Abstract
Phosphatidylcholine (PC) is the major phospholipid of pulmonary surfactant and it is hypothesized that PC and its subspecies modulate the functions of alveolar macrophages. The most abundant of these subspecies is dipalmitoylphosphatidylcholine (DPPC). This study was undertaken to determine the effect of PC on monocyte function using a human monocytic cell line, MonoMac-6 (MM6). This study showed that preincubation of MM6 cells with DPPC at 125 μg/ml for 2 h inhibited the oxidative response to either zymosan or phorbol-12-myristate-13-acetate (PMA) by 30% (P < 0·001). This inhibition with DPPC was independent of LPS priming. When DPPC was replaced with 1-palmitoyl-2-arachidonoyl phosphatidylcholine (PAPC) there was no inhibition and in contrast a significant increase in oxidant production was observed. We also demonstrated that total PC (tPC; a heterogeneous species of PC from egg) and DPPC but not PAPC significantly inhibited the release of TNF-α from MM6 cells (P < 0·05). DPPC did not inhibit phosphorylation of the mitogen activated protein kinases (MAPKs) p44/p42 or p38 in stimulated cells. Measurements of membrane fluidity with spin label EPR spectroscopy indicate that DPPC incorporation significantly alters the membrane fluidity of MM6 cells. These results suggest that DPPC, the major component of pulmonary surfactant, may play a role in modulating leucocyte inflammatory responses in the lung. This may in part be related to membrane effects but does not include alterations in p44/p42 or p38 MAPK signalling.
Keywords: phospholipids, monocyte, respiratory burst, signal transduction, mitogen activated protein kinases (MAPK)
INTRODUCTION
The thin epithelial barrier, which comprises the alveolar surface for gaseous exchange, is uniquely vulnerable to damage related to inflammatory changes. These may result from particulate insults, oxidant gases or damage secondary to infection. The immune response within this region of the lung must carefully balance pro- and anti-inflammatory responses without compromising host defences. It is well established that surfactant plays a major role in preventing lung collapse at the end of expiration, however, it has been suggested that the lipid component within surfactant may have a role in the regulation of lymphocyte function [1]. There is evidence indicating pulmonary surfactant lipids and surfactant proteins (SP-A, B, C and D) alter the bactericidal activity of the alveolar macrophage [2–4]. Recent reports suggest surfactant proteins (SP-A and SP-D) regulate a variety of immune cell functions in vitro including enhanced chemotaxis and phagocytosis and alterations in the production of free radicals and cytokines [5,6].
Phospholipids are the major component of surfactant, accounting for approximately 90% composition by weight [7]. The most abundant phospholipid in pulmonary surfactant is phosphatidylcholine (PC) comprising approximately 70% (by weight) of the total phospholipids which is mainly in the form of a diacyl species dipalmitoylphosphatidylcholine (DPPC) [8]. The immunomodulatory role of DPPC within the lung is not fully elucidated but a number of studies have indicated its importance with respect to inflammatory cell function as opposed to its primary role in reducing surface tension [9,10]. An early study by Juers, provided evidence that alveolar-lining material could enhance bactericidal killing and this was specifically attributed to its lipid content [11]. Subsequent studies have provided conflicting reports on the bactericidal modulating activity of surfactant and surfactant components on phagocytic cells, possibly due to differences in experimental designs [9,12]. Phagocyte oxidative responses as well as production of other inflammatory mediators are altered in ARDS and may be related to changes in surfactant or surfactant phospholipid composition [13].
The inhibitory effect of pulmonary surfactant on ROI generation in neutrophils and monocytes has been attributed to alterations in NADPH assembly [10,14,15]. It has also been shown that mitogen activated protein kinases (MAPK) may participate in the regulation of oxidant production by phosphorylation of p47phox, a component of the NADPH oxidase [16,17]. The mechanisms of respiratory burst inhibition by surfactant lipids have not been investigated in terms of signalling pathways. Three MAPK signal pathways have been identified in mammals including extracellular signal-regulated kinases (ERK) p44/p42, c-Jun NH2-terminal kinase and p38 MAPK [18–20]. Activation of p44/p42 and p38 MAPKs have shown to be involved in respiratory burst activation by opsonized zymosan [21].
Therefore this study examines the possible regulatory effects of PC (including total tPC, a heterogeneous sample of PC subspecies in which DPPC accounts for approximately 33%) and its subspecies on the respiratory burst and tumour necrosis factor alpha (TNF-α) release in the human monocytic cell line MonoMac6 (MM6). In addition this study seeks to identify the regulatory properties that DPPC may have on ERK p44/p42 or p38 MAPK activity.
