Abstract
The aetiology of IgA nephropathy (IgAN) is closely related with abnormality of mucosal immunity. We investigated the roles of γδ T cells in the regulation of IgA production by B cells in IgAN patients. The proportion of γδ T cells in peripheral blood mononuclear cells (PBMNC) was higher in IgAN patients than in the controls and was found to be correlated with the proportion of surface IgA-positive (sIgA+) B cells, which are precursors of IgA-secreting plasma cells. After in vitro PWM stimulation, sIgA expression on B cells and IgA production were significantly enhanced in PBMNC obtained from IgAN patients, whereas the enhancements were abolished by removal of γδ T cells from the PBMNC. Purified γδ T cells from IgAN patients induced surface IgA expression on naïve sIgD+ B cells more effectively than did αβ T cells. Moreover, stimulated γδ T cells from IgAN patients produced a larger amount of TGF-β1, which is one of the main cytokines that induces IgA class switching on B cells, as compared with αβ T cells and control γδ T cells. The expanded γδ T cells from IgAN patients exclusively expressed Vγ9, and the nucleotide sequences of junctional regions of Vγ9 showed very limited TCR diversities. It was therefore concluded that γδ T cells, which are expanded in response to specific antigens, enhance IgA class switching on B cells in IgAN patients.
Keywords: γδ T cells, IgA nephropathy, IgA switching, TGF-β
INTRODUCTION
IgA nephropathy (IgAN) is the most common type of glomerulonephritis and is characterized by mesangial IgA deposits [1]. The pathogenesis of IgAN remains unknown, although many studies have demonstrated that the underlying abnormalities in the disease are in humoral and cellular immunity rather than in the kidney [2–8]. In particular, an increase in IgA-specific helper T cells and a decrease in IgA-specific suppressor T cells in IgAN are attributed to increased in vitro, and probably in vivo, IgA synthesis from peripheral blood lymphocytes [7,9]. The IgA-specific helper T cells are reported to have receptors for the Fc-portion of IgA (FcαRs) and to enhance switching of naïve B cells to IgA-producing plasma cells. The FcαRs on T cells, however, have not been characterized and therefore the nature of the IgA-specific helper T cells in IgAN is therefore still obscure.
IgA is unique in that it is the major immunoglobulin in external secretions. The fact that deposited IgA in the glomerular mesangium in IgAN is predominantly polymeric IgA1, which is usually derived mainly from the mucosal immune system, suggests that the pathogenesis of this disease is related to abnormality of mucosal immunity [10]. In fact, many studies have suggested the involvement of mucosa in the immunological abnormalities in IgAN [11–16]. In the mucosal immune system, a unique type of T cell bearing γδ TCR (γδ T cell) is distributed more abundantly than are αβ TCR-positive T cells (αβ T cells), which are more common in peripheral blood and other lymphoid organs. Interestingly, mucosal IgA responses are impaired in γδ T cell-deficient mice, suggesting that γδ T cells are involved in the regulation of synthesis of IgA from B cells [17].
The purpose of this study was to determine whether γδ T cells play roles in increased IgA synthesis in IgAN. We therefore undertook a series of experiments, which revealed that γδ T cells in IgAN patients are IgA-specific switching cells and have some distinct characteristics from those in normal controls.
MATERIALS AND METHODS
Subjects
Fifty-two patients with IgAN proven by renal biopsy were studied. They varied in age from 8 to 17 years (mean age, 13·5 years) and included 35 males and 17 females. None of them were on corticosteroid or any immunosuppressive therapies at the time of blood collection. The serum creatinine levels in all patients were below 1·5 mg/dl. As controls, we studied 66 healthy volunteers, aged from 8 to 17 years (mean age, 13·6 years) and included 36 males and 30 females. They had no history or clinical features of renal diseases. This study was approved by the Ethics Committee of the Niigata University Hospital. Informed written consent was obtained from the parents of all individuals.
Phenotypic analysis of peripheral blood mononuclear cells (PBMNC)
Heparinized blood was obtained from the patients and controls. PBMNC were separated by Ficoll-Hypaque gradient centrifugation. Immunofluorescence analysis was performed as described elsewhere with some modifications [18]. Briefly, PBMNC were stained with FITC- or PE-labelled antiαβ-TCR, antiγδ-TCR, anti-Vγ9, anti-Vδ2, anti-CD20, F(ab)′ fragment of anti-IgA, F(ab)′ fragment of anti-IgG, and F(ab)′ fragment of anti-IgM antibodies (Pharmingen, San Diego, CA) for 20 min on ice. After washes, cells were analysed using FACScan (Beckton Dickinson, Franklin Lakes, NJ). For the negative control in each flow cytometric analysis, cells were stained with FITC-and PE-conjugated isotype control mAb. Basal fluorescence levels of the cells were estimated from the results.
Cell preparation
Cells positive for antiαβ-TCR, antiγδ-TCR, and F(ab)′ fragment of anti-IgD antibodies (Pharmingen, San Diego, CA) were separated by a preparative magnetic cell sorter (MACS, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according the manufacturer's instructions. Briefly, 2 × 106 PBMNC were stained with the biotinylated antibodies. After washing with PBS, the cells were labelled with Streptavidin MicroBeads. Then the cells were separated using a high gradient magnetic separation column placed in a strong magnetic field. The magnetically labelled cells were retained in the column. When the column is removed from the magnetic field, the magnetically retained cells are eluted. The sorted cells were stained with FITC-or PE-conjugated antibodies and analysed using FACScan. The positively sorted αβ T cells, γδ T cells and IgD+ B cells were all > 97% pure. Cells negative for antiγδ-TCR antibody were also separated using MACS. The nonlabelled fractions passed through the column and these negative sorted cells included <0·5% γδ T cells.
