Sigma), 0·03 m NaCl at pH 1·2. 170 ng/µl protein was digested in 100 µl aliquots of 0·01x SGF (pepsin diluted 1/100). Samples were preheated to 37°C. Incubations were done in a 37°C water bath and the enzymatic reaction was stopped by addition of 75 µl of 160 mm Na2CO3 at the following times: 0, 5, 30, 45 s and 1, 3, 30 min. Laemmli SDS-PAGE sample loading buffer with 2-Mercaptoethanol was added and the samples were heated to 56°C for 45 min
SDS-PAGE and immunoblot
Digestion of BSA was evaluated by SDS-PAGE using 18% polyacrylamide minigels. Proteins were visualized by Coomasie brilliant blue colloidal staining (Pierce, Rockford, IL). For immunoblotting, gels run in parallel were not stained, but electroblotted onto PVDF membranes (Millipore, Bedford, USA). To reduce nonspecific binding, blotted membranes were incubated in blocking buffer (PBS/Tween-20 0·05%, 2% cold water fish gelatine (Sigma)). Albumin was detected with monoclonal antibody (MoAb) 7A1 or 2F2 recognizing domain B1, respectively, domain B3 and a secondary antimouse Ig antibody coupled to horseradish peroxidase. The blots were developed by addition of diaminobenzidine (Sigma).
Statistical analysis
Statistical calculations were performed by nonparametric methods as antibody levels were not normally distributed. Correlations between age and antibody levels were analysed by Spearman rank. The Mann–Whitney U-test was used to compare data between populations. Comparisons between groups were done with Fisher's exact test. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, California, USA).
RESULTS
IgG and IgA antibodies to native BSA in an unselected population, in IDDM and atopic patients
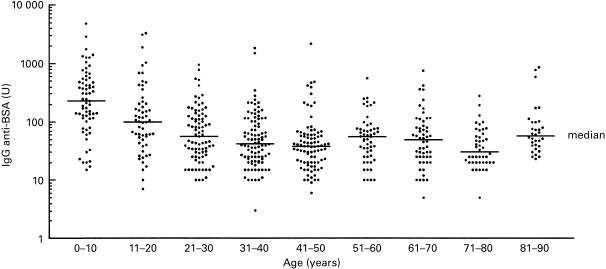
A population of 578 individuals was screened for IgG and IgA antibodies to BSA. Figure 1 shows the distribution of IgG antibodies to BSA. The highest titres were found in the age group 0–10 years, with a steady decline until 50 years. Levels of IgG and IgA antibodies are inversely correlated with age (r = − 0·303, P < 0·0001 for IgG and r = − 0·123, P = 0·0031 for IgA). A group of 84 IDDM patients as well as a group of 103 atopic patients were screened for IgG and IgA antibodies. IDDM patients had significantly higher titres of anti-BSA IgG and IgA antibodies (P = 0·0039 for IgG and P = 0·0003 for IgA) when compared to an age-matched subgroup of the control population, a finding that has already been reported by several authors [8, 9]. Atopic patients had slightly lower titres of IgG and IgA antibodies (Table 1).
Fig. 1.
Variation of anti-BSA IgG antibody titres in the population. 578 sera were screened for anti-BSA IgG antibodies by ELISA. Sera were divided into age groups of 10 years. Levels of anti-BSA antibodies were expressed as arbitrary units calculated from a standard serum pool. Horizontal bars mark median IgG titres for each group.
Table 1.
Humoral immune response to BSA
| IgG anti-BSA antibodies | IgA anti-BSA antibodies | ||||
|---|---|---|---|---|---|
| n | mean (U) | median (U) | mean (U) | median (U) | |
| Control population 0–93 years | 578 | 157·4 | 54·5 | 17·1 | 4·0 |
| control population 0–20 years | 126 | 389·9 | 146·5 | 46·8 | 5·0 |
| IDDM patients | 84 | 518·9 | 270·0 | 67·9 | 8·5 |
| atopic patients | 103 | 235·4 | 101·7 | 22·8 | 4·8 |
| P-value IDDM versus. controls 0–20 years | 0·0039 | 0·0003 | |||
| P-value atopics versus. controls 0–20 years | 0·0230 | 0·0322 | |||
Statistical analysis of IgG and IgA titres were performed with Mann–Whitney U-test, P-values are two-tailed.
