Abstract
Substance P is located in cutaneous nerve fibres and induces wheal and flare responses, accompanied by granulocyte infiltration, upon intradermal injection. Studies with animal skin and rat peritoneal mast cells have suggested that substance P induces the release of histamine and leukotriene B4 (LTB4), a potent chemoattractant for granulocytes, from skin mast cells. However, the release of LTB4 has not been detected from mast cells enzymatically isolated from human skin. In order to investigate the mechanism of granulocyte infiltration induced by substance P in human skin, we studied the release of LTB4 and histamine in response to substance P, and the effect of dexamethasone using human skin obtained from 22 nonallergic individuals. Histamine was released from all skin tissue samples in a dose-dependent manner. However, the amount of LTB4 release, both constitutive and inducible, was variable among skin preparations. Substance P induced a large release of LTB4 from the skin of eight donors (twice to six times that of the spontaneous release), but no or only negligible release from the skin of 14 donors. The amount of constitutive release of LTB4 correlated with the amount of tissue histamine. Dexamethasone selectively abolished the inducible release of LTB4, without an effect on histamine release and the constitutive release of LTB4. These results suggest that substance P induces the release of LTB4 in a certain population of human individuals by a glucocorticosteroid-dependent mechanism, and plays an important role in neurogenic inflammation with granulocyte infiltration.
Keywords: substance P, leukotriene B4, histamine, dexamethasone, human skin
INTRODUCTION
Substance P (SP), which is located in cutaneous sensory neurones [1], is thought to be a major mediator of neurogenic inflammation [2]. It induces the degranulation of mast cells isolated from the peritoneal cavity of rats [3] and human skin [4], releasing chemical mediators such as histamine. Intradermal injection of SP induces an immediate wheal and flare response [5–7] and granulocyte infiltration in both animal [8–10] and human skin [11]. Prior application of local anaesthetics, which prevents mast cell activation [7], and systemic administration of antihistamines reduce wheal and flare responses [5–7]. It is therefore well accepted that histamine released from mast cells mediates or enhances these reactions [12].
However, the exact mechanism of granulocyte infiltration induced by SP has yet to be determined. Using mast cell deficient mice, Matsuda et al. [8] and Yano et al. [9] have demonstrated that this reaction is dependent on mast cells. Furthermore, it has been reported that this reaction is inhibited by antagonists of leukotriene (LT) B4 [13], inhibitors of 5-lipoxygenase [10], and those of LT synthetase [14]. These studies suggest that the granulocyte infiltration induced by SP is critically mediated by mast cell-derived LTB4, a potent chemoattractant for neutrophils and eosinophils. In contrast, Robinson et al. [16] have reported that LTB4 is not released from mast cells isolated from human skin in response to anti-IgE antibody or calcium ionophore A23187. Moreover, Benyon et al. [17] have reported that the amounts of prostaglandin (PD) D2 and LTB4 released from mast cells in response to SP were 12-to 21-fold less than those released in response to anti-IgE antibody. A possible explanation for this discrepancy is mast cell heterogeneity among species or human individuals. In addition, in the studies reported by Robinson et al. [16] and Benyon et al. [17] mast cells were isolated by enzymatic digestion and stimulated in suspension. It is therefore possible that the function of mast cells might have changed during preparation under enzymatic digestion. In fact, we have recently demonstrated that house dust mite antigen induces the release of LTB4 from the surface of the skin of patients with atopic dermatitis and isolated skin tissues sensitized with IgE against the antigen [18].
In this study, we employed human skin slices of 22 individuals, rather than animal skin or dispersed human mast cells to investigate the release of LTB4 and histamine from human skin in response to SP. We demonstrate that there is a large variability of skin reactivity regarding the release of LTB4, and that dexamethasone selectively inhibits inducible release of LTB4.
MATERIALS AND METHODS
Chemicals
Substance P (SP) was purchased from Sigma Aldrich Co. (Tokyo, Japan). House dust mite antigen was obtained from Torii Co. (Tokyo, Japan). All other chemicals used in the present study were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Skin donors
Normal skin tissue was obtained from 22 individuals who were 15–85-years-old (13 men and 9 women) without allergic diseases, when they received skin surgery. The protocol of this study was approved by the ethics committee of Hiroshima University School of Medicine. Informed consent was obtained from all patients.