Cell culture
All cultures were performed at 37°C in 5% CO2 in RPMI1640 medium supplemented with 10% heat-inactivated calf serum in 96-well round-bottom tissue culture plates. Five 105 PBMNC or cells depleted of γδ T cells were cultured with 5 µg/ml pokeweed mitogen (PWM). In other experiments, 1 × 105 purified sIgD+ B cells were cultured with or without 1 × 105 autologous αβ T cells or γδ T cells, which were prestimulated with biotinylated anti-CD3 antibody plus streptoavidine for 24 h. After 7 days, cells were collected and the proportions of sIgG+, sIgA+, and sIgM+ B cells were analysed using FACScan. IgA, IgM, and IgG levels in culture supernatants were determined by ELISA. For the analysis of surface CD40L expression on activated T cells, purified αβ T cells or γδ T cells were stimulated with 100 ng/ml biotinylated anti-CD3 antibody plus streptavidin for 6 h. Cells were stained with PE-conjugated anti-CD40L mAb (Pharmingen) and analysed using FACScan. The results are shown as values of mean fluorescence intensity (MFI).
TGF-β1 ELISA
For assessment of TGF-β1 production from T cells, 1 × 105 purified αβ T cells and γδ T cells were stimulated with anti-CD3 antibody as described above for 48 h. The contaminated platelets in PBMNC were eliminated during the process of purification of αβ T cells or γδ T cells. The levels of active TGF-β1 in culture supernatants were measured by ELISA (Amersham Pharmacia Biotech, Uppsala, Sweden) according to manufacturer's instructions without any pretreatment.
Standardization of the amount of total cDNA
Total RNA was extracted from purified αβ T cells or γδ T cells using Isogen (Nippon gene, Tokyo, Japan) and was converted to cDNA with Moloney murine leukaemia virus reverse transcriptase (BRL Live Technologies, Gaithersburg, MD) using oligo (dT) primers (BRL). The β-actin cDNA were semiquantified by competitive PCR. Briefly, the internal standard for β-actin, which was about 50 bp shorter than the target sequence, was synthesized as described elsewhere [19]. Varying amounts of internal control were coamplified with a fixed quantity of cDNA using specific primers (sense primer, 5′-CGT GAC ATC AAA GAG AAG CTG TG-3′; antisense primer, GCT CAG GAG GAG CAA TGA TCT TGA-3′). Thirty-five cycles of denaturation (94°C for 45 s), annealing (60°C for 45 s), and elongation (75°C for 1·5 min) were performed in a thermocycler (Perkin-Elmer, Norwalk, CT). PCR products were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining. The point of equivalence in intensity of the competitor and wild-type band was designated the concentration of each cDNA. The amount of total cDNA was standardized according to the results of semiquantification of β-actin cDNA.
Analysis of TCR V gene usage by RT-PCR and sequencing of PCR products
Vγ (2, 3, 4, 8, and 9) and Vδ (1, 2, and 3) gene segments from cDNA obtained from γδ T cells were amplified by PCR with specific Vδ sense and antisense (Cγ and Cδ) primers [20]. PCR was performed for 30 cycles of denaturation (94°C for 45 s), annealing (60°C for 45 s), and elongation (75°C for 1·5 min) in a thermocycler (Perkin-Elmer, Norwalk, CT) in 20 µl of final reaction volume. Five µl of PCR products was separated by electrophoresis on 2% agarose gels, transferred to nylon membrane (Amersham Pharmacia Biotech) by Southern blotting and hybridized at 65°C for 15 h with 2 ng/ml digoxigenin-labelled Cγ-(5′-GGA AAC ATC TGC ATC AAG TTGT-3′) and Cδ-(5′-GAT GGT TTG GTA TGA GGC TGA-3′) specific probes, and visualized by chemiluminscence (Roche Diagnostics, Basel, Switzerland).
To assess the genetic diversity at the V-J junction, PCR products of Vγ9 were cloned and sequenced. The DNA bands separated by electrophoresis were excised from the gels and purified using Prep-A-Gene DNA purification system (Bio-Rad, Hercules, CA). The purified DNA fragments were cloned into the plasmid pBlue-Script SK(–) vector, and the nucleotide sequences were determined using BigDye Terminator Cycle Sequencing kit (PE Applied Biosystems, Foster City, CA) for an ABI PRISM310 sequencer (PE Applied Biosystems). At least 12 clones from each PCR product were sequenced.
Statistical analysis
Differences in the proportion of each cell population, the levels of immunoglobulin, and the MFI values of CD40L among groups were evaluated by the Mann–Whitney U-test. Spearman's rank coefficient was used to evaluate the correlation between the proportion of γδ T cells and that of sIgA+ B cells. All tests were accepted as statistically significant if P < 0·05.
RESULTS
Increased proportion of γδ T cells in PBMNC from IgAN patients
The proportion of γδ T cells in whole T cells in patients with IgAN was significantly higher than that in the controls (Table 1). The absolute numbers of γδ T cells were also higher in IgAN patients (data not shown). In accord with the results of previous studies [5,6,8], serum levels of IgA as well as the proportions of sIgA+ B cells were higher in IgAN patients than in the controls. In IgAN patients, the proportion of γδ T cells showed significant positive correlations with the proportions of sIgA+ B cells (r = 0·572, P < 0·01) and serum IgA levels (r = 0·301, P < 0·05). In contrast, the proportion of γδ T cells was not significantly correlated with that of sIgA+ B cells or serum IgA in the controls. These results suggest that there is a relationship between expanded γδ T cells and increased level of serum IgA.