MATERIALS AND METHODS
Materials
L-α-phosphatidylcholine (TPC; type XVI-E from egg yolk), l-α-phosphatidylethanolamine, l-α-phosphatidylcholine dipalmitoyl (DPPC), l-α-phosphatidylcholine β-arachidonoyl-γ-palmitoyl (PAPC), sphingomeylin, lyso-phosphatidylcholine, lipopolysaccharide (Escherichia coli O111:B4), phenylmethylsulphonyl fluoride, sodium orthovanadate, deoxycholate and tergitol NP-40 were purchased from Sigma Chemical Co. (Dorset, UK). chloroform, methanol and ammonium hydroxide were purchased from BDH (Dorset, UK). silica gel column was obtained from Jones Chromatography (Hengoed, UK)
Cell culture
The human monocytic cell line MonoMac-6 (MM6) was obtained from the German collection of microorganisms and cell cultures (DSM; Braunschweig, Germany). MM6 cells were maintained in RPMI 1640 medium without l-glutamine (Sigma, UK). RPMI was supplemented with 1% bovine insulin, 10% heat inactivated foetal bovine serum (FBS), 1% 2 mm l-glutamine, 1% nonessential amino acids, 1% penicillin (50 IU/ml)/streptomycin (100 μg/ml) and 1% sodium pyruvate (purchased from Gibco, Paisley, UK) at 37°C in 5% CO2 humidified atmosphere. Cells were subcultured every 3 days at a density of 0·4 × 106 cells/ml. Unless otherwise stated, prior to experimentation MM6 were ‘weaned’ onto a serum free medium, Ultraculture (supplemented as for RPMI 1640 media without FBS) as indicated by the manufacturer BioWhittaker UK Ltd, Wokingham, UK.
Isolation of human monocytes from peripheral blood was performed by density centrifugation over Ficoll Paque® and negative selection of monocytes using midiMACS (Miltenyi Biotec, Camberley, U.K.). Briefly, EDTA treated whole blood from healthy donors was diluted with 2–4 volumes of PBS. The diluted cell suspension was layered over Ficoll Paque® (1·077 density) and centrifuged at 400 × g for 30–40 min at 20°C. The interface cells (lymphocytes, monocytes and thrombocytes) were carefully aspirated, washed and resuspended in PBS containing 2 mm EDTA. The cells were indirectly magnetically labelled with a cocktail of hapten-conjugated CD3, CD7, CD19, CD45RA, CD56 and anti-IgE antibodies and MACS MicroBeads coupled to an antihapten monoclonal antibody. The magnetically labelled T-cells, NK cells, B cells, dendritic cells and basophils were retained on a MACS column in the magnetic field of the midiMACS. The isolated monocytes were resuspended at 1 × 106 cells/ml in supplemented RPMI medium and used immediately.
Viability of cells was assessed by trypan blue dye exclusion and the CellTiter AQueous-one solution proliferation assay (Promega, Southampton, UK). The viability of MM6 cells exposed to LPS and/or lipid was greater than 90% in all experiments.
Preparation of lipid media
The physiological concentrations of PC within the lung have been previously estimated to be between 100 and 350 μg/ml [2,22]. The concentrations of phospholipids utilized were approximately those encountered by alveolar macrophages within the lung. The desired amounts of lipids dissolved in chloroform (CHCl3) were dried as a thin film under nitrogen on ice in acid washed bijoux tubes treated with RepelCote (BDH, Dorset, UK) and stored in the dark at −70°C until required. Lipid preparations were hydrated in supplemented medium and sonicated on ice to minimize potential oxidation and breakdown of the lipid.
Preparation of stimulants
An opsonized zymosan (OpZ) suspension was prepared according to a method as previously reported by Allen, 1986 [23]. OpZ suspensions (2·5 mg/ml) were prepared in advance and stored frozen at −70°C. Phorbol 12-myristate 13-acetate (PMA; Sigma, UK) was dissolved in DMSO at a stock concentration of 1 mg/ml and diluted in RPMI 1640 to give a final working concentration of 100 ng/ml
Chemiluminescence
MM6 cells prepared at a density of 1 × 106/ml were preincubated with tPC, DPPC or PAPC at concentrations of 500, 250, 125, 63 and 2 μg/ml for 2 h at 37°C in 5% CO2 atmosphere. Cells were washed in PBS (×3) and resuspended in ultraculture, primed for 18 h with LPS (100 ng/ml). Luminol enhanced chemiluminescence (LCL) according to the method of Allen, 1986 [23] with minor modifications was used to quantify the release of ROIs. LCL was detected at room temperature using a standard luminometer. Experiments were performed in quadruplicate and each experiment was repeated three times. Briefly, following treatment, the cells were washed twice with phosphate buffered saline (PBS) and resuspended in standard buffer (4·58 mm KH2PO4, 8·03 mm Na2HPO4, 0·76% NaCl, 0·033% KCl 0·033, 0·1% glucose, 0·1% endotoxin free albumin 0·1, 0·5 mm MgCl2, and 0·45 mm CaCl2, pH 7·3) at 2·0 × 106 cells/ml. To every ml of cell suspension 100 ml of a 3·5-mm solution of luminol (Sigma, UK) was added. Following gentle mixing and a 10 minute adaptation period in the dark, 150 μl cell/luminol suspension were transferred to a 96 well plate (FluoroNunc-PolySorb, Gibco, UK). LCL was initiated by the addition of OpZ (125 μg/ml) or PMA (100 ng/ml). Temporal traces of evolving chemiluminescent reactions were recorded every 5 min for 60 min or until it demonstrated a definite decline. There was no appreciable light emission in this system in the absence of cells or stimulants. LCL results are expressed as relative light units (RLUs) or:
 |
Time course study of PC effects on LCL
In order to determine the effects of a period of incubation on productions of ROIs, preincubation of cells with lipids was performed using the optimal dose of lipid as determined by the previous experiment. MM6 cells were preincubated with the PC species for 0–18 h at 37°C in a 5% CO2 atmosphere.
