Abstract
Bullous pemphigoid (BP) is a subepidermal blistering disease associated with autoantibodies to the hemidesmosomal 180 kD BP autoantigen (BP180). However, the binding of autoantibodies to BP180 alone is not sufficient for blister formation in this disease and the infiltration of neutrophils into the skin is required. Dapsone and nicotinamide inhibit neutrophil chemotaxis and are used effectively in treating BP. IL-8 is a known chemoattractant for neutrophils and has been implicated in the inflammatory process of both human and experimental murine BP. We have recently shown that antibodies to BP180 mediate a dose and time-dependent release of IL-6 and IL-8 from cultured normal human epidermal keratinocytes (NHEK). In the present study, we addressed the question whether dapsone or nicotinamide influence this cytokine release. We demonstrate that dapsone, but not nicotinamide, in its pharmacological range, inhibits the IL-8, but not the IL-6 release from NHEK, induced by anti-BP180 IgG, in a dose-dependent fashion as detected by ELISA. IL-8 mRNA levels, as determined by RT-PCR, were the same in cells treated with BP IgG alone compared to cells treated with BP IgG plus dapsone. This observation suggests that dapsone inhibits the BP IgG-induced IL-8 release from cultured NHEK by mechanisms at the post-transcriptional level. Our findings contribute to the understanding how dapsone leads to a reduced influx of neutrophils into BP lesions and, finally, to the cessation of blister formation in this disease.
Keywords: autoantibody, BP180, cytokine, mRNA
INTRODUCTION
Bullous pemphigoid (BP) is a subepidermal blistering disease associated with autoantibodies to the hemidesmosomal proteins BP180 and BP230 [1]. The hemidesmosome connects the cytoskeleton of the basal keratinocyte with fibres of dermal collagen thus attaching epidermis and dermis. Intracellular proteins of the hemidesmosomal plaque, such as BP230, mediate interaction between keratin intermediate filaments and the transmembrane proteins BP180 and α6β4 integrin. Latter then interact via laminins 5 and 6 with type VII collagen that finally connects with the dermal collagens [reviewed in 2]. The binding of autoantibodies alone, however, is not sufficient for blister formation in BP. In addition, activation of complement, infiltration of neutrophil granulocytes into BP lesions, and the release of proteinases are essential for blister formation [3–7].
Dapsone (4,4′-diaminodiphenyl sulphone) has been used successfully in the treatment of subepidermal blistering autoimmune diseases associated with neutrophil infiltration, including BP [8,9]. Dapsone is thought to exhibit its anti-inflammatory effect by the suppression of various neutrophil and eosinophil functions. It leads to a reduction of neutrophil infiltration into the skin and inhibition of chemotaxis of these cells is considered as a major effect of this drug [10]. In addition, in vitro studies indicate that dapsone inhibits the activity of myeloperoxidase and other lysosomal enzymes, and the production of toxic oxygen intermediates in neutrophils [10–13]. Nicotinamide is another drug that is used in the treatment of BP, frequently in combination with tetracyclines [14–16]. Like dapsone, nicotinamide is thought to exhibit its anti-inflammatory action by interfering with neutrophil functions, including chemotaxis [15].
IL-8 is a known chemoattractant for neutrophils [17] and dapsone has been found to suppress the IL-8-mediated neutrophil chemotaxis in vitro [10]. In BP, IL-8 has been implicated to be important for the inflammatory response in both human and experimental murine BP. In blisters and sera of BP patients, abnormally high levels of IL-8 were detected [18], and in the experimental mouse model of BP, IL-8 injections facilitated blister formation in C5-or mast cell-deficient mice that were otherwise resistant to the induction of blisters [4,19]. In addition, we have recently shown that antibodies to BP180 mediate a dose-and time-dependent release of IL-6 and IL-8 from cultured normal human epidermal keratinocytes (NHEK) [20]. In the present study, we demonstrate that dapsone inhibits this release in a dose-dependent manner.