Table 1.
T cell subpopulations of PBMNC in IgAN patients
| IgAN patients (n = 52) | Control (n = 66) | P-value | |
|---|---|---|---|
| T lymphocyte subpopulation (%) | |||
| TCRγδ | 6·92 ± 5·98 | 4·53 ± 5·14 | <0·01 |
| CD3 | 48·1 ± 17·8 | 53·1 ± 15·5 | n.s. |
| TCRγδ/CD3 | 16·5 ± 9·2 | 9·2 ± 6·2 | <0·01 |
| Vγ9 | 5·8 ± 2·7 | 3·2 ± 1·8 | <0·01 |
| Vγ9/TCRγδ | 82·6 ± 8·9 | 73·0 ± 6·1 | <0·01 |
| Vδ2 | 3·8 ± 1·3 | 3·1 ± 2·3 | n.s. |
| Vδ2/TCRγδ | 59·8 ± 16·9 | 64·0 ± 16·2 | n.s. |
| Serum IgA (mg/ml) | 1·91 ± 1·37 | 1·44 ± 0·86 | <0·01 |
| sIgA+ B cells (%) | 5·92 ± 5·89 | 3·11 ± 3·80 | <0·01 |
PBMNC from IgAN patients (n = 52) and from the controls (n = 66) were stained with FITC-or PE-conjugated anti-CD3, γδ-TCR, Vγ9, and Vδ2 mAb and were analysed by FACScan.
Removal of γδ T cells from PBMNC abrogates increased IgA production in IgAN
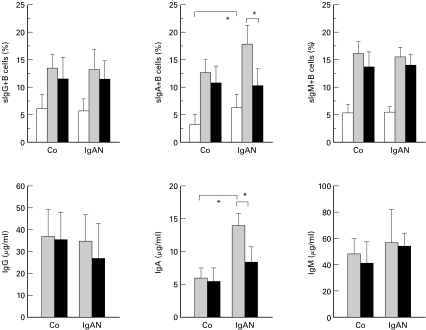
When the PBMNC from IgAN patients were cultured with PWM for seven days, proportions of sIgA+ B cells were significaltly higher than those in PBMNC from the controls (Fig. 1). Corresponding to the increase in the number of sIgA+ B cells, PBMNC from IgAN patients produced much larger amounts of IgA in culture supernatants than did PBMNC from the controls. There were no remarkable differences in the induction of sIgG+ and sIgM+ B cells as well as production of IgG and IgM by PBMNC between IgAN and controls.
Fig. 1.
Depletion of γδ T cells from PBMNC abrogated the increase in both the induction of sIgA on B cells and the production of IgA in IgAN. Whole PBMNC or PBMNC depleted of γδ T cells obtained from IgAN patients (n = 10) and controls (n = 10) were cultured with 5 µg/ml PWM for 7 days. The proportions of B cells expressing each class of Ig were analysed using FACScan. The levels of each class of Ig in culture supernatant were measured by ELISA. □ 0 days;  7 days; ▪ 7 days without γδ T cells.The proportions of sIgA+ B cells after cultivation were higher in PBMNC from IgAN patients than in PBMNC from the controls (*P < 0·01). Depletion of γδ T cells from PBMNC obtained from IgAN patients significantly decreased the induction of sIgA on B cells (*P < 0·01). Similarly, the levels of IgA in culture supernatants of PBMNC from IgAN patients were higher than those in culture supernatants of PBMNC from the controls (*P < 0·01), and they were decreased by the removal of γδ T cells (*P < 0·01).
7 days; ▪ 7 days without γδ T cells.The proportions of sIgA+ B cells after cultivation were higher in PBMNC from IgAN patients than in PBMNC from the controls (*P < 0·01). Depletion of γδ T cells from PBMNC obtained from IgAN patients significantly decreased the induction of sIgA on B cells (*P < 0·01). Similarly, the levels of IgA in culture supernatants of PBMNC from IgAN patients were higher than those in culture supernatants of PBMNC from the controls (*P < 0·01), and they were decreased by the removal of γδ T cells (*P < 0·01).
When γδ T cells were removed from PBMNC and cultured in the same manners as that described above, induction of sIgA+ B cells as well as the consequent IgA production by PBMNC from IgAN patients were significantly lower than those by PBMNC replete with γδ T cells. Unlike in IgAN, the depletion of γδ T cells has little effect on IgA synthesis in healthy individuals.
