Abstract
Susceptibility to rheumatoid arthritis (RA) is associated with defined HLA-DRB1 alleles. However the molecular basis of this association is not known. Peculiarities in the expression of disease-linked DRB1 alleles could be involved since in healthy controls HLA-DRB1 gene expression varies according to the alleles in B cells. Peripheral blood B cells of healthy controls and RA patients were examined for their level of allelic DRB1 transcripts using a competitive PCR approach. Levels of DRB1 transcripts were greatly modified in RA and influenced by HLA-DRB1 genotype: patients with double dose of RA-associated alleles displayed up-regulated amounts of DRB1 gene transcripts whereas patients carrying either a single or no at risk allele had low levels of DRB1 transcripts, compared to control individuals. These differential levels of DRB1 gene expression were not influenced in any way by clinical, biological or therapeutic features of the patients. Various amounts of DRB1 mRNA may be related to variations of the density of DR molecules on B cells and consequently could influence the response of CD4 T cells. This particular regulation of DRB1 gene expression in RA patients could therefore represent one of the molecular mechanisms involved in the association of HLA DRB1 genes to RA.
Keywords: B lymphocytes, MHC class II, mRNA, competitive PCR
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease defined as an autoimmune disorder. The aetiology of RA remains uncertain but different susceptibility factors are implicated, including environmental and genetic factors. A major genetic contribution to disease predisposition resides within the HLA class II region. More than 80% of patients with RA carry at least one of the disease-associated HLA-DRB1 alleles: DRB1*0101, DRB1*0401, DRB1*0404 and DRB1*0405.
Molecular basis for the association between at risk DRB1 alleles and RA remains elusive. RA-associated DRB1 alleles encoding for a common amino-acid sequence located within the peptide binding groove of the DRβ chain [1], could play a role in disease process either by shaping the T cell receptor repertoire and/or by presenting an inducing microbial or autoantigenic peptide to self-reactive T cells [2]. Since it has been demonstrated that variations of the density of MHC class II molecules on antigen presenting cells influence the intensity and the nature of T cell response [3,4], peculiarities in the expression of at risk DRB1 genes in RA could also play a role by influencing autoreactive T cell recognition.
We have previously shown that the level of DRB1 gene expression varies according to the alleles [5–7]. The polymorphism of the HLA-DRB1 proximal promoter region is directly responsible for differential transcriptional activities in vitro [5,6] and differential quantities of steady-state DRB1 mRNA in peripheral blood B cells [7]. A quantitative hierarchy of DRB1 mRNA in healthy individuals has been identified: DRB1 alleles of DR52 haplotype group (DRB1*03, DRB1*11, DRB1*12, DRB1*13, DRB1*14) > DRB1*04 > DRB1*01 > DRB1*08. Similar observations have been made for DQA, DQB, DRB3 and DRB4 genes [8–10].
In RA, an increased expression of HLA-DR molecules has been shown on various cell types present in synovial tissue but also on peripheral blood B cells using monoclonal antibodies against nonpolymorphic determinants of DR heterodimers [11–12].
The aim of this work was to study allelic DRB1 gene expression in RA patients compared to healthy controls and to see if a particular dysregulation of DRB1 genes could be evidenced in RA. Allelic DRB1 transcripts were quantified by competitive PCR. DRB1 gene expression in RA was found to be influenced by the HLA-DRB1 genotype and, more precisely, by the number of RA-associated alleles present in the DRB1 genotype configuration of patients.
PATIENTS AND METHODS
Patients
Fourteen healthy volunteer donors were recruited as controls and selected according to their DRB1 genotype (Table 1). Twenty-three RA patients fulfilling the 1987 American College of Rheumatology criteria [13] were also recruited on the basis of their HLA-DRB1 genotype (Table 1) independently of disease activity or therapeutic criteria. This study met local Ethical Committee approval.
Table 1.