Effect of DPPC on ROI production by human peripheral blood monocytes
To extend the effects seen with DPPC on a continuous cell line, ROI production was assessed in isolated human peripheral blood monocytes by the oxidation of 2′7′-dichlorofluoroscein diacetate (DCFH-DA) to the highly fluorescent 2′7′-dichlorofluoroscein (DCF) by ROIs. ROI production was measured using FACS analysis as basal levels of isolated monocytes were elevated using the luminol chemiluminescent technique. Briefly, isolated human peripheral blood monocytes (1 ×106/ml) were preincubated with DPPC (125 μg/ml) for 2 h at 37°C in 5% CO2 atmosphere. Cells were washed in PBS (×3) and resuspended in supplemented RPMI media at (2 × 106/ml). DCFH-DA (100 μm) was preincubated with the cells for 15 min at 37°C followed by stimulation with PMA (100 ng/ml) for 15 min at 37°C. Fluorescence (FL1) from single cells were collected using a logarithmic amplifier after gating on the combination of forward light scatter and perpendicular light scatter. A total of 10 000 cells were analysed per tube and data was acquired and analysed using Cellquest (Becton Dickinson, U.K.). The fluorescence distribution was analysed and displayed as a single histogram. Controls were monocytes incubated without lipids and/or DCFH-DA or PMA.
Control experiments
Two control experiments were carried out. The first to determine whether any observed oxidative response was independent of priming with LPS (100 ng/ml). MM6 cells prepared at a density of 1 × 106/ml were primed as above followed by washing in PBS (×3) and incubation with DPPC (125 μg/ml) for 2 h at 37°C in 5% CO2 atmosphere. Cells were washed and resuspended in standard buffer and chemiluminescence assays performed. The second experiment was to determine whether phospholipids interfered directly with the chemiluminescence assay. MM6 cells prepared at a density of 1 × 106/ml were primed as described above, followed by washing in PBS (×3). Cells were resuspended in standard buffer containing appropriate lipids and chemiluminescent assays were performed immediately.
Measurement of TNF-α release from MM6
MM6 cells prepared at a concentration of 1 × 106/ml, were preincubated with the same concentrations of PC subspecies and at the optimal preincubation time previously used. Following lipid preincubation, MM6 cells were stimulated with LPS (200 ng/ml) and PMA (100 ng/ml) for 4 h at 37°C in 5% CO2 atmosphere. Previous experiments have shown that PMA alone at this concentration does not stimulate TNF-α production from MM6 cells [24]. TNF-α in MM6 culture supernatants were quantified by a commercially available ELISA kit (R & D, Minneapolis, USA) according to the manufacturer's instruction.
Quantification of uptake of phospholipids into MM6 membrane by HPLC
MM6 cells prepared at a density of 1 × 106/ml were pretreated with 250 μg/ml DPPC for 2 h at 37°C in a 5% CO2 atmosphere. Cells were washed in PBS (×3) and membrane phospholipids were extracted using a modification to the method of Bligh and Dyer, 1959 [25]. The separation and quantification of membrane phospholipids was determined by HPLC on a silica gel column with a mobile phase consisting of chloroform, methanol and ammonium hydroxide. The uptake of DPPC into MM6 membranes was detected by a light scattering evaporating detector as described previously [26]. A standard phospholipid mixture containing DPPC, PC, sphingomyelin and phosphatidylethanolamine over a range of 5–200 μg/ml were used to obtain a standard curve. This was calculated by regressing the peak areas to obtain the best-fit quadratic line. The quantity of DPPC in MM6 cells after treatment was compared to the mean obtained from untreated cells and reported as a mean percentage increase by weight.
Measurement of membrane fluidity by EPR spectroscopy
MM6 cells at a density of 1 × 106/ml were preincubated with 125 or 250 μg/ml of DPPC for 2 h. After washing (×3) in PBS, the cells were resuspended in PBS at 5 × 106 cells/ml to which 10 μl of 20 mg/ml doxyl stearate in ethanol was added. The concentration of ethanol in the final cell suspension was less than 1%. The cells were incubated for 15 min at room temperature followed by washing in PBS (×3) to remove free spin label. Cells were re-suspended in 100 ml of PBS and membrane fluidity was measured by electron paramagnetic resonance (EPR) spectroscopy as previously described by Darmani, 1993 [27]. The order parameter (S) was calculated from the relationship, S = (A// −A⊥)/25G where A// and A⊥ are the maximal and minimal coupling constants, respectively, obtained from the EPR spectra of the spin labelled cells [27].