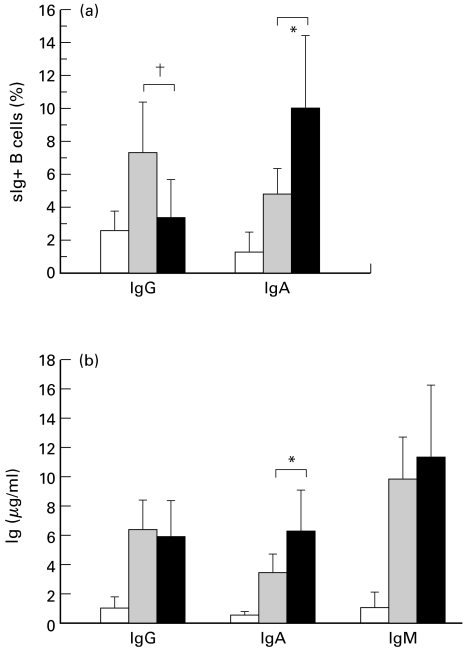
γδ T cells from IgAN patients induce IgA switching on naïve B cells
Purified sIgD+ naïve B cells from IgAN patients were cultured with prestimulated γδ or αβ T cells for 7 days, and the induction of sIgA+ B cells and production of IgA were analysed. Although activated αβ T cells induced the expression of sIgA+ on B cells to some extent, a significantly high proportion of sIgA+ B cells were induced when naïve B cells were cocultured with activated γδ T cells (Fig. 2). Correspondingly, IgA production by B cells was significantly higher in coculture with γδ T cells than with αβ T cells. On the contrary, control γδ T cells did not significantly enhance sIgA expression on naïve B cells and IgA production (data not shown). Unlike γδ T cells, activated αβ T cells of IgAN patients were prone to induce sIgG rather than sIgA on naïve B cells.
Fig. 2.
γδ T cells from IgAN patients could induce IgA switching on naïve IgD+ B cells. Purified γδ T cells and αβ T cells from IgAN patients (n = 10) were stimulated with biotinylated anti-CD3 mAb and streptoavidine for 24 h. Naïve IgD+ B cells were cultured with the prestimulated T cells for 7 days. After cultivation, surface expression of each Ig on B cells was analysed using FACScan, and the levels of each Ig were measured by ELISA. □ B;  B+αβ ▪ B+γδ. The γδ T cells from IgAN patients induced the expression of sIgA on B cells and production of IgA more effectively than did αβ T cells (*P < 0·01), whereas αβ T cells were prone to induce sIgG on B cells (†P < 0·05).
B+αβ ▪ B+γδ. The γδ T cells from IgAN patients induced the expression of sIgA on B cells and production of IgA more effectively than did αβ T cells (*P < 0·01), whereas αβ T cells were prone to induce sIgG on B cells (†P < 0·05).
CD40L expression on γδ T cells from IgAN patients
Since cognate interaction between T and B cells through CD40/CD40L is necessary for IgA switching [21,22], we investigated the expression of CD40L on activated γδ T cells. The levels of CD40L induced on activated γδ T cells were almost same as those on activated αβ T cells. There were no differences between the levels of surface CD40L expression on activated T cells from IgAN patients and the controls.
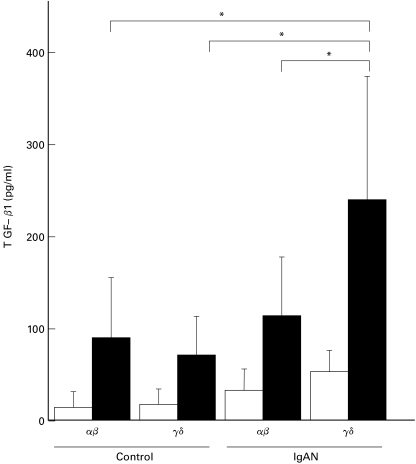
Increased TGF-β1 production by γδ T cells from IgAN patients
In addition to CD40/CD40L interactions, switching to IgA in B cells requires specific cytokines. Since TGF-β is the most potent cytokine that induces IgA class switching on B cells [21,22], we studied TGF-β production by γδ T cells that had been stimulated with anti-CD3 for 48 h (Fig. 3). The levels of TGF-β1 produced by activated γδ T cells from IgAN patients were significantly higher than those by αβ T cells from IgAN patients and by both types of T cells from the controls.
Fig. 3.
Increased TGF-β1 production by γδ T cells from IgAN patients. Purified γδ T cells or αβ T cells from IgAN patients (n = 10) and the controls (n = 10) were stimulated with anti-CD3 antibody and streptoavidine for 48 h. The levels of TGF-β1 in culture supernatants were measured by ELISA. The amount of TGF-β1 produced by γδ T cells from IgAN patients was much larger than that produced by αβ T cells from IgAN patients and the amounts produced by both types of T cells from controls (* P < 0·01). □ without stimulation; ▪ with stimulation.
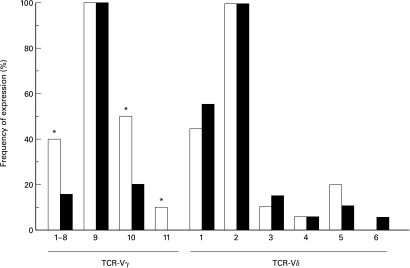
TCR V region repertoire of γδ T cells in IgAN patients
Since we found that γδ T cells from IgAN patients had characteristics different to those of γδ T cells from the controls, we investigated whether there were any differences in the TCR repertoire in IgAN patients and the controls. In most adults, the larger population of peripheral γδ T cells coexpress Vγ9 and Vδ2 gene segments [23]. We therefore analysed the proportions of Vγ9+ and Vδ2+ T cells by flow cytometry using specific mAbs (Table 1). It was found that Vγ9+ T cells were more abundant in IgAN patients than in the controls, whereas the proportion of Vδ2+ T cells in IgAN patients was almost same as that in the controls. To analyse the TCR V region repertoire other than Vγ9 and Vδ2, RT-PCR analyses using specific primers for each V gene segment were performed, and then Southern blot analyses using Cγ-and Cδ-specific probes were performed. As shown in Fig. 4, Vγ9 and Vδ2 gene segments were most frequent in both IgAN patients and the controls. Vγ genes other than Vγ9 were expressed with less frequency in IgAN patients than in the controls, suggesting that there is predominant usage of Vγ9 in IgAN patients. This is in agreement with the results of flow cytometric analysis using mAbs. On the other hand, the Vδ repertoire in IgAN patients was almost the same as that in the controls. In summary, γδ T cells in IgAN patients preferentially expressed Vγ9 among the various Vγ gene segments, but it was not clear what Vδ gene segment was paired to Vγ9 in IgAN patients.