HLA-DRB1 genotype distribution in patients (D) and healthy controls (C)
| DRB1*04 haplotypes | DRB1*01 haplotypes | DR52 haplotypes | Other DRB1 haplotypes | ||||
|---|---|---|---|---|---|---|---|
| Patients | |||||||
| D1 | DRB1*0401 | DRB1*0404 | |||||
| D2 | DRB1*0401 | DRB1*0404 | |||||
| D3 | DRB1*0401 | DRB1*0401 | |||||
| D4 | DRB1*0401 | DRB1*0401 | |||||
| D5 | DRB1*0401 | DRB1*0404 | |||||
| D6 | DRB1*0401 | DRB1*0401 | |||||
| D7 | DRB1*0401 | DRB1*0404 | DRB1*03 | ||||
| D8 | DRB1*0402 | DRB1*13 | |||||
| D9 | DRB1*0401 | DRB1*13 | |||||
| D10 | DRB1*0404 | DRB1*07 | |||||
| D11 | DRB1*0404 | DRB1*03 | |||||
| D12 | DRB1*0402 | DRB1*11 | |||||
| D13 | DRB1*0401 | DRB1*03 | |||||
| D14 | DRB1*0401 | ||||||
| D15 | DRB1*0401 | DRB1*0102 | |||||
| D16 | DRB1*0408 | DRB1*0101 | |||||
| D17 | DRB1*0101 | DRB1*0101 | |||||
| D18 | DRB1*0101 | DRB1*0101 | DRB1*13 | DRB1*07 | |||
| D19 | DRB1*03 | DRB1*10 | |||||
| D20 | DRB1*11 | DRB1*07 | |||||
| D21 | DRB1*03 | DRB1*16 | |||||
| D22 | DRB1*03 | ||||||
| D23 | DRB1*0101 | ||||||
| Controls | |||||||
| C1 | DRB1*0401 | DRB1*0401 | |||||
| C2 | DRB1*0401 | DRB1*0404 | DRB1*03 | ||||
| C3 | DRB1*0402 | DRB1*03 | |||||
| C4 | DRB1*0401 | DRB1*03 | |||||
| C5 | DRB1*0404 | DRB1*03 | |||||
| C6 | DRB1*0403 | ||||||
| C7 | DRB1*0401 | DRB1*0101 | |||||
| C8 | DRB1*0101 | DRB1*0101 | |||||
| C9 | DRB1*0101 | DRB1*0102 | DRB1*03 | ||||
| C10 | DRB1*0101 | DRB1*03 | DRB1*15 | ||||
| C11 | DRB1*03 | DRB1*07 | |||||
| C12 | DRB1*13 | DRB1*13 | |||||
| C13 | DRB1*03 | DRB1*03 | |||||
Low resolution PCR-based HLA-DRB1 typing was performed for all patients and controls. In addition, DRB1*04 and DRB1*01 subtypes were determined by SSP-PCR-based methods.
For RA patients, the mean age of symptom onset was 50·2 years and the mean disease duration was 9·1 years. Disease activity was evaluated on the basis of clinical criteria (morning stiffness, number of tender joints, number of swollen joints) and biological features reflecting inflammatory status (erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)). Active RA was defined by at least 3 of the following criteria: morning stiffness >45 min, number of swollen joints >6, number of tender joints >9, ESR > 28 mm/hour or CRP > 15 mg/l. Disease severity was also evaluated on the basis of articular damage on X-rays. Patients with severe disease had Larsen scores ≥4 on wrists, hands, feet and at least one other joint. Patients with benign disease had a score <2 on all joints [14]. In between, disease severity was defined as intermediary. Other criteria reflecting disease severity were also evaluated: disease duration, seropositivity for rheumatoid factor, surgical treatment of articular lesions and extra-articular manifestations.
Disease-modifying drugs (DMD) received by 17 patients included: methotrexate (patients D2, D3, D7, D9, D11, D17, D21), methotrexate with hydroxychloroquine (D13-D14) or cyclosporin (D16-D23), gold salts (D1, D6), sulfalazine (D18, D20), hydroxychloroquine (D19) and leflunomide (D5). Six patients (D4, D8, D10, D12, D15, and D22) received no disease-modifying drug. Systemic corticotherapy was used in 11 patients (D3, D5, D7, D8, D10, D11, D15, D16, D20, D21, D23) at dose equal or less than 10 mg/day. None of the patients had received local corticosteroids for at least 2 months.
RNA extraction and reverse transcription
Fresh peripheral blood was drawn up from controls and patients and buffy coats were recovered. B cells were then separated with anti-CD19 mAb-coated magnetic beads (Dynabeads, Dynal, Compiègne, France) and used directly for RNA extraction. Total RNA was extracted with RNA Quick II extraction kit and RNA Catch resins (Bioprobe Systems, Montreuil, France). RNA samples were stored at −70°C.
Nine microlitres of extracted RNA, corresponding approximatively to 106 cells, were added to 500 µm dNTP, 10 mm DTT, 50 pmol of random hexadeoxyoligonucleotide primers (Pharmacia, Saclay, France), 200 U of reverse transcriptase from Moloney murine leukaemia virus (Life Technologies, Cergy-Pontoise, France), 1X corresponding buffer and 20 U RNase inhibitor (RNAguard, Pharmacia, Saclay, France). Reverse transcription was performed in a 25-µl volume reaction for 1 h at 37°C. cDNA was immediately used for quantification of HLA-DRB1 transcripts. For each RNA sample, at least two reverse transcriptions were done.