MATERIALS AND METHODS
Human and rabbit sera
Rabbit serum R594 was raised against GST-NC16A2–4 containing a 42 amino acid stretch of human BP180 NC16A [21] which represents the immunodominant region within the BP180 ectodomain [22]. Serum/IgG preparations from R594 stained the epidermal side of NaCl-split human skin by indirect immunofluorescence (IF) microscopy at a titre of 5,120/10 240 and reacted with recombinant BP180 NC16A by Western blotting at a dilution of 1:20,000/1:64 000. In addition, serum samples were obtained from a BP patient with linear deposits of IgG and C3 at the basement membrane zone (BMZ) by direct IF of perilesional skin before treatment was initiated. By indirect IF on 1 m NaCl-separated normal human skin, autoantibodies in the patient's serum/purified IgG preparations bound to the epidermal side of the artificial split with a titre of 2,560/5120. By immunoblotting of epidermal extracts [23], the patient's IgG preparation exclusively labelled BP180 but not BP230 and by Western blotting with recombinant BP180 NC16A, specific reactivity was detected at dilutions of 1:60 000. Within the NC16A domain, the patient's autoantibodies strongly bound to regions 1 and 2, and weaker to regions 2·5 and 3. No reactivity was observed with regions 4 and 5 [20]. Normal human and preimmune rabbit sera were used as controls.
Keratinocyte culture
Normal human epidermal keratinocytes (NHEK) were isolated from human neonatal foreskin and grown in tissue culture flasks (Becton Dickinson Labware, Franklin Lakes, NJ, USA) in keratinocyte growth medium (KGM; Clonetics, La Jolla, CA, USA) containing 0·15 mm Ca2+ at 37°C in a humidified atmosphere containing 5% CO2 [20]. Serum R594 and the BP patient's serum were previously shown to stain third passage NHEK with a membrane binding pattern by indirect IF [20].
Isolation of IgG
IgG was isolated from human and rabbit sera by Protein G Sepharose 4 Flow affinity column chromatography (Pharmacia AB, Uppsala, Sweden) [20]. Eluted IgG fractions were concentrated under extensive washing with PBS (pH 7·2) using an Ultrafree-15 filter device (Millipore, Bedford, MA, USA). Concentrated IgG was sterile filtered (Schleicher & Schuell, Dassel, Germany) and the final concentration determined by photometry at 280 nm and Bradford protein assay (BioRad, Hercules, CA, USA). IL-6 and IL-8 levels in IgG preparations were below the detection limit of our ELISA.
Stimulation of keratinocytes
Third passage keratinocytes were grown to 70–80% confluence in 24-well plates (Becton Dickinson Labware). Hydrocortisone was omitted 12 h prior to stimulation to exclude interference with IL-8 production. For optimal IL-8 release, NHEK were treated with 4 mg/ml IgG for 12 h as reported [20]. At the beginning of this incubation, dapsone and nicotinamide were added to the culture medium. Dapsone (Fatol, Schiffweiler, Germany) and nicotinamide (Nicobion®, Merck, Darmstadt, Germany) were solubilized in PBS by over-night rotation at room temperature [12] to stock solutions of 4000 µg/ml (dapsone) and 20 000 µg/ml (nicotinamide). Additional dilutions were prepared in KGM for both dapsone and nicotinamide. Culture supernatant or cells from three wells were pooled and then subjected to ELISA or RT PCR analysis.
Cell proliferation assay
To test for chemosensitivity of the different dapsone and nicotinamide dilutions on cultured NHEK, a cell proliferation assay was performed (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay, Promega, Madison, WI, USA). Briefly, after a 12-h treatment of NHEK, grown in 200 µl KGM in 24-well plates (Becton Dickinson Labware), with various concentrations of dapsone or nicotinamide plus 4 mg/ml normal IgG, 40 µl of a mixture of the tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and the electron coupling reagent phenazine methosulphate (PMS) was added to each well. After a second incubation time of 4 h at cell culture conditions, the formazan production in the culture supernatant was measured at 490 nm using an ELISA plate reader. To analyse cell viability, OD490 readings of the dapsone and nicotinamide containing culture supernatants were compared to those with medium alone.