Fig. 4.
TCR-γδ repertoire of PBMNC from IgAN patients. Total RNA was extracted from peripheral blood γδ T cells from IgAN patients (▪; n = 12) and controls (□; n = 12) and reverse-transcribed to cDNA using oligo (dT) primers. The amount of each cDNA sample was standardized according to the results of semiquantitative PCR of β-actin cDNA. Subsequently, each Vγ and Vδ cDNA were amplified by PCR using specific sense and antisense primers. The PCR products were electrophoresed, transferred to nylon membranes, hybridized with digoxigenin-labelled Cγ and Cδ probes, and visualized by chemiluminiscence. The frequency of expression of each V gene product indicates the percentage of subjects with detectable V gene products in Southern blots. Vγ9 and Vδ2 gene segments were predominantly expressed in samples from both IgAN patients and controls, whereas Vγ genes other than Vγ9 were less frequently expressed in samples from IgAN patients than in samples from the controls (*P < 0·05), reflecting preferential usage of Vγ9 in IgAN patients.
To analyse the clonality of increased Vγ9+ T cells in IgAN patients, PCR-amplified cDNA of Vγ9 gene segments were cloned, and the sequences of the junctional region of each Vγ9 clone were determined. Since oligoclonality of γδ T cells appeared with advance of age [24], 24 subjects including 12 IgAN patients and 12 controls less than 13 years of age were analysed. The deduced amino acid sequences showed that all of the Vγ9 clones analysed corresponded to an in-frame rearrangement. The sequences of Vγ9 clones obtained from the controls were variable (data not shown). In contrast, one or two kinds of dominant Vγ9 clones were detected in each IgAN patient (Table 2). Four kinds of these dominant Vγ9 clones were obtained repeatedly from different IgAN patients. In particular, one Vγ9 sequence that was dominant in three patients occupied more than 90% of Vγ9 clones that were obtained from each patient. In summary, expanded Vγ9+ T cells in the peripheral blood of IgAN patients showed oligoclonal or monoclonal pattern.
Table 2.
Junctional sequences of Vγ9 clones from IgAN patients
| Patient | V-region | D-region | J-region | Number of clones | Clones obtained repeatedly from different donors |
|---|---|---|---|---|---|
| 1 | TYYCALWEV | QEDER | ..ELGKKIKVFGPGTKLIIT | 4/12 | *1 |
| 2 | TYYC..... | CLPQG | .QELGKKIKVFGPGTKLIIT | 3/12 | |
| TYYC..... | RILPQG | ..ELGKKIKVFGPGTKLIIT | 2/12 | ||
| 3 | TYYCALWE. | GG | ..ELGKKIKVFGPGTKLIIT | 4/12 | *2 |
| TYYCALWE. | PL | .QELGKKIKVFGPGTKLIIT | 2/12 | ||
| 4 | TYYCAL... | VT | NYYKKLFGSGTTLVVT | 3/12 | *3 |
| TYYCALWE. | GG | ..ELGKKIKVFGPGTKLIIT | 3/12 | *2 | |
| 5 | TYYCALWEV | QEDER | ..ELGKKIKVFGPGTKLIIT | 3/12 | *1 |
| TYYCALWE. | DA | .QELGKKIKVFGPGTKLIIT | 2/12 | ||
| 6 | TYYCAL... | VT | NYYKKLFGSGTTLVVT | 3/12 | *3 |
| TYYCALWE. | DRR | ....KLFGSGTTLVVT | 2/12 | ||
| 7 | TYYCAL... | PSPD | ...KKLFGSGTTLVVT | 12/12 | *4 |
| 8 | TYYCALWE. | GG | ..ELGKKIKVFGPGTKLIIT | 4/12 | *2 |
| TYYCALWEV | QEDER | ..ELGKKIKVFGPGTKLIIT | 3/12 | *1 | |
| 9 | TYYCAL... | PSPD | ...KKLFGSGTTLVVT | 11/12 | *4 |
| 10 | TYYCALWEV | L | ..ELGKKIKVFGPGTKLIIT | 2/12 | |
| TYYCALW. | RT | ..ELGKKIKVFGPGTKLIIT | 2/12 | ||
| 11 | TYYCAL... | PSPD | ...KKLFGSGTTLVVT | 12/12 | *4 |
| 12 | TYYC..... | LQVPQG | ..ELGKKIKVFGPGTKLIIT | 2/12 |
The cDNAs of Vγ9 gene segments were amplified by RT-PCR and cloned and then their sequences were determined. At least 12 clones from each IgAN patient (n = 12) were analysed. The deduced amino acid sequences of the most frequent clones in each IgAN patient are shown. Numbers on the right
1 to *4 indicate the clones that were obtained repeatedly from different donors.