IgG and IgA antibodies to recombinant BSA and BSA fragments
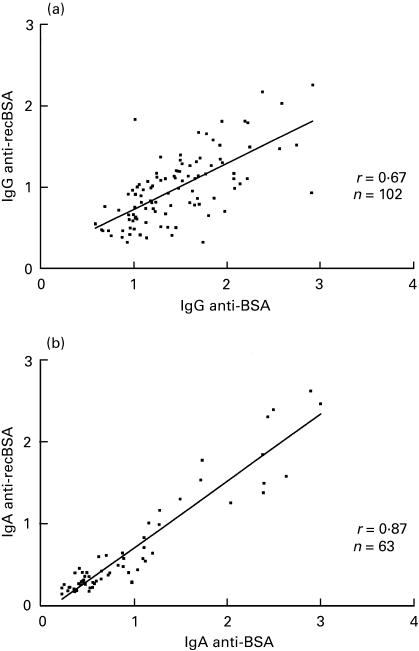
To map the BSA determinants recognized by the IgG and IgA responses the cDNA coding for BSA was divided into 3 fragments coding for 194, 175 and 214 aa, respectively (Fig. 2) and covering the 3 BSA domains. Individuals with medium and high levels of IgG and IgA antibodies to native BSA were screened with recombinant BSA and BSA fragments B1-B5. IgG and IgA antibody titres obtained with recombinant BSA in comparison to BSA are shown in Fig. 3. All sera positive for BSA reacted also with recombinant BSA, although at a lower level. Statistical analysis of the data gives a correlation coefficient of r = 0·67 for IgG antibodies and r = 0·87 for IgA antibodies.
Fig. 2.
Schematic representation of BSA gene fragments used for protein expression. Fragments B1 to B5 are located relative to the cDNA coding for BSA. Fragment B codes for the mature BSA molecule without its signal peptide. The nucleotide sequence of the cDNA has been deposited into the EMBL database under accession No Y17769. Recombinant proteins were produced in E. coli and proteins were purified by binding of the histidine tails on a Ni-chelating resin. Numbers above the boxes indicate nucleotide map positions, numbers below mark corresponding amino acid positions on the premature BSA molecule. His indicates a 5′ or 3′ nucleotide stretch coding for the hexahistidine tail.
Fig. 3.
Correlation between anti-BSA and antirecombinant BSA IgG (a) and IgA (b) reactivity in patients and controls.OD405 values obtained with 1/40 diluted sera in ELISA wells coated with BSA or recombinant BSA (recBSA) are plotted on x-axis and y-axis, respectively. A linear regression line was added to the graph.
The response to individual recombinant BSA fragments was classified as positive, if the reactivity was at least 20% of the reactivity to recombinant BSA. Table 2 shows the IgG and IgA responses to recombinant BSA and BSA fragments. Data show a dissociation between the IgG and the IgA response to fragment B1. Most sera have an IgG response to fragment B1. However only 42% of the control (0–93 years), 37% of the IDDM and 47% of the atopic sera have IgA antibodies to fragment B1. The differences are significant in the three groups.
Table 2.