Competitive PCR
PCR were performed as previously described [7]. Forward DRB primers annealed to the first hypervariable region of HLA-DRB1 second exon, and were specific for 3 groups of alleles, respectively: DRB1*01, DRB1*04 and DR52 group referred as DRB1*X. Reverse primer, identical for all DRB1 amplifications, annealed to a conserved region of DRB genes located between the second and third hypervariable regions. The specificity of DRB1 amplification had been previously demonstrated [7] and was confirmed in this study. Specific internal standards for HLA-DRB1*0101, DRB1*0401, and DRB1*X, as well as GAPDH external standard [7] were also used in this study. Reverse DRB and GAPDH primers were 5′ end-labelled with fluorescent dye carboxy-tetramethyl-rhodamine N-hydroxysuccinimide (TAMRA). Each competitive PCR consisted in coamplification of three or four different known quantities of a given internal or external standard with a constant volume of the same cDNA sample (3 µl). Each cDNA sample was submitted to competitive PCR at least in duplicate. Co-amplified fluorescent PCR products were electrophoresed for 5 h on an Applied Biosystem 373 A DNA and separated according to their size. Peaks of fluorescence were displayed using the Genescan software (Version 673, Applied Biosystems) and intensities of fluorescence, corresponding to the calculated area of each peak, were determined.
Ratios of fluorescence intensities between each coamplified PCR product (internal standard and cDNA to analyse) were calculated and plotted on a logarithmic scale as a function of the quantity of internal standard added to reaction. The resultant lines allowed the determination of the initial specific DRB1 cDNA amount in the sample, corresponding to the quantity of internal standard for which the ratio of fluorescence intensities was equal to 1. This amount was then related to the amount of GAPDH cDNA determined in a same way in order to normalize yields of RNA extraction and reverse transcription between samples. These corrected cDNA amounts were multiplied by 100 and expressed in arbitrary units (a.u.).
In controls carrying DRB1*04/04, DRB1*01/01, or DRB1*X/X genotypes, corrected cDNA amounts were divided by two, in order to reflect the mRNA amount of a single allele [7]. Similarly, in DR homozygous patients, cDNA amounts were also arbitrarily divided by two to estimate the level of transcripts corresponding to a given allele.
Statistical methods
Mean comparisons on quantitative data between groups of alleles or groups of patients were performed with the Mann Whitney unpaired t-test. The influence of quantitative variables (CRP, ESR) on DRB1 gene expression was assessed also with the Mann Whitney unpaired t-test. The influence of qualitative variables (disease activity, nature of treatment) was analysed in contingency tables with Fisher's exact test. Differences among groups were considered significant in all cases when P < 0·05.
RESULTS
A hierarchy of allelic DRB1 gene expression is observed in healthy controls
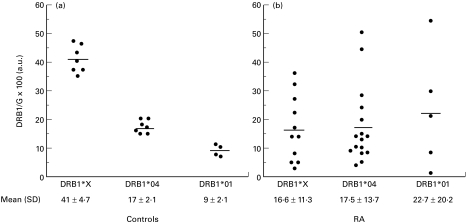
Quantification of DRB1*01, DRB1*04 and DRB1*X transcripts was performed in peripheral B cells of 14 healthy controls carrying various HLA-DRB1 genotypes (Table 1,Fig. 1a). DRB1*X were found significantly more abundant than DRB1*04 transcripts (P = 0·0006) and even more abundant than DRB1*01 transcripts (P = 0·0061). Total amount of DRB1 transcripts in DRB1*13 homozygous C13 individual was found equivalent to that of DRB1*03 homozygous control C14 (92 a.u. and 82 a.u., respectively) and around two fold higher than the amount of DRB1*03 transcripts in heterozygous controls (C3 = 47 a.u., C6 = 37 a.u., C10 = 37 a.u., C11 = 43 a.u., C12 = 35 a.u.). Similarly, around two times more DRB1*04 transcripts were found in homozygous controls C1 and C2 (30 a.u. and 40 a.u., respectively) compared to heterozygous controls (C3 = 15 a.u., C4 = 20 a.u., C5 = 18 a.u., C6 = 17 a.u.), irrespectively of the other DRB1 haplotype. DRB1*04 transcripts were expressed at significantly (P = 0·0061) higher levels than DRB1*01 transcripts. Altogether these results confirm our previously published data [7] by showing on an additional group of controls that, first, there is a quantitative hierarchy of DRB1 transcripts (DRB1*03, DRB1*13 > DRB1*04 > DRB1*01), second, DRB1 gene expression is allele-dependent and is not influenced by the nature of DRB1 allele on the other haplotype.
Fig. 1.
DRB1 corrected cDNA amounts in controls (a) and in RA patients (b). In homozygous control individuals and patients, cDNA amount of a single DRB1 allele is represented (see materials and methods). Alleles of the DR52 group are referred as DRB1*X. Means and standard deviations are indicated.