Western blot analysis of p44/p42 or p38 MAP kinase
The phosphorylated forms of the P44 (ERK1)/P42 (ERK 2) or P38 MAPK proteins were analysed by a commercially available kit (phosphoplus P44/P42 MAPK or phospho P38 Antibody kit; New England Biolabs UK Ltd, Hertfordshire, UK). Analysis of these MAPKS was performed according to the manufacturer's instructions. MM6 (1 × 106 cells/ml) were preincubated with or without 500, 100 or 10 μg/ml of DPPC for 2 h. Cells were washed in PBS (×3) and primed with or without LPS (100 ng/ml) for 18 h. Cells were stimulated with 100 ng/ml of PMA for 10 min or OpZ for 60 min. These times were determined as a result of experiments to ascertain the optimal conditions for MAPK expression (data not shown). Following stimulation with PMA or OpZ the cells were washed twice in ice cold PBS and lysed in lysing buffer (containing 100 μl of 100 mm NaCl, 10 mm tris-HCL (pH 7·2), 2 mm EDTA, 0·5% (w/v) deoxycholate, 1% (v/v) NP40, 10 mm MgCl2, 1 mm phenylsulphonyl fluoride and 100 mm sodium orthovanadate) for 15 min on ice, followed by sonication (Soniprep, Sanyo, Watford, UK) for 10s on ice to reduce sample viscosity. Cells were centrifuged at 13 500 ×g. For 5 min at 4°C and the lysate supernatant fraction was stored at −70°C. Protein concentration of the soluble cytosolic extract was estimated using a modified lowry method (Bio-rad, California, USA). Protein concentration of sample lysate supernatants were adjusted to 5 mg/ml with LDS sample buffer and heated for 10 min at 70°C. Samples were then loaded onto precast 10% SDS PAGE gels (Novex, Groningen, Netherlands) and electrophoresed at 200 V for 50 min. Gels were electroblotted on nitrocellulose membranes (Amersham, UK) and washed in washing buffer (Tris-buffered saline with 0·1% Tween-20). To prevent non-specific protein binding, the membrane was incubated in blocking buffer (Tris-buffered saline, 5% w/v non-fat dry milk and 0·1% Tween-20) for 1 h at room temperature. The appropriate phosphorylated anti-MAPK antibody was incubated with the membrane overnight at 4°C. The membrane was then rinsed with wash buffer (3 × 5 min). Detection of MAPK was achieved by incubating the membrane with horseradish peroxidase (HRP)-conjugated anti-mouse antibody (1:2000 dilution) for one hour at room temperature. The membrane was rinsed (3 × 5 min) in wash buffer. Visualization of antibody complex was achieved by enhanced chemiluminescence (ECL) LumiGLO® (1 minute, room temperature) and subsequent exposure on hyperfilm (Amersham, UK). MAPKS were identified by comparison with pre-stained molecular weight markers and MAPK control proteins.
Statistics
For multiple group comparisons, the data were subjected to one way analysis of variance (anova) to determine overall difference between the group means and Tukey's honestly significant difference (HSD) for pairwise differences for within group comparisons. Differences in medians were analysed by Mann–Whitney. Minitab software version 12·0 (Minitab Inc.) was used for all analyses.
RESULTS
Effect of phosphatidylcholine tPC, DPPC and PAPC on luminol enhanced chemiluminescence (LCL)
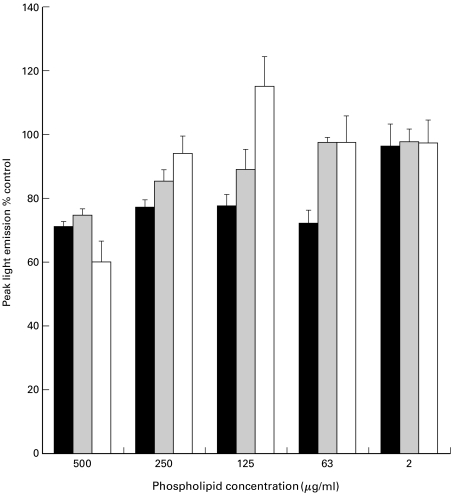
Control experiments showed that lipid preparations did not interfere with the chemiluminescent assay under these conditions (data not shown). LCL generation peaked at approximately 55 min after stimulating cells with OpZ. PC exerted no significant effect on the time to peak responses when compared to the primed MM6 control. However, the luminescence generated from MM6 pretreated in this manner with tPC, DPPC and PAPC exhibited significantly (P < 0·0001) different LCL than untreated MM6 primed with LPS. The modulation of peak light emitted was found to be dose dependent in MM6 preincubated with tPC, DPPC and PAPC (Fig. 1). Further analyses of this data using Tukey's pairwise comparison, demonstrated a significant difference (P < 0·001) amongst the means as the concentration of the phospholipid increased to 63, 125 and 250 μg/ml. There were significant differences between tPC and PAPC when compared to DPPC (P < 0·01) again when using Tukey's pairwise comparisons. Using PMA as a stimulant, preincubation of each PC subspecies at 125 μg/ml for 2 h gave a significant reduction in the release of ROIs as determined by LCL (P < 0·0001 using anova) (Fig. 2). Tukey's indicated that DPPC was responsible for the significant decrease in LCL in this part of the study.
Fig. 1.
Dose response of phospholipid treatment on respiratory burst activity of MM6 cells primed with LPS and stimulated with OpZ. Results are expressed as mean (± SD) of 3 separate experiments. Control values (100%) are MM6 cells incubated in the absence of lipid before priming with LPS. ▪ DPPC;  tPC; □ PAPC.
tPC; □ PAPC.
Fig. 2.
Effect of phospholipid treatment on respiratory burst activity in MM6 cells primed with LPS and stimulated with PMA. Results are expressed as mean (± SD) of 3 separate experiments. Control value represents MM6 cells incubated in the absence of lipid before priming with LPS. All phospholipids were used at a concentration of 125 μg/ml. *P < 0·001 analysed by anova.