IgG and IgA antibody responses to recombinant BSA and BSA fragments
| B1 (aa 25–218) | B2 (aa 219–393) | B3 (aa 394–607) | B4 (25–393) | B5 (aa 219–607) | B (aa 25–607) | |
|---|---|---|---|---|---|---|
| Controls 0–93 years | ||||||
| IgG | 43/46 (93·5%) | 38/46 (82·6%) | 43/46 (93·5%) | 44/46 (95·7%) | 46/46 (100%) | 46/46 (100%) |
| IgA | 10/24 (41·7%) | 18/24 (75%) | 17/24 (70·8%) | 21/24 (87·5%) | 22/24 (91·7%) | 24/24 (100%) |
| P-value | < 0·0001 | 0·5336 (ns) | 0·0256 | nd | nd | nd |
| Controls 0–20 years | ||||||
| IgG | 31/33 (93·9%) | 27/33 (81·8%) | 30/33 (90·9%) | 31/33 (93·9%) | 33/33 (100%) | 33/33 (100%) |
| IgA | 5/16 (31·3%) | 11/16 (68·8%) | 10/16 (62·5%) | 14/16 (87·5%) | 14/16 (87·5%) | 16/16 (100%) |
| P-value | < 0·0001 | 0·4663(ns) | 0·0432 | nd | nd | nd |
| IDDM | ||||||
| IgG | 24/26 (92·3%) | 23/26 (88·5%) | 21/26 (80·8%) | 26/26 (100%) | 26/26 (100%) | 26/26 (100%) |
| IgA | 9/24 (37·5%) | 17/24 (70·8%) | 14/24 (58·3%) | 21/24 (87·5%) | 22/24 (91·7%) | 24/24 (100%) |
| P-value | < 0·0001 | 0·1642 (ns) | 0·1239 (ns) | nd | nd | nd |
| Atopics | ||||||
| IgG | 24/30 (80%) | 21/30 (70%) | 24/30 (80%) | 27/30 (90%) | 30/30 (100%) | 30/30 (100%) |
| IgA | 7/15 (46·7%) | 7/15 (46·7%) | 8/15 (53·3%) | 11/15 (73·3%) | 11/15 (73·3%) | 15/15 (100%) |
| P-value | 0·0393 | 0·1932 (ns) | 0·0864 (ns) | nd | 0·0474 | nd |
Statistical analysis was done by Fisher's exact test. P-values are two-sided. Ns, not significant; nd, not done
Digestion of BSA in simulated gastric fluid
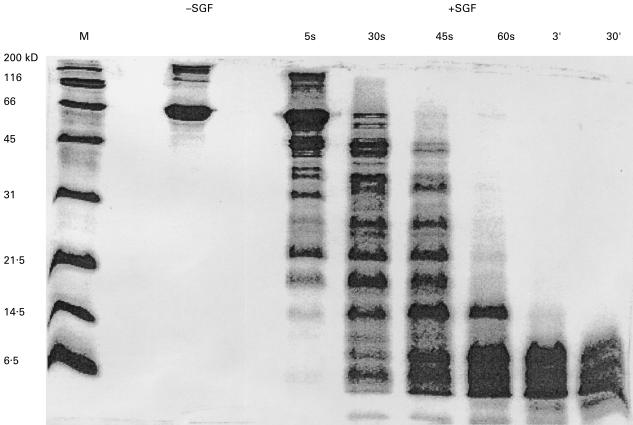
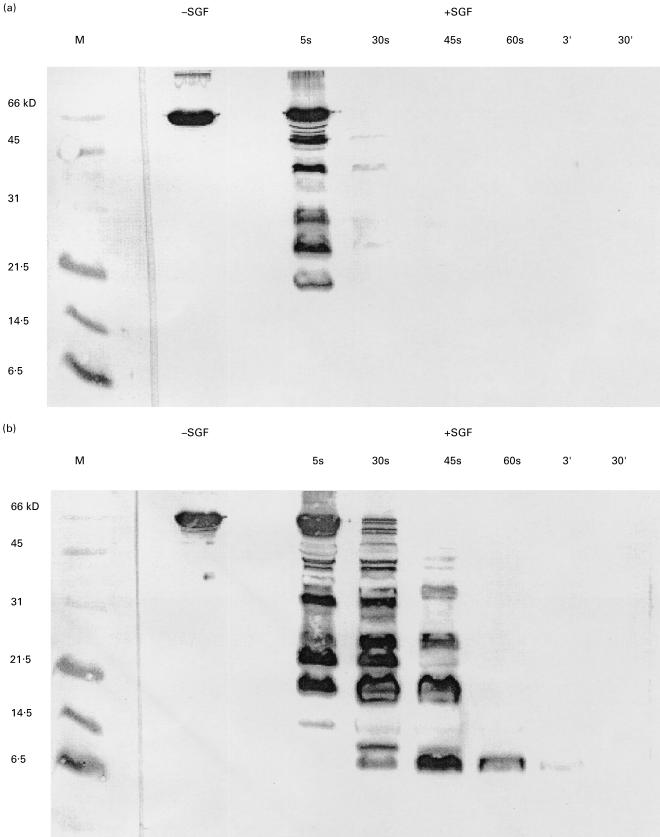
BSA is very rapidly degraded in SGF. At the 30 s timepoint, most of the BSA molecule has been degraded and at 60 s all high molecular weight protein bands have disappeared (Fig. 4). Two mouse monoclonal antialbumin antibodies were used to analyse the BSA digest at different time points (Fig. 5). Monoclonal antibody 7A1 is directed against an epitope in the region aa position 25–130 which is included in fragment B1. This antibody recognizes only BSA fragments generated during the first 5 s of incubation in SGF. Antibody 2F2 recognizes an epitope in fragment B3 and it is still capable of recognizing protein bands after 60 s of incubation in SGF. Under these experimental conditions the N-terminus of BSA is more rapidly degraded than the C-terminus.
Fig. 4.