Expression of DRB1 genes is highly variable in RA patients
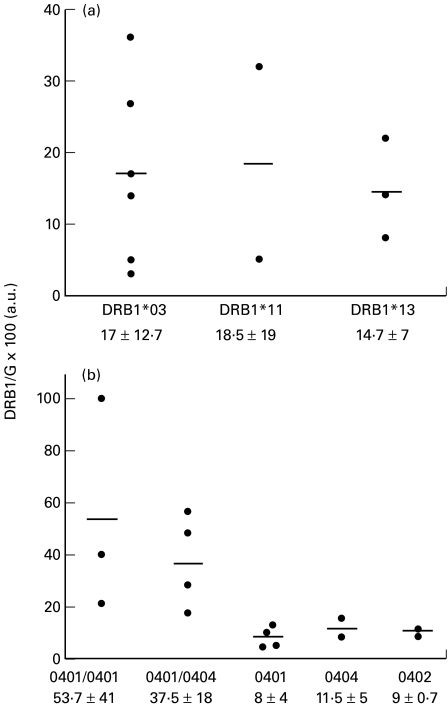
HLA-DRB1 transcripts were similarly quantified in 23 RA patients. By contrast to healthy individuals, the amount of a given DRB1 mRNA was rather variable from one patient to another (Fig. 1b). This heterogeneity could be illustrated by the magnitude of transcript quantity variations among patients sharing same DRB1 allele: 15 fold for DRB1*04, 12 fold for DRB1*X and up to 35 fold for DRB1*01. In contrast to what was observed in healthy controls, levels of DRB1 expression in RA patients were not statistically different among the three groups of alleles. When considering independently the alleles within DRB1*X group, no difference in the expression of DRB1*03, DRB1*11 and DRB1*13 genes could be evidenced (Fig. 2a). Similarly, levels of DRB1*04 transcripts were similar in heterozygous patients whatever the DRB1*04 subtype (Fig. 2b). In DRB1*0401/0401 or DRB1*0401/0404 patients, much more than twice the amount of DRB1*04 transcripts observed in heterozygous patients was found (Fig. 2b). Lower amount of DRB1 transcripts was found in DRB1*0401/0404 compared to DRB1*0401/0401 patients (53·7 ± 41·2 a.u. and 37·5 ± 18·3 a.u., respectively). However this slight difference did not reach statistical significancy (P = 0·85) Thus, the quantitative hierarchy of DRB1 mRNA described for normal individuals was not conserved for RA patients.
Fig. 2.
Expression of DRB1*X and DRB1*04 genes in RA patients.DRB1 corrected cDNA amounts in DRB1*X (A) and DRB1*04 (B) patients are represented. (a) Levels of DRB1*03, DRB1*11 and DRB1*13 gene expression are compared in heterozygous DR52 patients. (b) Expression of the various DRB1*04 subtypes is quantified in homozygous (cDNA of both genes) and heterozygous (cDNA of a single gene) patients. Means and standard deviations are indicated.
Expression of DRB1 genes is either increased or decreased in RA patients compared to controls
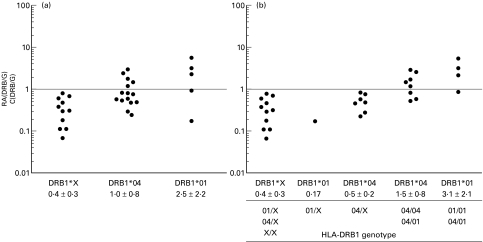
We then compared at single allele level, the amounts of DRB1 transcripts between RA patients and control individuals. For homozygous individuals, level of transcripts corresponding to a given DRB1 allele was estimated as described (see Patients and methods). In order to provide an easy representation of these comparisons, we defined a ‘RA/C ratio’ (Amount of DRB1 mRNA (patient)/Mean amount of DRB1 mRNA (controls)) for each DRB1 allele. Since mRNA amount for a defined DRB1 allele is stable in control individuals and not influenced by the genotype configurationmean values of mRNA amounts found in controls were used for further calculation of RA/C ratios.
The mean value of DRB1 mRNA amounts for each group of alleles was as follows: DRB1*X = 41 a.u., DRB1*04 = 17 a.u. and DRB1*01 = 9 a.u.The mean RA/C ratios were 1·0 + 0·8 for DRB1*04, 2·5 + 2·2 for DRB1*01 and 0·4 + 0·3 for DRB1*X gene. However, depending on the patients, DRB1 genes were differently expressed as illustrated by important variation of RA/C ratios (Fig. 3a). For DRB1*04 transcripts the RA/C ratios varied from 0·23 to 2·9, reflecting an expression rate from 4 times lower to almost 3 times higher than in controls. No effect of particular DRB1*04 subtypes on DRB1*04 gene expression could be evidenced. The amount of DRB1*01 transcripts were from 6 fold less to 6 fold more abundant than in control individuals (RA ratios from 0·17 to 5·9). By contrast, DRB1*X genes were always under-expressed (RA/C ratios from 0·07 to 0·8). For these alleles, the DRB1 transcript amounts were from 1·2 to 13 times lower than in control individuals.