Time course of phosphatidylcholine effects on LCL
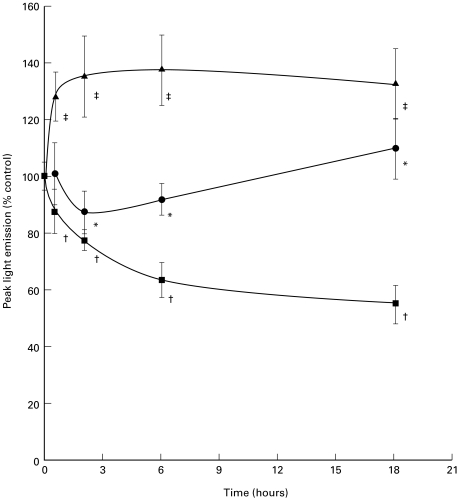
Using opsonized zymosan as stimulus (125 μg/ml), peak light emission of MM6 cells treated with tPC and DPPC for 2, 6 and 18 h was significantly inhibited (P < 0·01 and P < 0·001, respectively) from that of untreated MM6 (Fig. 3) when analysed by Mann–Whitney. However, 125 μg/ml PAPC significantly enhanced LCL (P < 0·001) at 30 min, 2, 6 and 18 h again when comparing the medians against control.
Fig. 3.
Time course of phospholipid treatment on respiratory burst activity in MM6 cells primed with LPS and stimulated with OpZ for production of ROIs. Results are expressed as mean (± SD) of 3 separate experiments. Control values (100%) are MM6 cells incubated in the absence of lipid before priming with LPS. • tPC; ▪ DPPC; ▴ PAPC. *P < 0·01, †P < 0·001, ‡P < 0·001 analysed by Mann–Whitney.
Control experiments
Experiments were performed to ascertain if the DPPC was affecting the LPS priming process or the stimulation of the cells with PMA or OpZ. Incubation of MM6 cells with DPPC (125 μg/ml) after they were primed with LPS gave significant inhibition of LCL (P < 0·005). This suggests that the DPPC was not affecting the priming process. In addition incorporation of phospholipids into the chemiluminescence buffer had no direct effect on the subsequent LCL from MM6 cells stimulated with PMA or OpZ. This showed that DPPC does not inhibit or quench the LCL once it has been produced.
Effect of DPPC on ROI production in isolated human peripheral blood monocytes
DCF-DA treated human peripheral blood monocytes were stimulated with PMA to induce ROI production and analysed by FACS for the fluorescent oxidized DCF product. Treatment of these cells with DPPC (125 μg/ml) inhibited the ROI response by approximately 30% (P < 0·05 analysed by Mann–Whitney) (data not shown). This is a similar inhibition to that obtained from chemiluminescence assays in which DPPC inhibited the LCL response of the human monocytic cell line MM6.
Secretion of TNF-α in MM6 cells precultured with PC and its subspecies
Total PC and DPPC significantly reduced TNF-α release by MM6 when compared to controls (P < 0·05). A significant reduction of TNF-α release was seen after preincubation with DPPC and tPC at a concentration of 125 and 250 μg/ml (Table 1). No significant reduction was seen in PAPC treated cells at any of the concentrations tested.
Table 1.
Dose dependent suppression of TNF-α release by LPS stimulated MM6 cells exposed to a range of concentrations of PC*
| TNF-α release (pg / ml) | |||
|---|---|---|---|
| Concentration of lipid (μg / ml) | TPC | DPPC | PAPC |
| Control (no lipid) | 532 ± 56 | 527 ± 32 | 545 ± 64 |
| 32 | 446 ± 51 | 483 ± 53 | – |
| 63 | 450 ± 103 | 469 ± 33 | 548 ± 134 |
| 125 | 395 ± 56† | 373 ± 70† | 542 ± 77 |
| 250 | 371 ± 58† | 382 ± 22† | 574 ± 89 |
| 500 | 446 ± 64 | 363 ± 106 | 576 ± 108 |
Data shown represents mean (± SD) for 3 experiments performed in triplicate.
P < 0.05 by anova with Tukey's multiple comparison method (n = 3).
Uptake of DPPC by MM6 cells and effects on membrane fluidity
HPLC analysis of MM6 cell extracts demonstrated that total membrane DPPC was increased by 30 ± 2·8% by weight (n = 3) after 2 h incubation with 250 μg/ml of DPPC (results not shown). No measurable increase in any other quantified phospholipids was seen. EPR spectroscopy showed that the order parameter (S) increased from 0·655 in untreated cells to 0·783 and 0·787 in cells preincubated for 2 h with 125 and 250 μg/ml DPPC, respectively (n = 3). This represents a significant decrease in membrane fluidity (results not shown).
The effect of DPPC on p44 (ERK 1)/p42 (ERK 2) or p38 MAPK activity
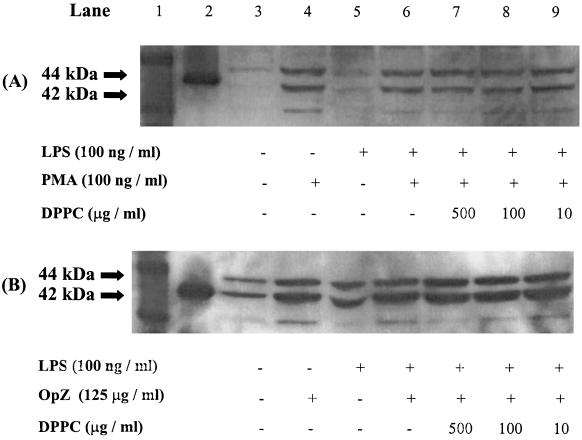
The preincubation times shown to affect LCL and TNF-α release were used to study MAPK signalling in DPPC treated cells. There was no observable effect of 10, 100 or 500 μg/ml of DPPC on p44 (ERK 1)/p42 (ERK 2) phosphorylation (Fig. 4) when primed with LPS and stimulated with PMA or OpZ. No observable effect of DPPC on p38 phosphorylation was seen using similar experimental conditions (Fig. 5). Similar results were obtained in cells that were not primed with LPS. Cells not stimulated with PMA or OpZ had little or no activated MAPK.