Serum for sensitization of human skin in vitro
Serum for passive sensitization of human skin was obtained from a patient with atopic dermatitis. The level of serum specific IgE antibody against house dust mite antigen (Der f-1) measured by the CAP-RAST method [18] exceeded 100 UA/ml.
Release of histamine and LTB4
Human skin slices were prepared as described before [18]. Briefly, after removing subcutaneous tissue, a piece of human skin was cut into 500 µm thick slices, using a hand microtome. The slices were washed three times in free Tyrode solution [18] and passively sensitized for 120 min with 20% human serum with high titre of IgE antibody against house dust mite antigen in RPMI-1640. After sensitization and washing three times in free Tyrode solution, 80 mg of the slices (wet weight) were divided into each tube and incubated in 300 µl of Tyrode solution in the presence or absence of various concentrations of SP or 50 µg/ml house dust mite antigen at 37°C for 20 min. The reaction was terminated by cooling the tubes in ice. After centrifugation at 1500 ×g for 5 min, LTB4 was extracted from supernatants as previously described [19]. Briefly, the supernatant was acidified by the addition of an equal volume of 0·1 m sodium acetate buffer (pH 3·5), lipids were extracted with two volumes of ethyl acetate. The ethyl acetate phases were evaporated by centrifugal concentrator, reconstituted with absolute ethanol and stored at −80°C until use.
Measurement of histamine
Histamine in the supernatants of the reaction mixtures and that in residual tissues was extracted with 2% perchloric acid, and the amounts of histamine in the samples were assayed using an automated fluorometric-HPLC system (Tosoh Corporation, Tokyo Japan) as previously described [20]. The magnitude of the histamine release was expressed as a percentage of the total histamine content of the skin slices in each tube.
Measurement of LTB4
The amount of LTB4 in the samples was measured using enzyme-immunoassay kits (Amersham International, Buckinghamshire, U.K.). The measurements were performed according to the manufacturer's instructions. The minimum detectable concentration of LTB4 was 6·0 pg/ml. Cross-reactivities for other related substances were less than 0·03%. The range of the assay that we employed in this study was from 10 pg/ml to 500 pg/ml.
High performance liquid chromatography
In order to verify the measurement of LTB4 by enzyme-immunoassay, the sera of three patients and the supernatants of the skin reaction mixture in 5 representative experiments were fractionated by reverse-phase HPLC. Separations were achieved as described before [18]. The amount of LTB4 in each fraction was measured as described above.
Pretreatment of skin tissues with dexamethasone
The sensitized skin slices were divided into each tube, incubated in αMEM with or without 1 µm dexamethasone at 37°C for 16 h, and challenged with 100 µm SP or 50 µg/ml house dust mite antigen as described above. The release of histamine and LTB4 was measured as described above.
Statistical analyses
Data were analysed by the Student's t-test for paired samples.
RESULTS
Release of histamine from human skin by SP
SP induced the release of histamine from sensitized human skin tissue in a dose-dependent manner over concentrations from 4 to 100 µm (Fig. 1a). The magnitude of histamine release induced by 100 µm SP, 23·6 ± 14·4% (mean ±SD, n = 21) was slightly smaller than that by antigen, 25·4 ± 13·8% (mean ±SD, n = 13), but the difference was not statistically significant.
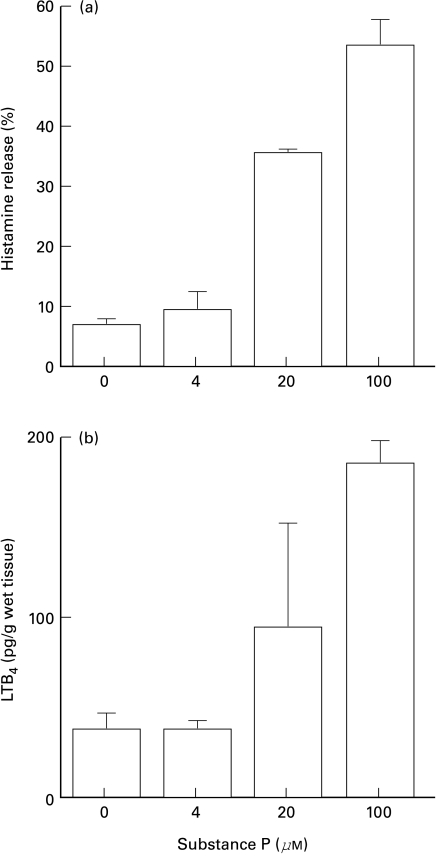
Fig. 1.