Fig. 3.
DRB1 gene expression in RA patients compared to HLA-matched controls. RA/C ratios (see materials and methods) are represented for groups of patients defined according to DRB1 haplotype (A) or to DRB1 genotype (B). An RA/C ratio value equal to 1 (dotted line) is obtained when the DRB1 transcript amounts are identical in RA patients and in control individuals. Represented RA/C ratios reflect the mRNA amounts of a single DRB1 gene. The mean RA/C ratio and standard deviation found in each group are indicated.
The under-expression of DRB1*X genes was statistically significant in RA patients compared to controls (mean RA/C ratio significantly different of 1·00 value; P = 0·0038).
DRB1 gene expression is influenced by DRB1 genotype in RA
We then attempted to establish a relationship between the HLA-DRB1 genotypes and the level of DRB1 transcripts in RA patients. A strong correlation (P = 0·0013 − O.R. = 41·6) was found between the level of DRB1 expression and the number of RA-associated genes present in the genotype configuration (Table 2). All of the seven patients displaying increased levels of DRB1 transcripts carried a double dose of RA-associated alleles (DRB1*04/01 or DRB1*04/04 or DRB1*01/01). By contrast, 75% of the patients with decreased levels of DRB1 transcripts carried either a single or no disease-associated haplotype (DRB1*04/X, DRB1*01/X or DRB1*X/X). This is in contrast with our results obtained in healthy controls [7].
Table 2.
HLA-DRB1 gene expression (RA/C), according to DRB1 genotype, disease status and drug regimen in RA patients
| RA/C > 1 n = 7 | RA/C < 1 n = 16 | P-value | |
|---|---|---|---|
| Number of RA-associated DRB1 alleles | |||
| 2 (double dose) | 100% | 25% | 0·0013 |
| 1 or 0 (single or no dose) | 0% | 75% | 41·7 (odds ratio) |
| Mean age (years) | 50 | 50·3 | 1 |
| Disease duration (years) | 8 (2·8) | 9·3 (8·8) | 0·79 |
| Disease activity | |||
| Active | 43% | 19% | |
| Non active | 57% | 81% | 0·32 |
| CRP | 13·4 (12) | 6·6 (5·2) | 0·18 |
| ESR | 22·5 (13·9) | 14·4 (9·3) | 0·27 |
| Disease severity | |||
| Articular damage | |||
| Severe | 29% | 31% | 0·67 |
| Benign | 14% | 31% | |
| Severe | 29% | 31% | 1 |
| Intermediary + benign | 71% | 69% | |
| Benign | 14% | 31% | 0·62 |
| Intermediary + severe | 86% | 69% | |
| Larsen Score (hands) | 53·3 (27·2) | 41·2 (33) | 0·46 |
| Larsen score (feet) | 16·6 (14·7) | 16·6 (19·7) | 0·81 |
| RF | |||
| Seropositivity | 71% | 69% | 1 |
| Seronegativity | 29% | 31% | |
| Surgical treatment | |||
| Yes | 14% | 31% | 0·37 |
| No | 86% | 69% | |
| Extra-articular manifestations | |||
| Present | 29% | 25% | |
| Absent | 71% | 75% | 1 |
| Treatment | |||
| Corticosteroids | |||
| Present | 43% | 50% | 1 |
| Absent | 57% | 50% | |
| Methotrexate | |||
| Present | 57% | 44% | 0·84 |
| Absent | 43% | 56% | |
In order to analyse the expression of DRB1 genes at a single allele level for these various genotypes, we then defined two groups of patients according to their DRB1 genotype: patients with a double dose of RA-associated alleles and patients with a single or no RA-associated genes. As shown in Fig. 3b, we observed that expression of DRB1*04 was significantly higher in DRB1*04/DRB1*04 and DRB1*04/DRB1*01 patients than in DRB1*04/DRB1*X patients (P = 0·007). Similarly, DRB1*01 transcripts were more abundant in DRB1*01/01 and DRB1*04/01 patients than in the single DRB1*01/X patient.