Quantification of IL-8 mRNA expression by RT PCR
IL-8 mRNA levels were quantified using a previously reported RT-PCR protocol in which samples were normalized on beta-actin mRNA levels [20,24]. Quantification of IL-8 mRNA was done semiquantitatively. A low threshold cycle (Ct) corresponded to a high concentration of IL-8 mRNA and vice versa. Slopes of standard curves for various target sequences were in the range of −3·4 to −3·6, i.e. a difference in Ct of 3·4–3·6 between two samples corresponded to a 10-fold difference in cDNA concentration. Samples were assayed in triplicate.
ELISA protocol
IL-6 (Pharmingen, San Diego, CA, USA) and IL-8 (Biosource, Fleurus, Belgium) were measured by ELISA according to the manufacturer‘s instructions. Samples were run in quadruplicate.
Statistics
For statistical analysis, the Mann–Whitney U-test was used.
RESULTS
In their pharmacological range, dapsone and nicotinamide do not reduce viability of NHEK
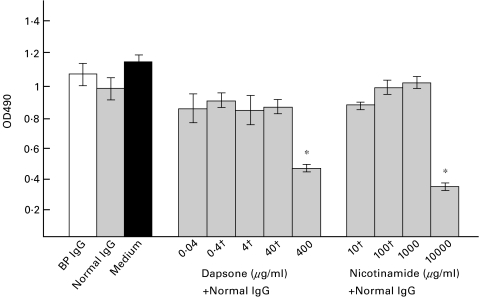
To study a possible effect of dapsone and nicotinamide on cell viability, NHEK were treated with various concentrations of the 2 drugs together with 4 mg/ml IgG, affinity-purified from a healthy control subject. No statistically significant difference in cell viability was observed between NHEK that were stimulated with dapsone concentrations ranging from 0·04 to 40 µg/ml or nicotinamide concentrations between 10 and 1000 µg/ml compared to NHEK stimulated with BP IgG, normal IgG or medium alone (Fig. 1). However, when NHEK were treated with 400 µg/ml dapsone or 10 000 µg/ml nicotinamide, cell viability was reduced by approximately 50%.
Fig. 1.
In their pharmacological range, dapsone or nicotinamide do not reduce cell viability of NHEK. NHEK were stimulated with 4 mg/ml IgG from a BP patient (BP IgG), IgG from a healthy volunteer (Normal IgG), and with medium alone (Medium). In addition, 4 mg/ml normal human IgG was incubated with various concentrations of dapsone or nicotinamide (pharmacological ranges are indicated with †) for 12 h. Cell viability was analysed by a nonradioactive cell proliferation assay. Samples were tested in duplicate, bars show mean ±SD. *statistical significant difference in cell viability compared to normal IgG-treated NHEK (P < 0·01). □ BP IgG;  Normal IgG; ▪ Medium.
Normal IgG; ▪ Medium.
Dapsone inhibits the IL-8, but not the IL-6 release from NHEK, mediated by rabbit or human IgG against human BP180, in a dose-dependent fashion
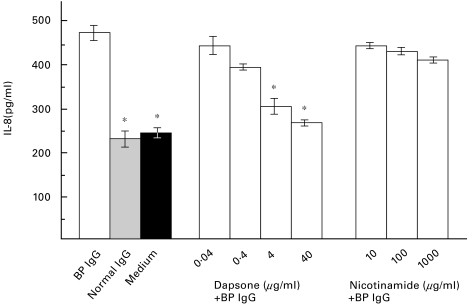
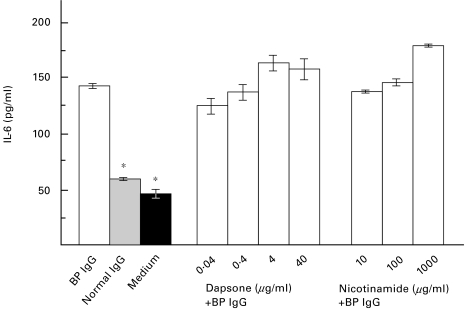
NHEK were stimulated with 4 mg/ml rabbit R594 IgG (raised against GST-BP180 NC16A2–4) or with BP IgG, affinity-purified from the BP patient’s serum, that contained antibodies to BP180 but not to BP230. At the same time, dapsone in concentrations of 40, 4, 0·4, and 0·04 µg/ml or nicotinamide in concentrations of 1,000, 100, and 10 µg/ml were added to the culture medium. After a 12-h incubation time, a dose dependent inhibition of the IL-8, but not the IL-6 release into the culture medium of dapsone-treated cells compared to normal rabbit or human IgG-treated cells or medium alone was observed (data in response to BP IgG shown in Fig. 2 and Fig. 3). In contrast, nicotinamide concentrations of 1,000, 100, and 10 µg/ml did not reduce the IL-6 and IL-8 release from NHEK treated with IgG from R594 or from the BP patient (Fig. 2 and Fig. 3; data not shown for R594 IgG).