The release of (a) histamine and (b) LTB4 from human skin tissue in response to SP. Human skin slices were passively sensitized and incubated with various concentrations of SP at 37°C for 20 min, as described in the materials and methods section. The amounts of histamine and LTB4 released in supernatants were measured by HPLC (histamine) or enzyme immunoassay (EIA) system (LTB4) and expressed as percentage release of total histamine or picograms per gram wet tissue in each samples. Values are mean ±SD from one representative set of experiments with triplicate samples. Similar results of histamine release were observed in all independent experiments with skin of 21 different donors. The release of LTB4 in a similar dose-dependent manner was observed in eight out of 22 independent experiments, with the variation of absolute amounts of LTB4, as shown in Fig. 3(a).
Release of LTB4 from human skin by SP
SP also caused the release of various amounts of LTB4 from human skin tissue in a dose-dependent manner, at concentrations from 4 to 100 µm (Fig. 1b).
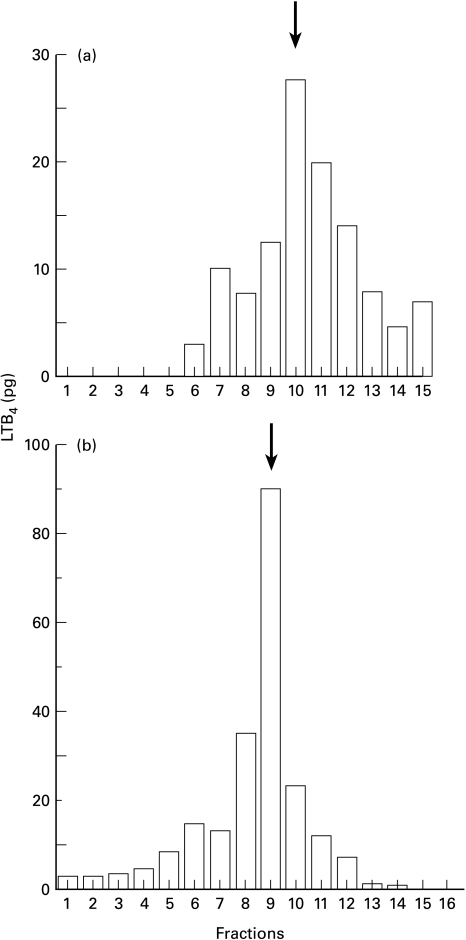
In order to ensure that the LTB4-like immunoreactivity detected by enzyme-immunoassay was LTB4 itself, we combined the supernatants of the reaction mixtures in five experiments, where large amounts of LTB4-like immunoreactivity were detected. We then fractionated them by reverse-phase HPLC. Sera of three patients that showed high levels of LTB4-like immunoreactivities were also fractionated individually. As shown in Fig. 2a,b, LTB4-like immunoreactivity was detected with the main peak in the tenth and ninth fraction, respectively, in which authentic LTB4 was eluted under the same conditions.
Fig. 2.
Detection of LTB4-like immunoreactivity in the fractions of HPLC. a, The supernatants of the reaction mixture in 5 representative experiments were combined and fractionated by reverse-phase HPLC. b, Sera of three patients that showed high levels of LTB4-like immunoreactivities were also fractionated individually. The data shows a representative result of one serum sample. The amount of LTB4 in each fraction was measured by EIA system as described in the materials and methods section. LTB4-like immunoreactivity was detected with the main peak in the tenth (a) and ninth (b) fraction, in which authentic LTB4 (shown by arrow) was eluted under the same conditions.
