Abstract
The cellular and humoral acquired immune responses to Schistosoma haematobium 28 kD gluthathione S-Transferase (Sh28GST) antigen were evaluated in a Senegalese population chronically infected with S. haematobium parasite. We show a gender-dependent immune response in adult individuals presenting similar intensities of infection. Indeed, the specific IgA response and production of TGF-β and IL-10 were found significantly higher in females compared to males. In addition, we showed that this profile was combined with a weak production of Th1-related cytokines (TNFα and IFNγ) and was associated with an absence of proliferation to the antigen. A significantly higher Nuclear Matrix Protein 41/7 secretion, an apoptosis marker, was specifically observed in mononuclear blood cell cultures of females suggesting that a specific cell death process was engaged in a gender-dependent manner. This specific profile could be associated with the so-called T helper type-3 (Th3) immune response specifically promoting the production of IgA and would be developed upon the down-regulation of the specific Type-1 response by a probable cell death mechanism. This gender-dependent immune regulation, which may be under the influence of nonimmunological factors like sexual hormones, may be related to the chronicity of the infection.
Keywords: human, gender, infectious immunity-parasites, antibodies and cytokines, helminths infection
INTRODUCTION
The regulation between Th1-type (characterized by IFNγ, IL-2, IL-12 and TNFα production) and Th2-type (characterized by IL-4, IL-5, IL-10 and IL-13 secretion) immune profiles during parasite infection such as schistosomiasis is well documented [1]. In humans, resistance to Schistosoma infection is associated with IL-4, IL-5 and IgE production to whole parasite antigens [1,2]. The activation of this Th2 profile during infection down-modulates specific Th1-type responses [3] which suggests that the development of protective immunity follows a maturation towards a Th2-like profile [1,4]. This is supported by recent studies which show that IL-10 production suppresses IFNγ responses to whole parasite antigens in chronically infected humans, in contrast to acute infections [5].
The 28 kD glutathione S-Transferase (28GST) antigen of Schistosoma is one of the most promising vaccine candidate against schistosomiasis [6] and the 28GST of S. haematobium (Sh28GST) is the first candidate to reach actually phase II clinical trials [4]. Studies of humoral responses in infected children have demonstrated the presence of IgG, IgE and IgA antibodies specific to the 28GST [7]. However, no difference in the specific isotypic responses was observed between resistant and susceptible children to reinfection, after treatment by praziquantel. This is in contrast with the responses observed after chemotherapy in infected adults where 28GST-specific IgA appears to play a crucial role in acquired immunity against reinfection [8].
Immunity against schistosomiasis appears very strongly age-dependent [9]. One possibility is that the development of the protective acquired immunity is slow, depending on prolonged and cumulative exposure to relevant antigens, and that age-dependent immunity simply reflects this requirement. Indeed, susceptibility to infection decrease with age and in particular, intensities of infection are very low for adults compared to children suggesting a key role of the maturity of protective immunity with age [10]. An alternative explanation is that the responses are intrinsically age-dependent, being modified by other age-related physiological processes such as the sexual maturation of the host [9,10]. The latter hypothesis is supported by recent data from endemic countries which provide evidence for gender-dependent development of immunity directed to whole parasite antigens [10,11]. In these studies, the authors suggest that sex steroid could be involved in the orientation of the immune response against schistosomes but however, indicate that intensity of infection appearead different according to sex. In a previous study in human S. mansoni infection, we have demonstrated that biological activity of antibody responses directed to S. mansoni 28GST (i.e. inhibition of GST enzymatic activity) was gender-dependent, before any chemotherapy [12].
Therefore, the present study was undertaken to compare the profile of antibody responses directed to Sh28GST but also the acquired cellular response in adult males and females chronically infected with S. haematobium. To avoid the effect of a different infection level on specific immunity, we had the opportunity in the studied village to assess the specific immune profile in males and females presenting similar intensities of infection.