Variation of DRB1 gene expression cannot be related to the disease status or drug regimen of the patients
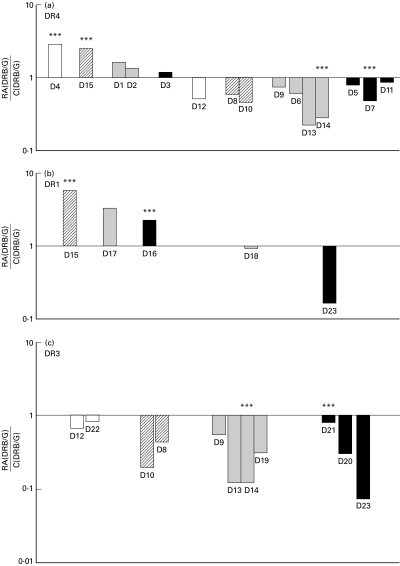
To search for a possible influence of inflammatory process, drug regimen or severity of the disease on DRB1 gene expression in RA patients, we analysed clinical and biological characteristics of patients displaying either an increased or a decreased of DRB1 gene expression. The activity of disease at the time of analysis was evaluated by clinical and biological indexes reflecting the inflammatory status of patients. Patients displaying an over-expression of DRB1 genes included three patients with active disease (one untreated, one receiving corticosteroids only, and one treated with disease-modifying drugs (DMD) and corticosteroids) and four patients with nonactive disease all treated with DMD (Fig. 4). Patients with a lower DRB1 gene expression included three patients with active disease all treated with DMD and 13 patients with nonactive disease either treated or untreated (Fig. 4).
Fig. 4.
DRB1 gene expression, disease activity and drug regimen in RA patients. (a) DRB1*04, (b) DRB1*01 and (c) DRB1*X gene expression is represented on a logarithmic scale as RA/C ratios calculated in indicated patients displaying either active disease (***) or non active disease. Patients may be represented twice according DRB1 genotype. □ No treatment; ▪ Disease-modifying drugs (DMD);  Corticosteroids; ▪ DMD and corticosteroids.
Corticosteroids; ▪ DMD and corticosteroids.
Among 8 patients receiving only DMD, increased DRB1 gene expression was found in three patients all displaying non active disease (D1, D2, D17) whereas decreased expression was found in 5 patients with non active disease and in a single patient with active disease (D14). The sole difference between these patients was their DRB1 genotype.
Two patients both with active disease and treated with DMD and corticosteroids, exhibited opposite DRB1 gene expression and genotype: increased DRB1 gene expression and double dose of susceptible DRB1 genes in patient D16, vs. decreased DRB1 gene expression and single dose of susceptible genes in patient D21. Patient D7, also with active disease and treated like patient D21, displayed low DRB1 cDNA amounts despite double dose of RA-associated alleles, indicating that the correlation between DRB1 genotype and DRB1 gene expression in RA was not absolute. Taken together, these results, summarized in Table 2, show that there is no relationship between the level of DRB1 gene expression and the activity of disease, or drug regimen. Similarly, no influence of the patients' age and disease duration can be observed.
The severity of disease was also evaluated based on articular damage on X-rays. Patients were classified with either benign, severe or intermediary disease (see Patients and methods) [14]. Seropositivity for rheumatoid factor, surgery and extra-articular manifestations were evaluated too. As shown in Table 2, correlation between disease severity and DRB1 gene expression in RA patients could not be demonstrated.
DISCUSSION
In this work, we have shown that the hierarchy of DRB1 mRNA amounts according to the alleles described in peripheral blood B cells of healthy individuals was not observed in RA patients. Important variations of DRB1*01 and DRB1*04 gene expression were seen in RA patients independently of DRB1*04 and *01 subtypes. On the contrary, all the DRB1*X genes were constantly expressed at a lower level than in healthy individuals. Seventy-five percent of the patients with double dose of RA-associated DRB1 genes expressed high amounts of DRB1 transcripts, whereas patients with a single or no RA-associated DRB1 gene constantly displayed lower levels of DRB1 transcripts.
In autoimmune diseases, variation of HLA class II gene expression in APC and other cell types could be induced by inflammatory mediators, although it has not been directly demonstrated. In this study, patients with active disease displayed either high or low levels of DRB1 transcripts, irrespectively to their drug regimen (Fig. 4,Table 2), suggesting that there is no relationship between inflammatory status or disease activity in our patients and their DRB1 gene expression. Similarly, when evaluated by analysis of cell surface markers, no difference in the state of activation or differentiation of the B cell population could be evidenced among patients and between patients and controls (data not shown) [15].
DRB1 genotype is now thought to act mostly on disease phenotype. The presence of double dose of RA-associated genes is associated to severe disease with cartilage destruction and increased frequency of extra-articular manifestations [14,16,17]. IL-1 is the dominant cartilage destructive cytokine [18] and its impact on cartilage destruction can be reduced by regulatory cytokines such as IL-4 and IL-10 [19]. Increased frequency of particular polymorphism of IL-1 and IL-10 genes has been recently identified in RA [20,21] and the simultaneous presence of susceptible DRB1 genes and a specific polymorphism of the exon 5 of IL-1β gene has been suggested to be predictive of erosive arthritis [22]. Thus, the influence of DRB1 genotype on RA phenotype could be related to genetically controlled patterns of production of cytokines involved in cartilage erosions. Since IL-1 and IL-10 modulate MHC class II expression in human and murine B cells [23–25], differential patterns of IL-1 and IL-10 production could feature distinctly our two groups of patients and thus could be responsible for differential DRB1 gene expression. Nevertheless, though this possibility cannot be completely ruled out, in our study DRB1 gene expression was not correlated to disease severity, since erosive arthritis was found both in patients displaying either increased or decreased expression of BRD1 gene (Table 2).