Fig. 4.
Immunoblot of p44/p42 MAPkinase activity. A, PMA stimulated MM6 cells; B, OpZ stimulated MM6 cells. Data shown are representative of three independent experiments. Lane 1, molecular weight markers; 2, p42 control protein.
Fig. 5.
Immunoblot of p38 MAPkinase activity. A, PMA stimulated MM6 cells; B, OpZ stimulated MM6 cells. Data shown are representative of three independent experiments. Lane 1, molecular weight markers; 2, p38 control protein.
DISCUSSION
This study provides evidence of the ability of the phospholipid PC to modulate the release of ROIs in a monocytic cell line and in isolated human peripheral blood monocytes. In addition, we have demonstrated that tPC, DPPC, but not PAPC reduce TNF-α release from MM6 cells. The data presented also provides new insights on the mechanisms by which DPPC down regulates inflammatory responses that may be associated with the fatty acid component of PC. Additionally, we have shown that DPPC is incorporated into the cell membrane and causes significant alterations in membrane fluidity.
This study utilized the human monocytic cell line MM6. This is a well characterized cell line which displays many characteristics of mature peripheral blood monocytes and macrophages. Monocytes, the circulating precursors of macrophages play an essential role in maintaining the sterility of tissues by the production of a range of cytokines, including TNF-α, and the release of ROIs and vasoactive lipids. The microenvironment in which monocytes mature into macrophages may be important in determining their functional characteristics. A recent study has shown that alveolar macrophages have a greater capacity for leukotriene synthesis than peripheral blood monocytes. This elevated capacity only occurs after entry into the alveolar space and declines over time when cells are cultured outside this environment [28]. Pulmonary surfactant is a complex mixture of phospholipids, neutral lipids and cholesterol and four genetically distinct surfactant specific proteins [6]. The major surfactant phospholipid is PC of which 90% of the disaturated species within the lung is dipalmitoyl [29]. The concentrations of phospholipids used in this study approximate to the levels known to occur in pulmonary surfactant [2,22].
Activation of the respiratory burst can occur in response to both particulate and soluble agents. The mechanism of signal transduction however, varies according to the type of agent used. The phorbol ester PMA, an analogue of the intracellular messenger diacylglycerol (DAG) activates the respiratory burst through activation of protein kinase C (PKC) [30]. In many cells, activation of PKC leads to activation of MAPK [20,31]. The yeast cell wall extracts zymosan, is a particulate and activates the respiratory burst via receptor–mediated interactions. It has been reported that in rat alveolar macrophages, opsonized zymosan stimulates tyrosine phosphorylation and activation of ERK and p38 MAPK pathways [21]. MM6 cells can respond to an activating stimulus with a greater capacity for ROI production if they are first primed with an agonist (LPS), by a mechanism that is not fully elucidated. It has been suggested that the priming process may involve both p44/p42 and p38 MAPKs [32]. The production of ROIs was impaired in MM6 cells that were exposed to tPC, DPPC and PAPC between a concentration range of 0–500 μg/ml and stimulated to release ROIs with OpZ. These effects were seen within 30 min incubation in the presence of both DPPC and PAPC but only after 2 h incubation in the presence of tPC. In addition PAPC demonstrates an ability to promote ROI production only at a concentration of 125 μg/ml. This was not observed with tPC and DPPC treatment. Using PMA as a stimulant of oxidative activity, we observed that only DPPC is capable of inhibiting the respiratory burst. These experiments were extended to isolated human peripheral blood monocytes where a similar reduction in ROI production by DPPC treated cells was seen. Interestingly, this appears to be independent of cell priming with LPS as significant inhibition was seen in MM6 cells primed with LPS prior to incubation with DPPC. These effects are directly related to the phospholipid interaction with MM6 cells since control experiments performed with the same lipid preparations did not interfere with the chemiluminescence assay itself. Cytotoxicity studies confirmed that the viability of MM6 cells exposed to lipid was greater than 90% at all concentrations of lipids used.
We have demonstrated in this study that tPC and DPPC significantly reduce TNF-α release from MM6 cells after 2 h preincubation with these lipids. However, PAPC was not seen to affect TNF-α release in the same way as DPPC. The maximum reduction in oxidative metabolism and TNF-α release was seen after incubation with DPPC for 2 h in the concentration range of 125–250 μg/ml. Quantification of DPPC by HPLC in MM6 cells indicated that there was an increase of DPPC uptake by MM6 cells following preincubation with this lipid. This increase suggests cellular uptake of this particular phospholipid during incubation. Coupled with this, EPR spectroscopy from spin-labelled MM6 cells showed an increase in order parameter, suggesting a significant change in membrane composition associated with preincubation with DPPC. These membrane changes may be associated with incorporation of saturated phospholipids into the membrane. A primary effect of changing the acyl chain saturation is to change the phase transition of the lipid [33]. Functional consequences of lipid heterogeneity have started to emerge such as the nonrandom mixing of lipids and phospholipids in the membrane bilayer leading to the formation of lipid microdomains [34]. These microdomains are thought to be involved in numerous signalling events associated with the cell membrane [35]. Ordered domains within the membrane are normally associated with saturated lipids, increased amounts of saturated lipids may cause perturbations in these domains and consequently affect such responses. It has been reported that surfactant inhibits the assembly of cytoplasmic and membraneous components of the NADPH oxidase into a functional enzyme [14]. The mechanism of surfactant inhibition of NADPH oxidase assembly is unknown.