DISCUSSION
In the present study, we found that γδ T cells in IgAN patients are IgA-specific switching cells. Circulating γδ T cells were increased in IgAN patients and the proportion of γδ T cells was significantly correlated with serum IgA level as well as the proportion of sIgA+ B cells. Since sIgA+ B cells are precursors of IgA-secreting plasma cells, it is thought that γδ T cells might participate in the switching to IgA in naïve B cells in IgAN patients. The fact that removal of γδ T cells from PBMNC of IgAN patients diminished the induction of sIgA+ B cells and IgA synthesis supports this speculation. Moreover, γδ T cells from IgAN patients prone to induce IgA switching on naïve B cells rather than IgG as compared with patients' αβ T cells and control γδ T cells, supporting the notion that γδ T cells in IgAN patients are IgA-specific switching cells.
Although previous studies have shown increased numbers of circulating IgA-specific switching cells in IgAN patients [7,9], there are some differences between the characteristics of these cells and those of γδ T cells, which induce IgA switching. In several studies, IgA-specific switching T cells of IgAN patients were found to be positive for CD4 antigen and had receptors for the Fc portion of IgA (FcαRs) on their surfaces. These cells were named Tα4 cells [7,9]. However, FcαRs have not been identified on T cells despite the successful cloning of myeloid FcαR (CD89), which is expressed on phagocytic cells such as granulocytes and monocytes [25,26]. It is possible that FcαRs other than CD89 are expressed on the surfaces of Tα4 cells. Nevertheless, we could not find any detectable IgA binding or CD89 expression on the surfaces of γδ T cells by flow cytometric analysis using anti-IgA mAb and anti-CD89 mAb A59 (data not shown), indicating that γδ T cells in IgAN patients do not have FcαRs on their surfaces. Moreover, most of the γδ T cells of IgAN patients do not express CD4 on their surfaces (data not shown). These results suggest that γδ T cells in IgAN patients do not correspond to Tα4 cells and that IgA-specific switching cells in IgAN patients therefore consist of heterogeneous cell populations.
γδ T cells in IgAN patients produced high levels of TGF-β, the cytokine that was known to induce IgA switching. It is generally accepted that switching to IgA in naïve B cells requires at least two signals. One signal is given by direct interaction between T and B cells via CD40 on B cells and CD40L on activated T cells [21,22]. As for CD40L on γδ T cells, the level of CD40L on activated γδ T cells was comparable to that on αβ T cells from IgAN patients and was comparable to the levels on both types of T cells obtained from the controls. Another important signal that induces IgA switching on naïve B cells is delivered by cytokines that are produced from activated T cells. Among the various cytokines produced from activated T cells, TGF-β1 is the most potent cytokine that stimulates naïve B cells to undergo IgA class switching [21,22]. Our results showed that the amount of active form of TGF-β1 produced by γδ T cells from IgAN patients after stimulation with anti-CD3 was higher than that produced by αβ T cells from IgAN patients as well as that produced by both types of T cells from the controls. Interestingly, PBMNC from IgAN patients have been demonstrated to express more abundant transcripts for TGF-β1 than PBMNC from controls [27], although it was not clear what kind of cells principally produced TGF-β mRNA. In PBMNC of healthy subjects, monocytes and NK cells have been considered as the principal source of TGF-β [26]. Normal T cells can produce only a small amount of TGFβ1 [28] and γδ T cells usually tend to behave as Th1 cells that produce IFN-γ predominantly [29]. However, it has been reported that γδ T cells can produce a large variety of cytokines such as IL-4, IL-6, TNF-α and TGF-β under certain circumstances [30]. Our results showed that activated γδ T cells from IgAN patients could also be a source of TGF-β.
The expanded γδ T cells from IgAN patients exclusively expressed TCR-Vγ9 on their surfaces. CDR3 sequences of Vγ9 of these cells showed oligoclonal and monoclonal patterns. Even in healthy donors, adults often display oligoclonal expansion of γδ T cells [24]. In the present study, we therefore selected subjects less than 13 years of age for analysing clonality of Vγ9+ T cells. In all of the control subjects less than 13 years of age, Vγ9+ T cells showed a polyclonal pattern, whereas the IgAN patients showed both an oligoclonal pattern (in nine of the 12 patients) and a monoclonal pattern (in three of the 12 patients). Clones with the same CDR3 sequences were obtained from different IgAN patients. Notably, monoclonally expanded clones, which were obtained from three patients, had the same CDR3 sequences. These results suggest that expanded Vγ9+ T cells in IgAN patients respond to particular antigens. It has been reported that Vγ9+ T cells proliferated in response to various antigens, including mycobacterium tuberculosis, staphylococcus enterotoxin B, and bacterial heat shock protein (hsp) [31]. It has also been reported that a subset of γδ T cells in IgAN patients could recognize the epitopes of the human hsp65 [32]. Since human hsp shares a high degree of homology with hsp of microorganisms, it is likely that Vγ9+ T cells in IgAN patients proliferate in response to bacterial hsp delivered from mucosal infection by microorganisms. Further studies are needed to clarify the antigens that are recognized by the oligoclonally expanding Vγ9+ T cells in IgAN patients.