Variability of LTB4 release among skin tissues
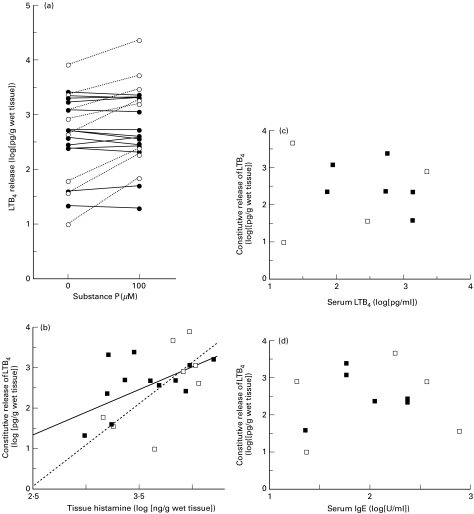
The amounts of both constitutive and induced release of LTB4 varied widely among the skin samples of the 22 donors (Fig. 3a). When converted to log values, the amount of spontaneous release ranged from 1·0 to 3·9 and appeared to be scattered with a normal distribution (2·6 ± 0·16, mean ±SEM). On the other hand, the amounts of induced release of LTB4 appeared to be separated into two clusters: inducible and noninducible. In skin obtained from 8 donors (responders), an increase in LTB4 release of not less than 200% above the constitutive level was induced in response to 100 µm SP, while an increase of less than 150% was induced in skin obtained from 14 donors (nonresponders). Figure 3b shows the relationship between the amounts of histamine contained in tissues and the spontaneous release of LTB4. There was a statistically significant correlation, as analysed by assessing either all donors or responders only. No significant correlation was observed between spontaneous release of LTB4, serum concentrations of LTB4 and total IgE levels in sera of the skin donors (Fig. 3c,d).
Fig. 3.
The variability in LTB4 release among skin tissues and the relation to the amount of tissue histamine, serum LTB4 and total IgE levels of skin donors. The amounts of both constitutive and induced release of LTB4 varied widely among the skin samples of 22 donors (a). The results of responders and nonresponders, defined in the results section are depicted by open and filled circles (a) or squares (b, c, d). The relationship between the amounts of histamine contained in tissues and the constitutive release of LTB4 is shown in b. The correlation coefficient for all samples was 0·54 (P < 0·01) and is depicted by a solid line; that for responders was 0·67 (P < 0·025) and is depicted by a dotted line. No significant correlation was observed among spontaneous release of LTB4, serum concentration of LTB4, and total IgE levels in sera of the skin donors (c, d)
Dexamethasone inhibits inducible release of LTB4, but not constitutive release of LTB4 or the release of histamine from human skin
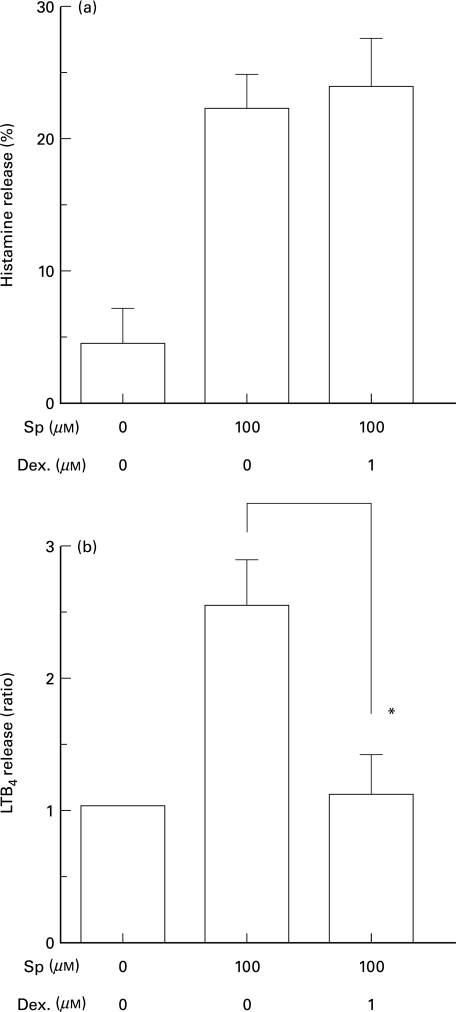
In order to study the mechanism of LTB4 release induced by SP, we studied the effect of dexamethasone on the release of histamine and LTB4 from skin tissue. Pretreatment of skin tissue with 1 µm dexamethasone at 37°C for 16 h significantly (P < 0·05, n = 3) inhibited SP-induced release of LTB4 (Fig. 4b). The release of LTB4 induced by antigen was also abolished by 1 µm dexamethasone (data not shown). However, the constitutive release of LTB4 of both responders (Fig. 4b) and nonresponders (n = 8, data not shown) was not affected, even when it was much larger than the SP-induced release of LTB4 in other skin tissue. The histamine release from skin tissue induced either by 100 µm SP (Fig. 4a) or house dust mite-antigen was not affected (data not shown).
Fig. 4.