An interesting finding was that these membrane changes did not appear to affect the major signalling pathways associated with the MAPKs p44 (ERK 1)/p42 (ERK 2). In this study DPPC did not alter the expression of these doubly phosphorylated MAPKs in MM6 cells primed with LPS and stimulated with PMA or OpZ. This effect was also seen with p38 MAPK. This suggests that DPPC inhibition of ROI generation and TNF-α release is not related to such signalling events.
Previous studies have used a number of different animal models and methodologies for estimating superoxide and ROI production, this may be responsible for conflicting reports [2,4,36]. We have shown that the dose of phospholipid and time of incubation has a critical effect on oxidative functions, e.g. PAPC has a stimulatory or inhibitory role on the respiratory burst that depends on lipid dose and time of incubation. Webb and Jeska [12] found an increase in the monocyte oxidative response when incubated with alveolar lining material and provided evidence that unsaturated lipids were responsible. The results of our study indicate that the different modulatory properties exerted by the phospholipids used in ROI production and TNF-α release after LPS stimulation appears to be selective. The only difference between DPPC and PAPC is in the esterified fatty acid at the sn-2 position of PC, it would appear that these modulations might be associated with this substitution.
Although DPPC reduced ROI production and TNF-α release, it did not inhibit phosphorylation of the MAPKs in MM6 cells stimulated with LPS and OpZ or PMA. However, preliminary membrane fluidity studies indicate that DPPC incorporation significantly altered the membrane of MM6 cells. These membrane alterations may affect the release of TNF-α from the interior of the cell, however, Baur et al. [37] have shown that DPPC pretreatment of monocytes results in a decrease in TNF-α mRNA production. Such changes may also affect enzyme systems associated with the membrane in particular NADPH oxidase, the enzyme responsible for the production of ROIs. Further studies are required to determine whether this is indeed the case.
In summary, this study has shown that DPPC, the major phospholipid species in pulmonary surfactant, can down-regulate oxidative functions in monocytes and may therefore act as an immunomodulator for leucocyte inflammatory responses in the lungs. Although observed reductions in release of inflammatory products were not complete, a 20–40% reduction in the release of these mediators would offer a considerable protection against inflammatory damage in the delicate alveolar region of the lung. Indeed, complete inhibition of such responses would be deleterious to the individual as macrophage mediated responses are the central immune defence mechanism in this region of the respiratory tract.
Acknowledgments
This study was supported in part by a grant from the Wales Office of Research and Development in Health and Social Sciences.
REFERENCES
- 1.Wilsher ML, Hughes DA, Haslam PL. Immunoregulatory properties of pulmonary surfactant: effect of lung lining fluid on proliferation of human blood lymphocytes. Thorax. 1988;43:354–9. doi: 10.1136/thx.43.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayakawa H, Myrvik QN, St. Clair RW. Pulmonary surfactant inhibits priming of rabbit alveolar macrophage. Evidence that surfactant suppresses the oxidative burst of alveolar macrophage in infant rabbits. Am Rev Respir Dis. 1989;140:1390–7. doi: 10.1164/ajrccm/140.5.1390. [DOI] [PubMed] [Google Scholar]
- 3.Milleron B, Costabel U, Teschler H, et al. Bronchoalveolar lavage cell data in alveolar proteinosis. Am Rev Respir Dis. 1991;144:1330–2. doi: 10.1164/ajrccm/144.6.1330. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa H, Giridhar G, Myrvik QN, Kucera L. Pulmonary surfactant phospholipids modulate priming of rabbit alveolar macrophages for oxidative responses. J Leukoc Biol. 1992;51:379–85. doi: 10.1002/jlb.51.4.379. [DOI] [PubMed] [Google Scholar]
- 5.Weissbach S, Neuendank A, Pettersson M, Schaberg T, Pison M. Surfactant protein A modulates release of reactive oxygen species from alveolar macrophages. Am J Physiol-Lung Cellular Mol Physiol. 1994;267:L660–L666. doi: 10.1152/ajplung.1994.267.6.L660. [DOI] [PubMed] [Google Scholar]
- 6.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–62. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 7.Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987;136:426–44. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- 8.Ansell GB, Hawthorne JN, Dawson RMC. Form and Function of Phospholipids. Amsterdam: Elsevier Scientific Publishing Co; [Google Scholar]