MATERIALS AND METHODS
Patients
The studied population was adult individuals who participated in a larger immuno-epidemiological study at the village of Ourou-Madiou (population estimate: 655) in the District of Podor, northern Senegal. Ourou-Madiou is located near the Senegal River, in an area where only urinary schistosomiasis due to S. haematobium is present [13]. The population (‘Toucouleurs’, a senegalese ethnic group) in this focus is sedentary. Men are mainly involved in cultivation and women, while they do help in the fields, are generally concerned with household duties [13]. Effective transmission in the region started after the construction of the dam of Diama – about 7 years before this study – and it is estimated that adults of both sexes have been exposed for the same duration [13]. Concomitant studies carried out in the focus have shown that the prevalence and intensity of infection level are low (< 55% &< 12 eggs/10 ml), with no differences between men and women [13].
For the present study, only every individual older than 35 years was considered. Our cohort included 12 males (average 45 years, range: 35–57 years) and 11 females (average 44 years, range: 35–51 years). This population sample was obtained from a large immuno-epidemiological study (108 infected persons, 7–57 years; detection of S. haematobium eggs using the urine filtration technique) who had not received treatment in the last 2 years. Only in this age group (above 35 years), males and females had similar low intensities of infection in term of egg count (Males: 6·6 ± 6·5 Eggs/10 ml of urine; Females: 10·6 ± 12·9 Eggs/10 ml; geometric mean ±SEM; NS by Mann-Withney U-test). In addition, detection of circulating anodic antigen (CAA) from schistosome adult worm, a sensitive assay to evaluate worm burden in Schistosoma infection, was performed in serum of patients as previously described [14]. No difference in CAA level was observed between both sexes (Males: 12·7 ± 14·34 ng/ml; Females: 19·46 ± 21·09 ng/ml; NS by Mann-Withney U-test).
An informed consent was obtained from all participants by the Senegalese Medical authority of the Region, as required by the Ethical Committee of the Senegalese Ministry of Health. All patients were treated with Praziquantel (40 mg/kg body weight) just after blood sampling and pregnant or lactating women were excluded from the study according to WHO recommendation.
Antigens
The recombinant Sh28GST was produced in recombinant Saccharomyces cerevisiae strain TGY73·4 containing the plasmid pTG8889 (provided by TRANSGENE S.A., Strasbourg, France) as previously decribed [8]. The purity of the rSh28GST (> 98%) was checked by SDS-polyacrylamide gel electrophoresis and Coomassie blue staining and its concentration measured by amino acid analysis.
Soluble Egg Antigens (SEA) was a total extract of S. haematobium eggs and was kindly provided by Pr. A. Wilson (University of York, U.K.).
Human Ab levels
Specific antibody levels to Sh28GST in individual sera were determined by enzyme-linked immunosorbent assay (ELISA) as previously described [12]. Sh28GST protein (10 µg/ml) or SEA (5 µg/ml) was coated on 96-well plates (Nunc, Roskilde, Denmark) at 4°C overnight. Plates were blocked then in phosphate-buffered saline containing 0·5% gelatin (Merck, Darmstadt, Germany), and individual sera were incubated at 4°C overnight at a 1/10 dilution for IgE, IgG4 or a 1/20 dilution for IgM, IgG1, IgG2, IgG3, IgA detection. Corresponding biotinylated mAbs to human Ig isotypes (SBA, Birgmingham, AL) were incubated at a 1/1000 dilution (2 h at 37°C). Peroxidase-conjugated streptavidin (1/1000; 30 min at 37°C) was then added (Amersham, Les Ulis, France). Colorimetric development was carried out by using ABTS (2,2′-azino-bis (3-ethylbenzthiazoline 6-sulphonic acid) diammonium; Sigma, St-Louis, MO) in 50 mm citrate buffer (pH 4) containing 0·003% H2O2, and absorbance (OD) was measured at 405 nm. Identical ELISAs were performed in parallel for 30 Europeans uninfected individuals. Individual results were expressed as δOD value calculated for each isotype test according to the formula: δOD = ODx-ODn, where ODx represented individual OD value of infected patient and ODn was the arithmetic mean of individual OD value for the 30 uninfected control individuals (ODn value for IgM = 0·3; IgE = 0·22; IgG1 = 0·13; IgG2 = 0·13; IgG3 = 0·34; IgG4 = 0·18; IgA = 0·24).