Drug regimens of RA patients could also influence DRB1 gene expression. Indeed, it has been shown that corticosteroids down-regulate HLA class II genes on human B cell lines and peripheral blood mononuclear cells [26,27]. In our study, corticosteroid treatment did not correlate with low levels of DRB1 gene transcripts in fresh peripheral B cells. This could be related to the low doses of corticosteroids received by all the patients. Methotrexate has been shown to increase production of Th2 cytokines (IL-4) and to decrease production of Th1 cytokines (IL-2 and IFN-γ) by in vitro activated PBMC of patients with active RA [28]. This in vitro modulation of T cells cytokines was suggested to play a role in the anti-inflammatory properties of methotrexate. Since IL-4 enhances HLA class II gene expression at the transcriptional level in B cells, methotrexate treatment could be associated with higher levels of DRB1 transcripts in RA patients. In our study, methotrexate was received by patients either over-expressing or under-expressing their DRB1 genes and no influence of methotrexate on DRB1 gene expression in B cells was found in vivo.
Altogether, no clinical or biological features could be shown to be associated with variations of DRB1 mRNA levels in RA patients' B cells, suggesting a DRB1 genotype-dependent regulation of DRB1 gene transcription in RA. However, factors linked to DRB1 genotype and able to modulate DRB1 gene expression, have not been described to date. The observation that in all patients, DRB1 genes on both haplotypes are simultaneously either over-or under expressed suggests the presence, during the course of RA, of trans-regulating factors modulating DRB1 gene expression. Such regulating factors could be dominant inhibitors of DRB1 gene transcription linked to non RA-associated DRB1*X alleles and/or recessive enhancers of DRB1 expression linked to RA-associated alleles.
The mechanisms of the association between the HLA-DRB1 component and RA remain currently uncertain. The presentation ability of at-risk alleles is likely related to their amino-acid sequence but could also be influenced by characteristics of their expression. It has been shown that HLA-DR membrane expression was directly related to DRB1 transcript amounts [29,30]. Allele-dependent variation of HLA heterodimer expression on B cells with functional consequences on T cell immune response has been specifically shown in RA patients [Kerlan-Candon et al. unpublished observation]. In this view, whatever the mechanism of DRB1 dysregulation is: primary or induced by cytokines for example, variation in the expression of DR molecules on B cells could have important consequences on immune response, possibly leading to autoimmune process.
In conclusion, we have shown in this study that the number of RA-associated DRB1 genes present in the genotype configuration of RA patients influences the level of DRB1 gene expression in peripheral B cells, independently of disease activity, severity and drug regimen. This particular regulation of DRB1 gene expression in RA, either genetically determined or resulting from unidentified modulating factors, may be part of the molecular mechanisms involved in the association of RA with the HLA class II component.
Acknowledgments
We wish to thank Jeanine Bourrel for her technical assistance.
REFERENCES
- 1.Gregersen P, Silver J, Winchester R. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 2.Fox D. The role of T cells in the immunopathogenesis of rheumatoid arthritis. Arthritis Rheum. 1996;40:598–605. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- 3.Janeway C, Bottomly K, Babish J, et al. Quantitative variation in Ia antigen expression plays a central role in immune regulation. Immunol Today. 1984;5:99–101. doi: 10.1016/0167-5699(84)90043-4. [DOI] [PubMed] [Google Scholar]
- 4.Lechler R, Norcross M, Germain R. Qualitative and quantitative studies of antigen-presenting cell function by using I-A-expressing L cells. J Immunol. 1985;135:2914–22. [PubMed] [Google Scholar]
- 5.Louis P, Eliaou J-F, Candon S, et al. Polymorphism in the regulatory region of HLA-DRB genes correlating with haplotype evolution. Immunogenetics. 1993;38:21–6. doi: 10.1007/BF00216386. [DOI] [PubMed] [Google Scholar]
- 6.Louis P, Vincent R, Cavadore P, et al. Differential transcriptional activities of HLA-DR genes in the various haplotypes. J Immunol. 1994;153:5059–67. [PubMed] [Google Scholar]
- 7.Vincent R, Louis P, Gongora C, et al. Quantitative analysis of the expression of the HLA-DRB genes at the transcriptional level by competitive polymerase chain reaction. J Immunol. 1996;156:603–10. [PubMed] [Google Scholar]