Fig. 2.
Dapsone inhibits the IL-8 release from NHEK, induced by IgG from a BP patient, in a dose-dependent manner. NHEK were incubated with a combination of 4 mg/ml IgG, affinity-purified from the BP patient's serum, and various concentrations of dapsone (BP IgG + dapsone) or nicotinamide (BP IgG + nicotinamide) for 12 h. IL-8 levels after stimulation with BP IgG alone (BP IgG), IgG from a healthy volunteer (Normal IgG), and culture medium alone (Medium) are shown on the left. IL-8 levels in the culture supernatant were analysed in quadruplicate by ELISA. Bars show mean ±SD (pg/ml). Asterisks indicate a statistical significant difference of the IL-8 release compared to BP IgG-treated NHEK (P < 0·01). This pattern is representative of the patterns seen in three separate experiments with keratinocytes from different donors. □ BP IgG;  Normal IgG; ▪ Medium.
Normal IgG; ▪ Medium.
Fig. 3.
Dapsone does not inhibit the IL-6 secretion from NHEK, induced by IgG from a BP patient. NHEK were incubated with a combination of 4 mg/ml IgG, affinity-purified from the BP patient's serum, and various concentrations of dapsone (BP IgG + dapsone) or nicotinamide (BP IgG + nicotinamide) for 12 h. IL-6 levels after stimulation with BP IgG alone (BP IgG), IgG from a healthy volunteer (Normal IgG), and culture medium alone (Medium) are shown on the left. IL-6 levels in the culture supernatant were analysed in quadruplicate by ELISA. Bars show mean ±SD (pg/ml). Asterisks indicate a statistical significant difference of the IL-6 release compared to BP IgG-treated NHEK (P < 0·01). □ BP IgG;  Normal IgG; ▪ Medium.
Normal IgG; ▪ Medium.
Dapsone does not reduce the IL-8 release from NHEK that were stimulated with normal IgG
When NHEK were treated with the combination of 4 mg/ml IgG from a healthy control subject or preimmune rabbit IgG and together with various dapsone concentrations, no difference of the IL-8 release into the culture medium was observed compared to treatment with normal IgG, but without any dapsone, or with medium alone (data not shown).
Dapsone does not effect IL-8 mRNA levels of NHEK that were stimulated with BP IgG
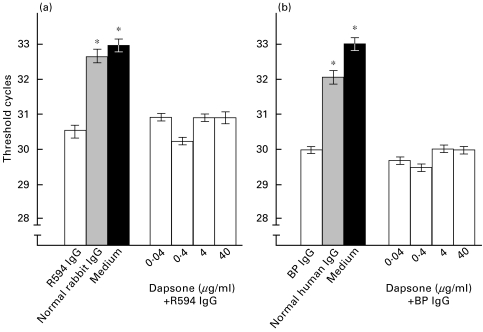
NHEK were cultured with 4 mg/ml R594 or BP patient's IgG together with dapsone concentrations between 0·04 and 40 µg/ml for an incubation period of 12 h. Samples were then analysed semiquantitatively by RT PCR. A difference of 3·5 cycles corresponds to a 10-fold difference in cDNA concentration. IL-8 mRNA levels of NHEK that were treated with R594 IgG or patient's IgG were 6·0-fold (R594 IgG) and 5·1-fold (patient's IgG) higher compared to levels of cells treated with normal IgG or with medium alone. When NHEK were stimulated with IgG from R594 or the BP patient together with various dapsone concentrations, IL-8 mRNA levels were not significantly different compared to levels in NHEK treated with the same IgG preparations but without dapsone (Fig. 4).
Fig. 4.