PBMC culture and cellular response measurement
Peripheral blood mononuclear cells (PBMC) from each individual were isolated from heparin-treated blood samples by density centrifugation on Histopaque 17–0840–02 (Pharmacia, Uppsala, Sweden). Cells were plated in RPMI 1640 medium (Gibco, Courbevoie, France) supplemented with 10% fetal calf serum (batch number:40F0567K, Gibco), at 1·5 × 106 per well in duplicate cultures or at 2 × 105 per well in triplicate cultures, in 24 or 96-well flat-bottomed plates (Nunc) for cytokine production analysis and proliferation analysis, respectively. PBMC were stimulated with Sh28GST antigen (10 µg/ml) and supernatants were harvested on day 3 for cytokines detection. To measure Ag-specific proliferation, cultures were pulsed with 0·5 µCi of [3H] thymidine (Amersham) and harvested on day 4. At first, proliferation was expressed as Stimulation Index (SI; stimulated/unstimulated mean count) and the mean of SI was different according to sex (males: 1·59 ± 0·41 and females: 0·78 ± 0·19). However, the values of SI were very low (range: 0·451–2·013). So, proliferation data were expressed as δcpm representing the difference between mean count per minute (triplicate culture) of stimulated versus unstimulated cultures, for each individual. For cytokines ELISA, the following reagents were purchased from Genzyme (Cambridge, MA): purified mouse mAbs to human TNF-α, IFN-γ; biotinylated rabbit mAbs to human TNF-α, IFN-γ; and recombinant human TNF-α, IFN-γ from Pharmingen (San Diego, CA): purified rat mAbs to human IL-5 (clone TRFK5), IL-10 (clone JES3–9D7); biotinylated rat mAbs to human IL-5 (clone JES1–5A10), IL-10 (clone JES3–12G8); and recombinant IL-5, IL-10. Quantitative ELISA for TNF-α, IFN-γ, IL-5 and IL-10 were performed with paired mAbs specific for corresponding cytokines under manufacturer's recommendations. We assessed IL-4 detection by EASIA Kits (Biosource, Fleurus, Belgium) and TGF-β release by TGF-β1 ELISA System Kit (Promega, Madison, WI) in day 3 supernatants. Cytokine concentrations were expressed as picograms or nanograms per milliliter after substrating the amount detected in unstimulated medium control cultures. Identical culture conditions were assessed with PBMC of 30 uninfected control individuals in which no cytokine production and no proliferation were detected after Sh28GST stimulation (data not shown).
Detection of the cell death marker NMP41/7
Several studies have demonstrated that the Nuclear Matrix Protein (NMP 41/7) was cleaved and solubilized during cell death process and especially after apoptosis [review in 15]. In addition, it has been recently described that the presence of NMP41/7, in culture supernatants, was correlated to cell DNA fragmentation and FAS-Ligand production [16]. To evaluate cell death process after in vitro stimulation of PBMC by Sh28GST, the presence of NMP41/7 marker was determined a posteriori by a specific ELISA (Calbiochem, San Diego, CA) in same day 3 supernatants used for cytokine detection. Results were expressed for each individual in units per milliliter (U/ml) and amounts detected in medium control culture have been substracted (unstimulated). No specific presence of NM41/7 was detected in supernatants from cultures of 30 uninfected individuals (data not shown).
Statistical analysis
All data were analysed with Abacus Concepts STATVIEW software (Berkeley, CA). Correlations between all the covariates were analysed using Kendall's rank correlation coefficient. The nonparametric Mann–Whitney U-test was used to compare the mean of the two independent groups. All differences were considered significant at P < 0·05.