- 8.Andersen LC, Beaty JS, Nettles JW, et al. Allelic polymorphism in trascriptional regulatory regions of HLA-DQB genes. J Exp Med. 1991;173:181–92. doi: 10.1084/jem.173.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal DP, Qiu X. Polymorphism in the upstream regulatory region and level of expression of HLADRB genes. Mol Immunol. 1994;31:1117–20. doi: 10.1016/0161-5890(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 10.Morzycka-Wroblewska E, Munshi A, Ostermayer M, et al. Differential expression of HLA-DQA1 alleles associated with promoter polymorphism. Immunogenetics. 1997;45:163–70. doi: 10.1007/s002510050185. [DOI] [PubMed] [Google Scholar]
- 11.Teyton L, Lotteau V, Turmel P, et al. HLA-DR, DQ and DP antigen expression in rheumatoid synovial cells: a biochemical and quantitative study. J Immunol. 1987;138:1730–5. [PubMed] [Google Scholar]
- 12.Eliaou J-F, Andary M, Favier F, et al. Increase of class II HLA molecules on the membrane of B lymphocytes from patients with rheumatoid arthritis. Autoimmunity. 1988;1:217–22. doi: 10.3109/08916938808997166. [DOI] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 14.Combe B, Eliaou J-F, Daures J-P, et al. Prognostic factors in rheumatoid arthritis. Comparative study of two subsets of patients according to severity of articular damage. Br J Rheumatol. 1995;34:529–34. doi: 10.1093/rheumatology/34.6.529. [DOI] [PubMed] [Google Scholar]
- 15.Louis-Plence P, Kerlan-Candon S, Morel J, et al. The down-regulation of HLA-DM genes expression in Rheumatoid Arthritis in not related to their promoter polymorphism. J Immunol. 2000;165:4861–9. doi: 10.4049/jimmunol.165.9.4861. [DOI] [PubMed] [Google Scholar]
- 16.Weyand C, Hicok K, Conn D, et al. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Int Med. 1992;117:801–6. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 17.Gough A, Faint J, Salmon M, et al. Genetic typing of patients with inflammatory arthritis at presentation can be used to predict outcome. Arthritis Rheum. 1994;37:1166–70. doi: 10.1002/art.1780370809. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Ladner U, Roberts CR, Franklin BN, et al. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J Immunol. 1997;158:3492–8. [PubMed] [Google Scholar]
- 19.Moreland AW, Heck LW, Koopman WJ. Biologic agents for treating rheumatoid arthritis. Arthritis Rheum. 1997;40:397–409. doi: 10.1002/art.1780400302. [DOI] [PubMed] [Google Scholar]
- 20.Jouvenne P, Chaudhary A, Buchs N, et al. Possible genetic association between interleukin-1alpha gene polymorphism and the severity of chronic polyarthritis. Eur Cytokine Netw. 1999;10:33–6. [PubMed] [Google Scholar]
- 21.Hajeer AH, Lazarus M, Turner D, et al. IL-10 gene promoter polymorphism in rheumatoid arthritis. Scand J Rheumatol. 1998;27:142–5. doi: 10.1080/030097498441029. [DOI] [PubMed] [Google Scholar]
- 22.Cantagrel A, Navaux F, Loubet-Lescoulie P, et al. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 1999;42:1093–100. doi: 10.1002/1529-0131(199906)42:6<1093::AID-ANR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Rohn W, Tang LP, Dong Y, et al. IL-1 beta inhibits IFN-gamma-induced class II MHC expression by suppressing transcription of the class II transactivator gene. J Immunol. 1999;162:886–96. [PubMed] [Google Scholar]
- 24.Go NF, Castle B, Barrett R, et al. IL-10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172:1625–31. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding L, Linsley PS, Huang L-Y, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 26.Glimcher LH, Kara CJ. Sequences and factors: a guide to MHC class II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 27.Czerwony G, Alten R, Gromnica-Ihle R, et al. Differential surface expression of HLA-DRB1 and HLA-DRB4 among peripheral blood cells of DR4 positive individuals. Hum Immunol. 1999;60:1–9. doi: 10.1016/s0198-8859(98)00096-2. [DOI] [PubMed] [Google Scholar]
- 28.Constantin A, Loubet-Lescoulié P, Lambert N, et al. Anti-inflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis. Arthritis Rheum. 1998;41:48–57. doi: 10.1002/1529-0131(199801)41:1<48::AID-ART7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.Fertsch D, Schoenberg DR, Germain RN, et al. Induction of macrophage Ia antigen expression by rIFN-γ and down-regulation by IFN-α/β and dexamethasone are mediated by changes in steady state levels of Ia mRNA. J Immunol. 1987;139:244–9. [PubMed] [Google Scholar]
- 30.Koerner TJ, Hamilton TA, Adams DO. Suppressed expression of surface Ia antigen on macrophages by lipopolysaccharide: evidence for regulation at the level of accumulation of mRNA expression by rIFN-γ. J Immunol. 1987;139:239–43. [PubMed] [Google Scholar]