Dapsone does not inhibit the BP180-mediated increase of IL-8 mRNA levels in NHEK as analysed by RT PCR. NHEK were treated with 4 mg/ml R594 (raised against recombinant human BP180 NC16A), preimmune rabbit IgG (Normal rabbit IgG), medium without any IgG (Medium), and with the combination of R594 IgG together with various dapsone concentrations (left side). In addition, NHEK were stimulated with 4 mg/ml IgG affinity-purified from a BP patient (BP IgG) or from a healthy volunteer (Normal human IgG), with medium alone (Medium), and with the combination of patient's IgG together with various dapsone concentrations (right side). Threshold cycles (Ct) for amplification of IL-8 mRNA are presented, bars show means of triplicate determinations ±SD. RT-PCR analysis was performed semiquantitatively. A difference in Ct of 3·5 cycles corresponds to a 10-fold difference in mRNA concentration. Asterisks indicate a statistical significant difference of the IL-8 release compared to R594 or BP IgG treated NHEK (P < 0·01). □ Anti-BP180 IgG;  Normal IgG; ▪ Medium.
Normal IgG; ▪ Medium.
DISCUSSION
Dapsone is used effectively in treating subepidermal autoimmune bullous diseases, including dermatitis herpetiformis and BP [8,9,25]. The drug does not influence the deposition of IgA or complement in the skin of patients with dermatitis herpetiformis [25,26], but critically reduces the number of neutrophils in skin lesions [11]. IL-8 is a strong chemoattractant for neutrophils and dapsone was shown to reduce the IL-8-induced chemotaxis in vitro [10]. Subsequently, suppression of neutrophil chemotaxis, at least in part mediated by IL-8, has been suggested as the major mode of action in the treatment of dermatitis herpetiformis [10,27]. Neutrophils have also been shown to be crucial for blister formation in both human and experimental murine BP [3,5]. In addition, the biological importance of IL-8 in the pathogenesis of BP has been implicated by i) the presence of abnormally high IL-8 levels in blister fluid and sera of BP patients [18] (ii) the observation that C5-deficient [4] or mast cell-deficient mice [19], that were otherwise resistant to the pathogenic effect of antimurine BP180 IgG, could be made susceptible to the IgG-mediated blistering by intradermal injections of IL-8.
We have recently demonstrated that antibodies to human BP180 NC16A mediate a dose-and time-dependent release of IL-6 and IL-8 from cultured human keratinocytes [20]. In the present study, we tested the effect of dapsone on the IL-8 release in this in vitro model which involved the treatment of NHEK with purified IgG from a rabbit (immunized against recombinant human BP180 NC16A2–4) and from a BP patient. The patient's serum reacted with the N-terminal 45 amino acid segment of BP180 NC16A that has previously shown to habor major antigenic sites for most BP sera [20] and was unreactive with BP230.
In a first set of experiments, NHEK were incubated with affinity-purified IgG from R594 and the BP patient in combination with various dapsone concentrations. Previously, stimulation of NHEK with 4 mg/ml IgG and an incubation period of 12 h have been determined as optimal conditions for the IL-8 release in our experimental model [20]. Background levels of cytokines that we saw with longer incubation periods in both medium alone and medium containing normal human or normal rabbit IgG may reflect the culture conditions on a plastic surface (and not human dermis), or may result from unspecific stimuli produced by the keratinocytes after some incubation time. The therapeutic serum concentration of dapsone ranges from 0·5 to 5 µg/ml but may reach higher levels in the skin [28]. In the present study, we observed a dose-dependent suppression of the IL-8 release, mediated by rabbit and human antibodies to BP180, at dapsone concentrations within the pharmacological range. In contrast, the IL-6 release was not decreased in response to the dapsone treatment under these conditions. Therapeutic serum concentrations of nicotinamide range from 1 to 40 µg/ml [29]. Unlike dapsone, pharmacological doses of nicotinamide did not affect the IL-8 release in our system. To exclude the possibility that dapsone alone, without costimulation with IgG to BP180, suppresses the IL-8 secretion, NHEK were stimulated with IgG affinity-purified from a normal human serum together with various dapsone concentrations. No effect on the IL-8 release was seen under these conditions. In addition, cell viability was affected only by unphysiological concentrations of dapsone or nicotinamide as high as 400 µg/ml or 10 000 µg/ml, respectively. At these drug concentrations, the reduced IL-8 secretion of NHEK compared to treatment of NHEK with anti-BP180 IgG but without drugs may be due to the decreased viability of NHEK. These findings suggest that the IL-8 release from NHEK, in response to antibodies to BP180, is suppressed by therapeutic concentrations of dapsone. The reduction of the IL-8 release appears to be mediated by a specific effect of this drug on NHEK.