The effects of dexamethasone on the release of histamine and LTB4 induced by SP. The human skin slices were treated with or without 1 µm dexamethasone for 16 h, followed by incubation in the presence or absence of 100 µm SP for 20 min. The amounts of histamine (a) and LTB4 (b) released into the medium were measured as described in the materials and methods section and expressed as percent release of total histamine and ratio to spontaneous release of LTB4 in each sample (1166·8, 2328·1, 7852·4 pg/g wet tissue), respectively. Data are expressed as means ±SEM of three independent experiments. *P < 0·05 (Student's t-test)
DISCUSSION
In this study, we have demonstrated that SP may induce release of LTB4 from human skin. The amount of inducible release of LTB4 by SP varied widely among skin donors and appeared to be distributed in two clusters. We tentatively defined donors whose skin released more than twice the constitutive release in response to SP as responders, and the others as nonresponders. According to such categorization, 8 out of 22 skin donors (36·4%) were classified as responders and 14 (63·6%) were nonresponders.
LTB4 is synthesized from arachidonic acid via the 5-lipoxygenase-LTA4 pathway, and has the ability to induce the aggregation, chemokinesis, and chemotaxis of granulocytes and their adhesion to endothelial cells at low concentrations [15,21]. It also has the potential to activate granulocytes to release lysosomal enzymes [25] and generate superoxide anions [27] and nitric oxide [28]. In 1997, Yokomizo et al. [25] reported the cloning of cDNA for the human LTB4 receptor in the HL-60 leukaemia cell-line. This was followed by the cloning of the mouse LTB4 receptor by Huang et al. [26]. The quantitative study of mRNA for the LTB4 receptor revealed that it was up regulated in IL-5-transgenic mice, suggesting the involvement of LTB4 in the accumulation of eosinophils by IL-5 [26]. Recently, Morita et al. [27] reported that an antagonist against the LTB4 receptor inhibited the proliferation and cytokine production of T cells, including IL-2, interferon-γ, and IL-4, suggesting that LTB4 was intrinsically involved in T cell activation by various stimuli. Other studies with LTB4 receptor antagonists have suggested that LTB4 is involved in a wide range of immune responses and host defense reactions, such as bronchial asthma [28], nephrotoxic serum nephritis [29] and allograft rejection [30]. More recently, Byrum et al. have demonstrated, using mice deficient of leukotriene A4 hydrolase, the enzyme which generates LTB4 from LTA4, that systemic shock induced by platelet-activating factor is crucially mediated by LTB4 [31].
In the skin, an increase in LTB4 release has been reported in psoriasis [32], allergic dermatitis [33] and delayed pressure urticaria [34]. We have recently demonstrated the release of LTB4 and histamine in response to antigen both in vivo and in vitro [18], suggesting a role for LTB4 released from mast cells in the late phase reaction of the skin accompanied by the infiltration of granulocytes. In this study, we demonstrated that the release of LTB4 might be also induced by SP in vitro. Several authors have reported that SP itself shows chemotactic activity for neutrophils or polymorphonuclear cells in vitro. SP also induces the release of TNFα, which is critically involved in inflammatory cell infiltrations [35]. However, the release of TNFα was detected only after six hours after the addition of SP [35], whereas LTB4 was detected in 20 min. Moreover, the direct chemotactic activity of SP for granulocytes in vivo is still controversial. Firstly, the concentrations of SP necessary to demonstrate such an activity are variable among reports [36–38]. Secondly, since mast cells reside closely around nerves and/or blood vessels in the skin [2], mast cells should be exposed initially to high concentrations of SP released from nerve endings, as compared with leucocytes in blood vessels. Therefore, mast cells around nerves may actually be exposed in vivo to such high concentrations of SP as those used in this study. In fact, Suzuki et al. [39] have recently demonstrated that the activation of nerve cells induces the activation of adjacent mast cells by SP in vitro. Taken together, this suggests that LTB4 may play a role in a relatively early stage of granulocyte infiltration induced by SP. The precise role and degree of contribution of SP-induced release of LTB4 in vivo needs to be elucidated in future studies using inhibitors specific for SP and LTB4.
The source of LTB4 in this system remains to be identified. Macrophages and neutrophils are potential candidates as a source of LTB4 released by SP, but they could never migrate into the dissected skin tissues employed in our system. Moreover, antagonists against NK1 receptors, through which SP activates macrophages, do not inhibit the release of LTB4 induced by SP (data not shown). Furthermore, the amounts of spontaneous release of LTB4 significantly correlated with the amounts of histamine contained within the tissues but did not correlate with concentrations of serum LTB4 or serum IgE, suggesting the involvement of mast cells, at least in the constitutive release of LTB4. Thus, although we cannot define the source of LTB4 released in our system, there is no other possible candidate except for mast cells, as previously discussed in the study with guinea-pig skin [40]. The fact that skin obtained from 13 out of 22 (63·6%) individuals did not show an apparent increase in LTB4 release in response to SP may account for the lack of detection of LTB4 observed in the study by Robinson et al. [16].