It is not clear why the oligoclonal expansion of Vγ9+ T cells, which induce IgA switching, did not result in the synthesis of oligoclonal IgA from B cells. It is known that serum IgA, which is increased in more than 50% of IgAN patients, is polyclonal in IgAN [33]. IgA can react against wide range of antigens including bacteria (Pneumococcus, Streptococcus mutans and Esherichia coli), viruses (herpes simplex, cytomegarovirus and EB virus) [34–38], and food allergens (bovine serum albumin, ovoalbumin, casein and wheat gliadin) [39–42]. If oligoclonal T cells had controlled IgA synthesis, the clonal diversities of IgA-secreting B cells would have been more limited. One possible explanation for this discrepancy is that there are several IgA-specific switching cells in IgAN other than Vγ9+ T cells and therefore the resulting IgA synthesis become polyclonal. Another possible explanation is that there are differences in the manner of antigen-recognition between γδ T cells and conventional αβ T cells [43]. The antigens recognized by γδ T cells do not have to be processed and digested to peptides by antigen-presenting cells, unlike those recognized by αβ T cells. Instead, protein antigens are recognized directly by γδ T cells. Moreover, γδ T cells can recognize nonpeptide antigens such as phosphate-containing nonpeptides. Differences in the properties of antigens recognized by each type of T cells may lead to the difference in the epitopes recognized by B cells and thus the difference in the clonality of antibodies. However, the true reason for the discrepancy remains unclear. Further studies are needed to clarify the roles of γδ T cells in IgA synthesis in IgAN.
REFERENCES
- 1.D'Amico G. The commonest glomerulonephritis in the world. Iga Nephropathy Q J Med. 1987;64:709–27. [PubMed] [Google Scholar]
- 2.Berger J. Recurrence of IgA nephropathy in renal allografts. Am J Kidney Dis. 1988;12:371–2. doi: 10.1016/s0272-6386(88)80027-1. [DOI] [PubMed] [Google Scholar]
- 3.Egido J, Sancho J, Rivera F, et al. The role of IgA and IgG immune complexes in IgA nephropathy. Nephron. 1984;36:52–9. doi: 10.1159/000183115. [DOI] [PubMed] [Google Scholar]
- 4.Egido J, Garcia-Hoyo R, Lozano L, et al. Immunological studies in familial and sporadic IgA nephropathy. Semin Nephrol. 1987;7:311–4. [PubMed] [Google Scholar]
- 5.Emancipator SN, Lamm ME. IgA nephropathy: pathogenesis of the most common form of glomerulonephritis. Lab Invest. 1989;60:168–83. [PubMed] [Google Scholar]
- 6.Nomoto Y, Sakai H, Arimori S. Increase of IgA-bearing lymphocytes in peripheral blood from patients with IgA nephropathy. Am J Clin Pathol. 1979;71:158–60. doi: 10.1093/ajcp/71.2.158. [DOI] [PubMed] [Google Scholar]
- 7.Sakai H, Miyazaki M, Endoh M, et al. Increase of IgA-specific switch T cells in patients with IgA nephropathy. Clin Exp Immunol. 1989;78:378–82. [PMC free article] [PubMed] [Google Scholar]
- 8.Schena FP, Mastrolitti G, Fracasso AR, et al. Increased immunoglobulin-secreting cells in the blood of patients with active idiopathic IgA nephropathy. Clin Nephrol. 1986;26:163–8. [PubMed] [Google Scholar]
- 9.Yasumoto Y, Suga T, Miura M, et al. Subpopulations of T alpha cells in patients with IgA nephropathy: correlation between T alpha 4 cells and in vitro IgA production. Clin Immunol Immunopathol. 1989;51:232–9. doi: 10.1016/0090-1229(89)90022-6. [DOI] [PubMed] [Google Scholar]
- 10.Conley ME, Cooper MD, Michael AF. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980;66:1432–6. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bene MC, De Hurault Ligny B, Kessler M, et al. Confirmation of tonsillar anomalies in IgA nephropathy: a multicenter study. Nephron. 1991;58:425–8. doi: 10.1159/000186474. [DOI] [PubMed] [Google Scholar]
- 12.Egido J, Blasco R, Lozano L, et al. Immunological abnormalities in the tonsils of patients with IgA nephropathy: inversion in the ratio of IgA: IgG bearing lymphocytes and increased polymeric IgA synthesis. Clin Exp Immunol. 1984;57:101–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Whitworth JA, Leibowitz S, Kennedy MC, et al. IgA and glomerular disease. Clin Nephrol. 1976;5:33–6. [PubMed] [Google Scholar]
- 14.Rostoker G, Pech MA, Petit-Phar M, et al. Mucosal immunity in adult primary glomerulonephritis. I Evaluation Salivary Iga Subclasses Components Nephron. 1990;54:42–6. doi: 10.1159/000185808. [DOI] [PubMed] [Google Scholar]
- 15.Rostoker G, Wirquin V, Terzidis H, et al. Mucosal immunity in primary glomerulonephritis. III. Study of intestinal permeability. Nephron. 1993;63:286–90. doi: 10.1159/000187211. [DOI] [PubMed] [Google Scholar]
- 16.Finlayson G, Alexander R, Juncos L, et al. Immunoglobulin A glomerulonephritis: a clinicopathologic study. Lab Invest. 1975;32:140–8. [PubMed] [Google Scholar]