In a final set of experiments, we investigated the effect of dapsone on IL-8 mRNA levels of NHEK treated with rabbit or human antibodies to BP180. Interestingly, dapsone did not influence IL-8 mRNA levels in response to any of the 2 antibody preparations. This finding suggests that dapsone exhibits its effect on the post-transcriptional level in our system. Although IL-8 secretion is regulated primarily at the level of gene transcription [30], post-transcriptional gene regulation of IL-8, including influence on mRNA stability, has also been reported [31].
In summary, our data demonstrate that pharmacological doses of dapsone inhibit the IL-8 release from cultured human keratinocytes, mediated by antibodies to BP180, in a dose-dependent fashion. We further show that this inhibition may involve post-transcriptional gene regulation. Our findings contribute to understand the mode of action of dapsone in the treatment of BP.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Zi 439/4–1) and the Interdisciplinary Center for Clinical Research at the University of Würzburg, Germany (IZKF-01KS9603, E.S.). We thank Drs George J. Giudice and Luis A. Diaz, Medical College of Wisconsin, Milwaukee, for providing us with rabbit serum 594.
REFERENCES
- 1.Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci. 1999;20:134–54. doi: 10.1016/s0923-1811(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering disease: advances in the clinic and the laboratory. Adv Dermatol. 2000;16:113–57. [PubMed] [Google Scholar]
- 3.Gammon WR, Merrit CC, Lewis DM, Sams WM, Carlo JR, Wheeler Ce., Jr An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol. 1982;78:285–90. doi: 10.1111/1523-1747.ep12507222. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, Diaz LA. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539–44. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, Till GO, Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–63. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Shipley JM, Thiennu HV, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475–82. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Shapiro SD, Zhou X, Twining SS, Senior RM, Giudice GJ, Fairley JA, Diaz LA. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. 2000;105:113–23. doi: 10.1172/JCI3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Person JR, Rogers Rs., III Bullous pemphigoid responding to sulfapyridine and the sulfones. Arch Dermatol. 1977;113:610–5. [PubMed] [Google Scholar]
- 9.Venning VA, Millard PR, Wojnarowska F. Dapsone as first line therapy for bullous pemphigoid. Br J Dermatol. 1989;120:83–92. doi: 10.1111/j.1365-2133.1989.tb07769.x. [DOI] [PubMed] [Google Scholar]
- 10.Booth SA, Moody CE, Dahl MV, Herron MJ, Nelson RD. Dapsone suppresses integrin-mediated neutrophil adherence function. J Invest Dermatol. 1992;98:135–40. doi: 10.1111/1523-1747.ep12555654. [DOI] [PubMed] [Google Scholar]
- 11.Harvath L, Yancey KB, Katz SI. Selective inhibition of human neutrophil chemotaxis to N-formyl-methionyl-leucyl-phenylalanine by sulfones. J Immunol. 1986;137:1305–11. [PubMed] [Google Scholar]
- 12.Stendahl O, Molin L, Dahlgren C. The inhibition of polymorphonuclear leukocyte cytotoxicity by dapsone. J Clin Invest. 1978;62:214–20. doi: 10.1172/JCI109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wozel G, Barth J. Current aspects of modes of action of dapsone. Int J Dermatol. 1988;27:547–52. doi: 10.1111/j.1365-4362.1988.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 14.Berk MA, Lorincz AL. The treatment of bullous pemphigoid with tetracycline and niacinamide. A preliminary report. Arch Dermatol. 1986;122:670–4. [PubMed] [Google Scholar]