RESULTS
Isotypic responses to Sh28GST antigen
Since the same intensity of infection, in terms of egg counts and serum CAA levels (see Materials and methods), was observed in both sexes of the cohort including all individuals older than 35 years, the comparison of responses to Sh28GST was assessed according to gender.
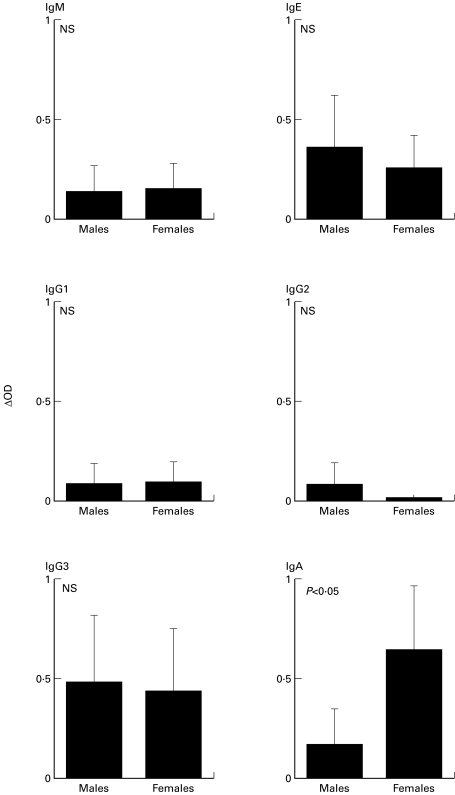
Mean of Ab levels of specific IgM, IgG1, IgG2, IgG3 and IgE responses were similar between male and female populations (Fig. 1). No specific IgG4 response was found in either of the sex groups (data not shown). In contrast, IgA response to Sh28GST were significantly higher in female individuals compared to males (Fig. 1; P < 0·05). To evaluate the specificity of the observed isotypic difference, IgA response against total parasite extract (Soluble Egg Antigens; SEA) was assessed. All individuals were responders but no difference was observed according to sex (Males: δOD = 1·83 ± 0·63; Females: δOD = 1·78 ± 0·37; P = 0·336; NS). This suggests that gender-dependent IgA response appeared to be specifically related to Sh28GST antigen.
Fig. 1.
Gender-dependent isotypic responses to Sh28GST in S. haematobium infected male (n = 12) and female (n = 11) individuals as determined by ELISA. Results represent means (± SEM) of δOD of each individual values and statistical significance between each group is indicated.
Cytokine production to Sh28GST
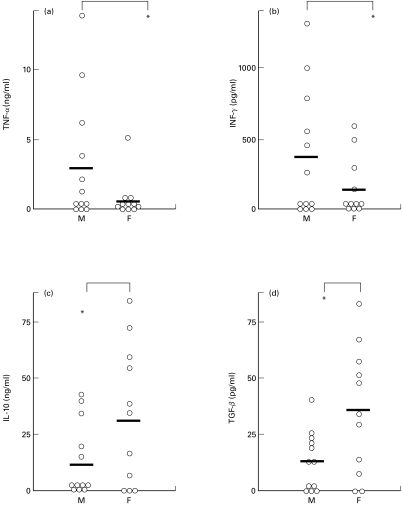
In vitro cytokine production (IFN-γ, TNF-α, IL-4, IL-5, IL-10, and TGF-β) of PBMC after stimulation with the recombinant Sh28GST antigen was evaluated. A specific production of IFN-γ, TNF-α, IL-10 and TGF-β was observed (Fig. 2). In contrast, no Th2-type cytokines (IL-4, IL-5) were detected (data not shown). The average of all individual values of TNF-α or IFN-γ production was significantly lower in females compared to males (Fig. 2; P < 0·05). In contrast, the secretion of both IL-10 and TGF-β was significantly higher in the female group (P < 0·05). The correlation between all values including parasitological data and age was analysed using Kendall's rank correlation coefficient but no significant correlation was observed.