The differences between the spontaneous release of LTB4 among the skin samples of each donors were of nearly a 1000-fold range. Such variability has also been reported in the release of LTC4 from human basophils in response to anti-IgE antibody [41] and concentrations of serum LTB4 [42]. The biological significance of such variability is not clear. Ford-Hutchinson et al. [43] showed that the degree of granulocytes chemotaxis induced by LTB4 was not necessarily correlated with the concentration of LTB4. Therefore, inducibility of LTB4 may be more important in terms of the pathogenesis of skin diseases. The heterogeneity of mast cell characteristics among species and organs has been well demonstrated [44]. Our results indicate that human mast cells are also substantially heterogeneous in terms of the release of LTB4 among individuals. Such a functional heterogeneity of mast cells may account for the dominant infiltration of neutrophils and eosinophils, occasionally observed in chronic urticaria [45], and possibly in other mast cell-mediated cutaneous diseases.
Glucocorticoids have potent anti-inflammatory activities and are used for the treatment of various diseases. However, their mechanisms of action on neurogenic inflammation, especially on human cell functions, have not been fully investigated. The release of preformed mediators, including histamine by exocytosis and the new synthesis of arachidonic acid metabolites and cytokines from rodent mast cells are all inhibited by pretreatment with dexamethasone in the range from 10 to 100 nm [46]. However, Schleimer et al. [47] have revealed that dexamethasone has no effect on the release of histamine from human lung mast cells. In this study, we have confirmed that a concentration of dexamethasone as high as 1 µm has no effect on histamine release or the constitutive release of LTB4 from human skin. On the other hand, the release of LTB4 by SP and that by antigen was abolished to basal levels by the same concentration of dexamethasone. These results suggest that inducible release of LTB4 may be regulated by a mechanism which is different from that for constitutive release of LTB4.
In conclusion, the effects of SP and dexamethasone on the release of histamine and LTB4 from human skin are heterogeneous, and further study of the mechanisms of cell activation by SP may allow us to develop a new therapy for diseases associated with neurogenic inflammation.
Acknowledgments
This work is supported in part by a Grant-in-Aid for Scientific Research (11670832 to M. H.) from the Ministry of Education, Science, and Culture, Japan.
REFERENCES
- 1.Hökfelt T, Kellerth JO, Nilsson G, et al. Substance P: Localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–90. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 2.Prenow B. Substance P. Pharmacol Rev. 1983;35:85–141. [PubMed] [Google Scholar]
- 3.Fewtrell CMS, Foreman JC, Jordan CC, et al. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benyon RC, Lowman MA, Church MK. Human skin mast cells: Their dispersion, purification, and secretory characterization. J Immunol. 1987;138:861–7. [PubMed] [Google Scholar]
- 5.Hägermark Ö, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1197;8:233–5. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- 6.Jorizzo JL, Coutts AA, Greaves MW. Vascular responses of human skin to injection of substance P and mechanism of action. Eur J Pharmacol. 1983;87:67–76. doi: 10.1016/0014-2999(83)90051-1. [DOI] [PubMed] [Google Scholar]
- 7.Foreman JC, Jordan CC, Oehme P, et al. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983;335:449–65. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda H, Kawakita K, Kiso Y, et al. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–31. [PubMed] [Google Scholar]
- 9.Yano H, Wershil BK, Arizono N, et al. Substance-P induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–86. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh DT, Weg VB, Williams TJ, et al. Substance P-induced inflammatory responses in guinea-pig skin: the effect of specific NK1 receptor antagonists and the role of endogenous mediators. Br J Pharmacol. 1995;114:1343–50. doi: 10.1111/j.1476-5381.1995.tb13354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CH, Barker JNWN, Morris RW, et al. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–82. [PubMed] [Google Scholar]