- 15.Fivenson DP, Breneman DL, Rosen GB, Hersh CS, Cardone S, Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol. 1994;130:753–8. [PubMed] [Google Scholar]
- 16.Hornschuh B, Hamm H, Wever S, Hashimoto T, Schröder U, Bröcker E-B, Zillikens D. Treatment of 16 patients with oral tetracycline and niacinamide and topical clobetasol. J Am Acad Dermatol. 1997;36:101–3. doi: 10.1016/s0190-9622(97)70336-0. [DOI] [PubMed] [Google Scholar]
- 17.Barker JNWN, Jones ML, Mitra RS, et al. Modulation of keratinocyte-derived interleukin-8 which is chemotactic for neutrophils and T lymphocytes. Am J Pathol. 1991;139:869–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt E, Ambach A, Bastian B, Bröcker EB, Zillikens D. Elevated levels of interleukin-8 in blister fluid of bullous pemphigoid compared with suction blisters of healthy controls. J Am Acad Dermatol. 1996;34:310–2. doi: 10.1016/s0190-9622(96)80146-0. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Zhou M, Fleming G, Giudice G, Diaz LA. Cellular events in experimental bullous pemphigoid: mast cells, but not T or B lymphocytes, natural killer cells, or macrophages, play a major role in the early stage of subepidermal blistering. J Invest Dermatol. 1999;112:556. (Abstract) [Google Scholar]
- 20.Schmidt E, Reimer S, Kruse N, Jainta S, Bröcker E-B, Marinkovich MP, Giudice GJ, Zillikens D. Autoantibodies to BP180 associated with bullous pemphigoid release IL-6 and IL-8 from cultured human keratinocytes. J Invest Dermatol. 2000;115:842–8. doi: 10.1046/j.1523-1747.2000.00141.x. [DOI] [PubMed] [Google Scholar]
- 21.Balding SD, Diaz LA, Giudice GJ. A recombinant form of the human BP180 ectodomain forms a collagen-like, homotrimeric complex. Biochemistry. 1997;36:8821–30. doi: 10.1021/bi970675n. [DOI] [PubMed] [Google Scholar]
- 22.Zillikens D, Rose PA, Balding SD, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997;109:573–9. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 23.Zillikens D, Kawahara Y, Ishiko A, et al. A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Invest Dermatol. 1996;106:1333–8. doi: 10.1111/1523-1747.ep12349283. [DOI] [PubMed] [Google Scholar]
- 24.Kruse N, Pette M, Toyka K, Rieckmann P. Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J Immunol Meth. 1997;210:195–203. doi: 10.1016/s0022-1759(97)00188-9. [DOI] [PubMed] [Google Scholar]
- 25.Katz SI. Commentary: Sulfoxone (Diasone) in the treatment of dermatitis herpetiformis. Arch Dermatol. 1982;118:809–12. doi: 10.1001/archderm.118.10.805. [DOI] [PubMed] [Google Scholar]
- 26.Dahl MV, Falk NJ, Carpenter R, Michael AF. Membrane attack complex of complement in dermatitis herpetiformis. Arch Dermatol. 1985;121:70–2. [PubMed] [Google Scholar]
- 27.Thuong-Nguyen V, Kadunce DP, Hendrix JD, Gammon WR, Zone JJ. Inhibition of neutrophil adherence to antibody by dapsone: a possible therapeutic mechanism of dapsone in the treatment of IgA dermatoses. J Invest Dermatol. 1993;100:349–55. doi: 10.1111/1523-1747.ep12471811. [DOI] [PubMed] [Google Scholar]
- 28.Zuidema J, Hilbers-Modderman ESM, Merkus FWHM. Clinical pharmocokinetics of dapsone. Clin Pharmacokinet. 1986;11:299–310. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]
- 29.Petley A, Macklin B, Renwick AG, Wilkin TJ. The pharmacokinetics of nicotinamide in humans and rodents. Diabetes. 1995;44:152–5. doi: 10.2337/diab.44.2.152. [DOI] [PubMed] [Google Scholar]
- 30.Roebuck KA. Regulation of interleukin-8 gene expression. J Interf Cytok Res. 1999;19:429–38. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 31.Villarete LH, Remick DG. Transcriptional and post-transcriptional regulation of interleukin-8. Am J Pathol. 1996;149:1685–93. 32. [PMC free article] [PubMed] [Google Scholar]