Fig. 2.
Gender-dependent cytokine production by peripheral blood mononuclear cells (PBMC) of infected male (M, n = 12) and female (F, n = 11) individuals in response to Sh28GST antigen. Supernatants were collected after Sh28GST stimulation and assayed for (a) TNF-α, (b) IFN-γ, (c) IL-10 and (d) TGF-β by ELISA. Results represent median values of duplicate cultures for each subject and bars are the arithmetic mean for each group. Statistical significance between groups is indicated. *P <0·05.
Specific proliferation and cell death process after stimulation by Sh28GST
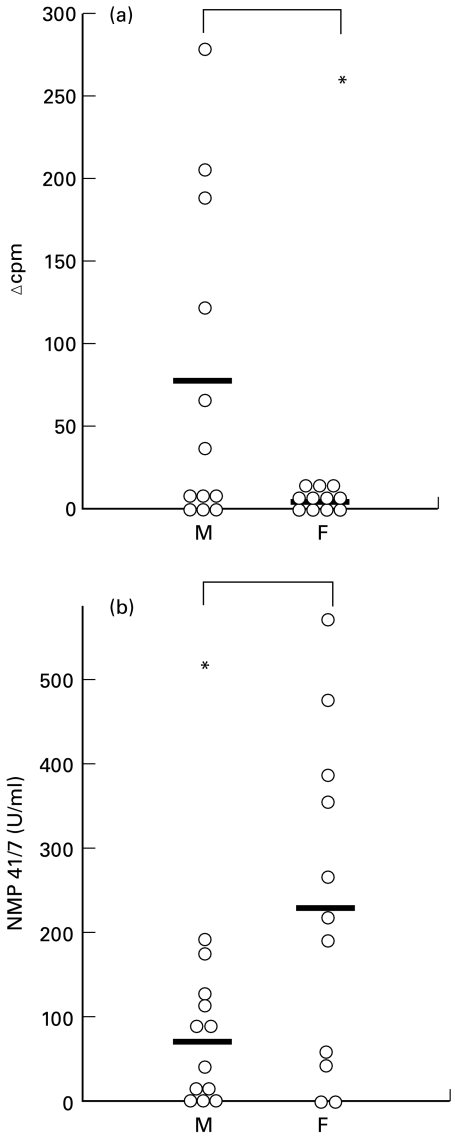
We have evaluated the specific proliferation of PBMC after stimulation by Sh28GST in cell cultures. We detected no specific cellular proliferation in the female group (Fig. 3a). In contrast, a low but positive proliferation was detected for 6 male individuals. The mean of individual values of specific proliferation was found significantly different according to sex (P < 0·05) whereas background levels were similar (males: 1201 ± 1176 cpm and females: 1408 ± 886 cpm, NS).
Fig. 3.
Proliferation (a) and presence of cell death marker NMP 41/7 (b) after stimulation by Sh28GST of PBMC of infected male (M, n = 12) and female (F, n = 11) individuals. Individual proliferation data are presented as the median counts per minute (cpm) of triplicate culture expressed as δcpm representing the difference between mean counts per minute of stimulated versus unstimulated cultures for each individual. NMP 41/7 was detected in the culture cell supernatants by ELISA and results represent median value of duplicate cultures for each subject Bars represent the arithmetic mean for each group and statistical significance between groups is indicated. *P <0·05.
It has been recently demonstrated that cell death by apoptosis induces the inhibition of TNF-α secretion during in vitro monocyte activation by favouring both immunoregulatory cytokine IL-10 production [17] and TGF-β secretion [18]. Subsequently to our observation of a lack of proliferation and TNF-α production associated with a marked secretion of TGF-β and IL-10 in female individuals, we have evaluated the specific cell death process after in vitro Sh28GST stimulation. Since this evaluation was scheduled a posteriori to the cell cultures, we assessed this phenomenon by measuring a cell death marker, the Nuclear Matrix Protein (NMP 41/7; see Materials and methods). The mean value of NMP41/7 secretion was significantly higher in supernatants from the female group than in those from males (Fig. 3b; P < 0·05). This indicates a more pronounced in vitro cell death process in female PBMC after antigen activation.