- 12.Petersen LJ, Poulsen LK, Søndergaard J, et al. The use of cutaneous microdialysis to measure substance P-induced histamine release in intact human skin in vivo. J Allergy Clin Immunol. 1994;94:773–83. doi: 10.1016/0091-6749(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto I, Tomoe S, Tomioka H, et al. Leukotriene B4 mediates substance P-induced granulocyte infiltration into mouse skin. J Immunol. 1993;151:2116–23. [PubMed] [Google Scholar]
- 14.Saban MR, Saban R, Bjorling D, et al. Involvement of leukotrienes, TNF-α and the LFA-1/ICAM−1 interaction in substance P-induced granulocyte infiltration. J Leukocyte Biol. 1997;61:445–51. doi: 10.1002/jlb.61.4.445. [DOI] [PubMed] [Google Scholar]
- 15.Ford-Hutchinson AW, Bray MA, Doig MV, et al. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–5. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 16.Robinson C, Benyon RC, Holgate ST, et al. The IgE- and calcium-dependent release of eicosanoids and histamine from human purified cutaneous mast cells. J Invest Dermatol. 1989;93:397–404. [PubMed] [Google Scholar]
- 17.Benyon RC, Robinson C, Church MK, et al. Differential release of histamine and eicosanoids from human skin mast cells activated by IgE-dependent and non-immunological stimuli. Br J Pharmacol. 1989;97:898–904. doi: 10.1111/j.1476-5381.1989.tb12030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koro O, Furutani K, Hide M, et al. Chemical mediators in atopic dermatitis: Involvement of leukotriene B4 released by a type I allergic reaction in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999;103:663–70. doi: 10.1016/s0091-6749(99)70240-x. [DOI] [PubMed] [Google Scholar]
- 19.Brain S, Camp RDR, Dowd P, et al. The release of leukotriene B4-like material in biologically active amounts from the lesional skin of patients with psoriasis. J Invest Dermatol. 1984;83:70–3. doi: 10.1111/1523-1747.ep12261712. [DOI] [PubMed] [Google Scholar]
- 20.Tsuruta Y, Kohashi K, Ohkura Y. Determination of histamine in plasma by high-speed liquid chromatography. J Chromatogr. 1978;146:490–3. doi: 10.1016/s0378-4347(00)81209-8. [DOI] [PubMed] [Google Scholar]
- 21.Gimbrone Ma, Jr, Brock AF, Schafer AI. Leukotriene B4 stimulates polymorphonuclear leukocyte adhesion to cultured vascular endothelial cells. J Clin Invest. 1984;74:1552–5. doi: 10.1172/JCI111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rae SA, Smith MJ. The stimulation of lysosomal enzyme secretion from human polymorphonuclear leukocytes by leukotriene B4. J Pharm Pharmacol. 1981;33:616–7. doi: 10.1111/j.2042-7158.1981.tb13884.x. [DOI] [PubMed] [Google Scholar]
- 23.Palmblad J, Gyllenhammar H, Lindgren JA, et al. Effects of leukotrienes and f-Met-Leu-Phe on oxidative metabolism of neutrophils and eosinophils. J Immunol. 1984;132:3041–5. [PubMed] [Google Scholar]
- 24.Larfars G, Lantoine F, Devynck MA, et al. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood. 1999;93:1399–405. [PubMed] [Google Scholar]
- 25.Yokomizo T, Izumi T, Chang K, et al. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–4. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 26.Huang WW, Gracia-Zepeda EA, Sauty A, et al. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med. 1998;188:1063–74. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita H, Takeda K, Yagita H, et al. Immunosuppressive effect of leukotriene B4 receptor antagonist in vitro. Biochem Biophys Res Commun. 1999;264:321–6. doi: 10.1006/bbrc.1999.1523. [DOI] [PubMed] [Google Scholar]
- 28.Sakurada T, Abe M, Kodani M, et al. Synergistic effects of pranlukast and leukotriene B4 receptor antagonist on antigen-induced pulmonary reaction. Eur J Pharmacol. 1999;370:153–9. doi: 10.1016/s0014-2999(99)00126-0. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Kuroda T, Kazama JI, et al. The leukotriene B4 receptor antagonist ONO-4057 inhibits nephrotoxic serum nephritis in WKY rats. J Am Soc Nephrol. 1999;10:264–70. doi: 10.1681/ASN.V102264. [DOI] [PubMed] [Google Scholar]