Digestion of BSA in SGF. SDS-PAGE and coomassie brilliant blue staining were used to assess digestion of BSA. BSA was incubated with SGF for the times indicated above the gel. M, molecular weight marker; -SGF, 1 µg of BSA without SGF; + SGF, 10 µl of each sample corresponding to 5·5 µg of BSA were loaded per lane.
Fig. 5.
Digestion of BSA in SGF assayed by immunoblot using monoclonal antibodies. The same samples as in Fig. 4 were run in 18% SDS-PAGE gels and blotted onto PVDF membranes. BSA was incubated with SGF for the times indicated above the gel. Membrane (a) was incubated with monoclonal antibody 7A1, membrane (b) with monoclon al antibody 2F2.
DISCUSSION
The existence of serum IgG antibodies to dietary antigens is a common finding. The frequency and titre of these antibodies tends to decline with age. This applies to BSA as shown by Rothberg and Farr [3], a finding which we could confirm in our study. BSA is an antigen which is often introduced very early in infancy when babies are fed cow's milk and dairy products. The introduction of beef in the menu will further increase the consumption of BSA. The decline in antibody titre with age has been related to tolerance induction and to a decrease in gut permeability [10, 11], but could also be related to a better enzymatic digestion of dietary proteins.
The IgG and IgA antibody binding capacity of recombinant BSA correlates well with the antibody binding capacity of native BSA, irrespective of the probable loss of some conformational epitopes on recombinant BSA. This allows a confident albeit careful interpretation of the reactivity pattern of patient and control sera with recombinant BSA fragments. The IgG antibodies of the control population and the IDDM patients recognize more or less equally well the three domains B1, B2, B3. This holds also true for atopic persons, although the overall recognition frequency is somewhat lower. In contrast IgA antibodies recognize the different domains less frequently. This is most significant for fragment B1 recognition by IgA antibodies of the 3 cohorts studied. One explanation could be that the BSA molecule is presented in a different form to the B cells giving rise to the IgA isotype than to the B cells leading to the IgG isotype. One could also speculate that the IgA and IgG antibody response pattern to the B1 domain differ due to some regulatory suppressive mechanism acting on the IgA production pathway only. IgG are systemic antibodies originating in the spleen and the lymphnodes while IgA originate in mucosal tissues. A major part of IgA is produced in the GALT. Antibody producing plasmacytes secrete antibodies which carry the recognition motive with which the precursor B cells first recognized the BSA molecule. The affinity of this recognition motive will be later refined by somatic mutation. According to this fundamental notion, one can assume that IgG antibodies which recognize in their variety all 3 domains of BSA equally well are secreted upon contact with intact BSA molecules which have escaped digestion by gaining the general circulation possibly already in the upper digestive tract. However as IgA antibodies recognizing the B1 domain are mainly lacking, one can speculate that the gut B lymphocytes which give rise to IgA secreting plasmacytes encounter BSA from which the B1 domain has been eliminated by digestion.
It has been shown that the speed with which dietary proteins are digested varies greatly [7]. This might have consequences for their immunogenic capacity. In a simulated gastric fluid assay BSA is completely digested within minutes, whereas β-lactoglobulin, another milk protein, remains almost completely intact after 60 min [7]. In our digestion assay, the B1 domain becomes undetectable after 30 s, suggesting that this part of the molecule is already completely digested at that time. The B3 domain of BSA can still be recognized at one minute, suggesting that the part carrying the C-terminus is still not completely degraded at that time. If these in vitro observations have some relevance to the in vivo digestive process, this could be an explanation for the lower frequency of IgA antibodies recognizing the B1 domain. Whether these observations made for BSA hold true for other food antigens remains to be proven. Indeed BSA is somehow special in the sense that first it is very rapidly digested by gastric fluid possibly explaining the incomplete recognition pattern by IgA, in opposition to a systemic IgG response which recognizes equally well the three BSA domains on an intact molecule. Further studies of the IgG and IgA response to recombinant domains of rapidly digested dietary proteins in comparison to that of slowly or hardly digested dietary antigens are necessary.
Besides the digestive hypothesis the lower frequency of IgA antibodies against the B1 domain could also be explained by regulatory mechanisms. Tolerance induction to ingested antigens is a common finding and has been experimentally induced [12]. IgG and IgA antibodies are produced against molecules that have apart from a series of B cell epitopes one or possibly several T cell epitopes. This applies to intact native molecules, but also applies to moieties which have been split off by digestion. If the T cell epitope is tolerogenic, cellular immune responses and humoral responses to the protein are blocked. The IgA antibody reponse to a split off B1 domain could be turned off if this domain harboured one or several T cell epitopes tolerogenic in the IgA pathway, but not in the IgG pathway. Of course both hypotheses are not mutually exclusive.