- 17.Fujihashi K, McGhee JR, Kweon MN, et al. gamma/delta T cell-deficient mice have impaired mucosal immunoglobulin A responses. J Exp Med. 1996;183:1929–35. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyabe S, Kuwano Y, Takeda K, et al. IgA nephropathy-specific expression of the IgA Fc receptors (CD89) on blood phagocytic cells. Clin Exp Immunol. 1997;110:226–32. doi: 10.1111/j.1365-2249.1997.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Celi FS, Zenilman ME, Shuldiner AR. A rapid and versatile method to synthesize internal standards for competitive PCR. Nucl Acids Res. 1993;21:1047. doi: 10.1093/nar/21.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Hanlon TP, Messersmith WA, Dalakas MC, et al. Gamma delta T cell receptor gene expression by muscle-infiltrating lymphocytes in the idiopathic inflammatory myopathies. Clin Exp Immunol. 1995;100:519–28. doi: 10.1111/j.1365-2249.1995.tb03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerutti A, Zan H, Schaffer A, et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 22.Zan H, Cerutti A, Dramitinos P, et al. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-beta: evidence for TGF-beta but not IL-10-dependent direct S mu→S alpha and sequential S mu→S gamma, S gamma→S alpha DNA recombination. J Immunol. 1998;161:5217–25. [PMC free article] [PubMed] [Google Scholar]
- 23.Gilfillan S, Benoist C, Mathis D. The human T cell receptor repertoire. In: Bell JI, Owen MJ, Simpson E, editors. T Cell Receptors. Oxford: Oxford University Press; 1995. pp. 111–32. [Google Scholar]
- 24.Giachino C, Granziero L, Modena V, et al. Clonal expansions of V delta 1+ and V delta 2+ cells increase with age and limit the repertoire of human gamma delta T cells. Eur J Immunol. 1994;24:1914–8. doi: 10.1002/eji.1830240830. [DOI] [PubMed] [Google Scholar]
- 25.Maliszewski CR, March CJ, Schoenborn MA, et al. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990;172:1665–72. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteiro RC, Kubagawa H, Cooper MD. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med. 1990;171:597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Caestecker MP, Bottomley M, Telfer BA, et al. Detection of abnormal peripheral blood mononuclear cell cytokine networks in human IgA nephropathy. Kidney Int. 1993;44:1298–308. doi: 10.1038/ki.1993.382. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuka K, Gray JD, Stimmler MM, et al. Decreased production of TGF-beta by lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1998;160:2539–45. [PubMed] [Google Scholar]
- 29.Duhindan N, Farley AJ, Humphreys S, et al. Patterns of lymphokine secretion amongst mouse gamma delta T cell clones. Eur J Immunol. 1997;27:1704–12. doi: 10.1002/eji.1830270717. [DOI] [PubMed] [Google Scholar]
- 30.Lundqvist C, Baranov V, Teglund S, et al. Cytokine profile and ultrastructure of intraepithelial gamma delta T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–12. [PubMed] [Google Scholar]
- 31.Carding SR. Role of gamma delta T cells in immunity to infectious diseases and the regulation of hematolymphoid cell development. Immunol Res. 1998;17:13–22. doi: 10.1007/BF02786426. [DOI] [PubMed] [Google Scholar]
- 32.Warr K, Fortune F, Namie S, et al. T-cell epitopes recognized within the 65, 000 MW hsp in patients with IgA nephropathy. Immunology. 1997;91:399–405. doi: 10.1046/j.1365-2567.1997.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galla JH. IgA nephropathy. Kidney Int. 1995;47:377–87. doi: 10.1038/ki.1995.50. [DOI] [PubMed] [Google Scholar]
- 34.Nagy J, Uj M, Szucs G, et al. Herpes virus antigens and antibodies in kidney biopsies and sera of IgA glomerulonephritic patients. Clin Nephrol. 1984;21:259–62. [PubMed] [Google Scholar]
- 35.Davin JC, Malaise M, Foidart J, et al. Anti-alpha-galactosyl antibodies and immune complexes in children with Henoch-Schonlein purpura or IgA nephropathy. Kidney Int. 1987;31:1132–9. doi: 10.1038/ki.1987.119. [DOI] [PubMed] [Google Scholar]
- 36.Drew PA, Nieuwhof WN, Clarkson AR, et al. Increased concentration of serum IgA antibody to pneumococcal polysaccharides in patients with IgA nephropathy. Clin Exp Immunol. 1987;67:124–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Andre PM, Le Pogamp P, Griffais R, et al. Is Epstein-Barr virus involved in primary IgA nephropathy? Nephron. 1990;54:185–6. doi: 10.1159/000185845. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki S, Nakatomi Y, Sato H, et al. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet. 1994;343:12–6. doi: 10.1016/s0140-6736(94)90875-3. [DOI] [PubMed] [Google Scholar]
- 39.Russell MW, Mestecky J, Julian BA, et al. IgA-associated renal diseases: antibodies to environmental antigens in sera and deposition of immunoglobulins and antigens in glomeruli. J Clin Immunol. 1986;6:74–86. doi: 10.1007/BF00915367. [DOI] [PubMed] [Google Scholar]
- 40.Rostoker G, Laurent J, Andre C, et al. High levels of IgA antigliadin antibodies in patients who have IgA mesangial glomerulonephritis but not coeliac disease. Lancet. 1988;1:356–7. doi: 10.1016/s0140-6736(88)91147-6. [DOI] [PubMed] [Google Scholar]
- 41.Sato M, Kojima H, Takayama K, et al. Glomerular deposition of food antigens in IgA nephropathy. Clin Exp Immunol. 1988;73:295–9. [PMC free article] [PubMed] [Google Scholar]
- 42.Coppo R, Amore A, Roccatello D, et al. IgA antibodies to dietary antigens and lectin-binding IgA in sera from Italian, Australian, and Japanese IgA nephropathy patients. Am J Kidney Dis. 1991;17:480–7. doi: 10.1016/s0272-6386(12)80644-5. [DOI] [PubMed] [Google Scholar]
- 43.Chien YH, Jores R, Crowley MP. Recognition by gamma/delta T cells. Ann Rev Immunol. 1996;14:511–32. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]