- 30.Weringer EJ, Perry BD, Sawyer PS, et al. Antagonizing leukotriene B4 receptors delays cardiac allograft rejection in mice. Transplantation. 1999;67:808–15. doi: 10.1097/00007890-199903270-00005. [DOI] [PubMed] [Google Scholar]
- 31.Byrum RS, Goulet JL, Snouwaert JN, et al. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol. 1999;163:6810–9. [PubMed] [Google Scholar]
- 32.Greaves MW. Neutrophil polymorphonuclears, mediators and the pathogenesis of psoriasis. Br J Dermatol. 1983;109:115–8. doi: 10.1111/j.1365-2133.1983.tb04001.x. [DOI] [PubMed] [Google Scholar]
- 33.Barr RM, Brain S, Camp RD, et al. Levels of arachidonic acid and its metabolites in the skin in human allergic and irritant contact dermatitis. Br J Dermatol. 1984;111:23–8. doi: 10.1111/j.1365-2133.1984.tb04012.x. [DOI] [PubMed] [Google Scholar]
- 34.Czarnetzki BM, Meentken J, Rosenbach T, et al. Clinical, pharmacological and Immunological aspects of delayed pressure urticaria. Br J Dermatol. 1984;111:315–23. doi: 10.1111/j.1365-2133.1984.tb04729.x. [DOI] [PubMed] [Google Scholar]
- 35.Okabe T, Hide M, Koro O, et al. Substance P induces tumor necrosis factor-α release from human skin via mitogen-activated protein kinase. Eur J Pharmacol. 2000;398:309–15. doi: 10.1016/s0014-2999(00)00304-6. [DOI] [PubMed] [Google Scholar]
- 36.Carolan EJ, Casale TB. Effects of neuropeptides on neutrophil migration through noncellular and endothelial barriers. J Allergy Clin Immunol. 1993;92:589–98. doi: 10.1016/0091-6749(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 37.Haines KA, Kolasinski SL, Cronstein BN, et al. Chemoattraction of neutrophils by substance P and transforming growth factor-β1 is inadequately explained by current models of lipid remodeling. J Immunol. 1993;151:1491–9. [PubMed] [Google Scholar]
- 38.Locatelli L, Sacerdote P, Mantegazza P, et al. Effect of ibuprofen and diclofenac on the chemotaxis induced by substance P and transforming growth factor-β on human monocytes and polymorphonuclear cells. Int J Immunopharmacol. 1993;15:833–8. doi: 10.1016/0192-0561(93)90021-p. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki R, Furuno T, McKay DM, et al. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 1999;163:2410–5. [PubMed] [Google Scholar]
- 40.Furutani K, Koro O, Hide M, et al. Substance P- and antigen-induced release of leukotriene B4, prostagrandine D2 and histamine from guinea pig skin by different mechanisms in vitro. Arch Dermatol Res. 1999;291:466–73. doi: 10.1007/s004030050439. [DOI] [PubMed] [Google Scholar]
- 41.MacGlashan Dw, Jr, Peters SP, Warner J, et al. Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol. 1986;136:2231–9. [PubMed] [Google Scholar]
- 42.Seggev JS, Wiessner JH, Thornton WH, et al. Comparison of serum and plasma leukotriene B4 levels in normal and asthmatic subjects. Ann Allergy Asthma Immunol. 1995;75:365–8. [PubMed] [Google Scholar]
- 43.Ford-Hutchinson AW, Brunet G, Savard P, et al. Leukotrien B4, polymorphonuclear leukocytes and inflammatory exudates in the rat. Prostaglandins. 1984;28:13–27. doi: 10.1016/0090-6980(84)90110-2. [DOI] [PubMed] [Google Scholar]
- 44.Lowman MA, Ress PH, Benyon RC, et al. Human mast cell heterogeneity: Histamine release from mast cells dispersed from skin, lung, adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol. 1988;81:590–7. [PubMed] [Google Scholar]
- 45.Natbony SF, Phillips ME, Elias JM, et al. Histologic studies of chronic idiopathic urticaria. J Allergy Clin Immunol. 1983;71:177–83. doi: 10.1016/0091-6749(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 46.Daëron M, Sterk AR, Hirata F, et al. Biochemical analysis of glucocorticoid-induced inhibition of IgE-mediated histamine release from mouse mast cells. J Immunol. 1982;129:1212–8. [PubMed] [Google Scholar]
- 47.Schleimer RP, Schulman ES, MacGlashan Dw, Jr, et al. Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J Clin Invest. 1983;71:1830–5. doi: 10.1172/JCI110938. [DOI] [PMC free article] [PubMed] [Google Scholar]