- 9.Speer C, Götze B, Curstedt T, Robertson B. Phagocytic functions and tumor necrosis factor secretion of human monocytes exposed to natural porcine surfactant (Curosurf) Pediatr Res. 1991;30:69–75. doi: 10.1203/00006450-199107000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Chao W, Spragg RG, Smith RM. Inhibitory effect of porcine surfactant on the respiratory burst oxidase in human neutrophils. Attenuation of p47phox and p67phox membrane translocation as the mechanism. J Clin Invest. 1995;96:2654–60. doi: 10.1172/JCI118331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juers JA, Rogers RM, McCurdy JB, Cook WW. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976;58:271–5. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb D, Jeska E. Enhanced luminol-dependent chemiluminescence of stimulated rat alveolar macrophages by pretreatment with alveolar lining material. J Leukoc Biol. 1986;40:55–64. doi: 10.1002/jlb.40.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman R, Dauber J, Rogers R. Improvement in alveolar macrophage migration after therapeutic whole lung lavage in pulmonary alveolar proteinosis. Am Rev Respir Dis. 1989;139:1030–2. doi: 10.1164/ajrccm/139.4.1030. [DOI] [PubMed] [Google Scholar]
- 14.Geertsma MF, Broos HR, van den Barselaar MT, Nibbering PH, van Furth R. Lung surfactant suppresses oxygen-dependent bactericidal functions of human blood monocytes by inhibiting the assembly of the NADPH oxidase. J Immunol. 1993;150:2391–400. [PubMed] [Google Scholar]
- 15.Ahuja A, Oh N, Chao W, Spragg R, Smith R. Inhibition of the Human Neutrophil Respiratory Burst by Native and Synthetic Surfactant. Am J Respir Cell Mol Biol. 1996;14:496–503. doi: 10.1165/ajrcmb.14.5.8624255. [DOI] [PubMed] [Google Scholar]
- 16.El Benna J, Faust LP, Babior BM. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J Biol Chem. 1994;269:23431–6. [PubMed] [Google Scholar]
- 17.El Benna J, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 18.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 19.Davis RJ. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–6. [PubMed] [Google Scholar]
- 20.Han J, Lee JD, Bibbs L, Ulevitch RJA. MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 21.Torres M, Forman HJ. Activation of several MAP kinases upon stimulation of rat alveolar macrophages: role of the NADPH oxidase. Arch Biochem Biophys. 1999;366:231–9. doi: 10.1006/abbi.1999.1225. [DOI] [PubMed] [Google Scholar]
- 22.Rooney SA, Canavan PM, Motoyama EK. The identification of phosphatidylglycerol in the rat, rabbit, monkey and human lung. Biochim Biophys Acta. 1974;360:56–67. doi: 10.1016/0005-2760(74)90179-9. [DOI] [PubMed] [Google Scholar]
- 23.Allen R, Mead M, Kelly J. CRC Handbook Methods for Oxygen Radical Research. London: CRC Press; 1986. Phagocyte oxygenation activity measured by chemiluminesence and chemilumingenic probing; pp. 343–50. [Google Scholar]
- 24.Figueres A, Raetz C. Processing and secretion of TNF a in endotoxin treated Monomac 6 cells are dependent on Phorbol Myristate Acetate. J Biol Chem. 1992;267:23261–8. [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. Can.J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Becart J, Chevalier C, Biesse JP. Quantitative analysis of phospholipids by HPLC with a light scattering evaporating detector – application to raw materials for cosmetic use. J High Resolution Chromatogr. 1990;13:126–9. [Google Scholar]
- 27.Darmani H, Harwood JL, Jackson SK. Effect of interferon-gamma on membrane conformation in the macrophage- like cell line P388D. J Interferon Res. 1993;13:427–31. doi: 10.1089/jir.1993.13.427. [DOI] [PubMed] [Google Scholar]
- 28.Phare S. M, Peter-Golden, M, and Coffey, M. J. Alveolar lining fluid regulates mononuclear phagocyte 5-lipoxygenase metabolism. European Respiratory J. 1998;12:1141–6. doi: 10.1183/09031936.98.12051141. [DOI] [PubMed] [Google Scholar]
- 29.Daniels CB, Orgeig S, Wood PG, Sullivan LC. lopatko O, Smits A. The changing state of Surfactant Lipids; New Insights from Ancient Animals. Am Zool. 1998;38:305–20. [Google Scholar]
- 30.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–5. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 31.Chow CW, Grinstein S, Rotstein OD. Signaling events in monocytes and macrophages. New Horiz. 1995;3:342–51. [PubMed] [Google Scholar]
- 32.Yaffe MB, Xu J, Burke PA, Forse RA, Brown GE. Priming of the neutrophil respiratory burst is species-dependent and involves MAP kinase activation. Surgery. 1999;126:248–54. [PubMed] [Google Scholar]
- 33.Hamilton J. Interactions of Triglycerides with Phospholipids: Incorporation into the Bilayer Structure and Formation of Emulsions. Biochemistry. 1989;28:2514–20. doi: 10.1021/bi00432a025. [DOI] [PubMed] [Google Scholar]
- 34.Horejsi V, Drbal K, Cebecauer M, et al. GPI-microdomains: a role in signalling via immunoreceptors. Immunol Today. 1999;20:356–61. doi: 10.1016/s0167-5699(99)01489-9. [DOI] [PubMed] [Google Scholar]
- 35.Brown DA, London E. Structure and Origin of Ordered Lipid Domains in Biological Membranes. J Membr Biol. 1998;164:103–14. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 36.Geertsma MF, Zomerdijk TP, Nibbering PH, van Furth R. Pulmonary surfactant inhibits monocyte bactericidal functions by altering activation of protein kinase A and C. Immunology. 1994;83:133–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Baur FM, Brenner B, Goetze-Speer B, Neu S, Speer CP. Natural Porcine Surfactant (Curosurf) Down Regulates mRNA of Tumour Necrosis Factor-a (TNF-a) and TNF-a Type II Receptor in Lipopolysaccharide-Stimulated Monocytes. Pediatr Res. 1998;44:32–6. doi: 10.1203/00006450-199807000-00005. [DOI] [PubMed] [Google Scholar]