Dissociation of antibody response has been described for other isotypes. IgG and IgE antibody to full length or overlapping recombinant mite allergens have been analysed previously. In these studies IgG antibody response was found to be overall broader than IgE antibody response which sometimes failed to recognize some parts of the allergen moieties as for instance the N-terminus of the house dust mite allergen Der p 2 [13]. Recently dissociation of allergen-specific IgE and IgA responses in sera and tears of pollen allergic patients has been described [14]. In that study the authors showed a dissociated IgE and IgA response against natural and whole-length recombinant pollen allergens but did not use fragments.
In conclusion in this study we have analysed the humoral immune response to BSA, a dietary molecule in a unselected population, in IDDM patients and atopic patients. The results show that the domain recognition pattern for BSA differs between IgG and IgA antibodies. This suggests a dual and independent digestive and systemic immune response to a common food antigen.
Acknowledgments
This work was supported by grants from the CRP-Santé (94/05) and the Fondation Recherche Cancer et Sang (94/04) in Luxembourg.
REFERENCES
- 1.Strobel SM, Mowat A. Immune response to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–80. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 2.Husby S. Normal immune responses to ingested foods. J Pediatr Gastroenterol Nutr. 2000;30:S13–9. doi: 10.1097/00005176-200001001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Rothberg RM, Farr RS. Anti-bovine serum albumin and anti-alpha lactalbumin in the serum of children and adults. Pediatrics. 1965;35:571–88. [PubMed] [Google Scholar]
- 4.He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–15. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Hilger C, Grigioni F, Hentges F. Sequence of the gene encoding cat (Felis domesticus) serum albumin. Gene. 1996;169:295–6. doi: 10.1016/0378-1119(95)00851-9. [DOI] [PubMed] [Google Scholar]
- 7.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nature Biotechnol. 1996;14:1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 8.Karjalainen J, Martin JM, Knip M, Ilonen J, Robinson BH, Savilahti E, Akerblom HK, Dosch HM. A bovine albumin peptide as a possible trigger of insulin-dependent diabetes mellitus. N Eng J Med. 1992;327:302–7. doi: 10.1056/NEJM199207303270502. [DOI] [PubMed] [Google Scholar]
- 9.Saukkonen T, Savilahti E, Varaala O, Virtala T, Tuomilehto J, Akerblom HK. Children with newly diagnosed IDDM have increased levels of antibodies to bovine serum albumin but not to ovalbumin. Diabetes Care. 1994;17:970–6. doi: 10.2337/diacare.17.9.970. [DOI] [PubMed] [Google Scholar]
- 10.Scott H, Rognum TO, Midtvedt T, Brandtzaeg P. Age-related changes of human serum antibodies to dietary and colonic bacterial antigens measured by an enzyme-linked immunosorbent assay. Acta path. Microbiol Immunol Scand. 1985;93:65–70. doi: 10.1111/j.1699-0463.1985.tb02924.x. [DOI] [PubMed] [Google Scholar]
- 11.Vaarala O, Saukkonen T, Savilahti E, Klemola T, Akerblom HK. Development of immune response to cow's milk proteins in infants receiving cow's milk or hydrolyzed formula. J Allergy Clin Immunol. 1995;96:917–23. doi: 10.1016/s0091-6749(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald TT. Immunosuppression caused by antigen feeding. II. Suppressor T cells mask Peyer's patch B cell priming to orally administered antigen. Eur J Immunol. 1983;13:138–42. doi: 10.1002/eji.1830130209. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi I, Sakiyama Y, Tame A, Kobayashi K, Matsumoto S. IgE and IgG4 antibodies from patients with mite allergy recognize different epitopes of Dermatophagoides pteronyssinus group II antigen (Der p 2) J Allergy Clin Immunol. 1996;97:638–45. doi: 10.1016/s0091-6749(96)70309-3. [DOI] [PubMed] [Google Scholar]
- 14.Aghayan-Ugurluoglu R, Ball T, Vrtala S, Schweiger C, Kraft D, Valenta R. Dissociation of allergen-specific IgE and IgA responses in sera and tears of pollen-allergic patients: a study performed with purified recombinant pollen allergens. J Allergy Clin Immunol. 2000;105:803–13. doi: 10.1067/mai.2000.104782. [DOI] [PubMed] [Google Scholar]