DISCUSSION
In the present study, we have evaluated the acquired humoral and cellular immune responses to Sh28GST in individuals chronically infected by S. haematobium parasite in Northern Senegal, a focus where no systematic differences in the prevalence of infection were observed between males and females [13]. We showed a gender-dependent specific immune responses in adult individuals (above 35 years) presenting similar intensity of infection in term of egg counts and detection of circulating parasite antigens. Whereas no Th2-type IL-4 and IL-5 production was detected in sex groups, the mean value of secretion of IL-10 and TGF-β was higher in females than in males to the detriment of presence of Type-1 cytokines (TNF-α, IFN-γ). It is well established that TGF-β and IL-10 have a critical inhibitory effect on IFN-γ and TNF-α production by human leucocytes [19,20]. In particular, previous studies strongly suggest that IL-10 is responsible for the down-regulation of Th1 cytokine production in schistosome infections [5]. These reports suggest that concomitantly secreted TGF-β and IL-10 down-modulate Th1-type response as we observed in our study. In addition, it is generally agreed that TGF-β and IL-10 cooperate to induce IgA secretion by human B cells [21]. Although correlations between these cytokine productions and specific IgA levels were not found statistically significant (Kendall's test), our results suggest that, in addition to the down-modulation of the type-1 response, the specific secretion of TGF-β and IL-10 would play a predominant role in IgA production as it is observed in infected female individuals.
In a mouse model of oral tolerance, it has been described a distinct lineage of T helper cells, termed Th3 which would be derived from Th2-like subsets [22]. A so-called Th3 subpopulation has been also identified in human peripheral blood mononuclear cells after oral administration with antigen inducing systemic immune tolerance [23]. These Th3 cells mainly produce TGF-β and are dependent on autocrine IL-10 production for their growth [24]. In contrast to Th1 and Th2 cells, this Th3 subset would provide help for IgA production in the mechanism of oral tolerance [24]. In accordance to these reports, our results suggest that the specific profile observed in chronically infected females could be close to the definition of the Th3 response.
It has been demonstrated that the outcome of Th3 subsets have the ability to downregulate the specific Th1 immune responses, by apoptosis mechanism [22,24]. Subsequently to our observation of a consistant IL-10 and TGFβ production by female individuals, we have evaluated a posteriori the part of cell death process during in vitro stimulation by Sh28GST by dosage of NMP 41/7 protein, a late marker of apoptosis released during cell death [15,16]. We detected a higher concentration of NMP41/7 in female group indicating that the gender-dependent immune regulation observed could involved a cell death process. In addition, it has been demonstrated that T cell deletion of Th1 and Th2 clones mediated by cell death induced the appearance of an unique subpopulation of T helper cells of the type 3 phenotype [22,24]. The same authors showed that proliferation of Th3 lymphocytes was very weak. In accordance to this latter observation, we observed the absence of cellular proliferation in the PBMC cultures from female individuals after stimulation with Sh28GST. This result could be partly explained by the inhibitory effect of secreted TGF-β and IL-10 on human T cell proliferation [25,26]. Taken together, these results argue in favour of the expression of Sh28GST-specific Th3-like response in the female group which would be associated with down-regulation of the specific type-1 profile through a specific cell death process.
These specific regulation processes would promote IgA production directed towards the antigen [24] as we observed in the present study. IgA responses have been widely associated to long-standing infectious diseases in humans [27]. Thus IgA and to a certain extent the Th3-like response observed, could be considered to be a marker of a specific maturation of immune responses in chronic schistosomiasis. The outcome of Th3 response was previously associated to oral tolerance [24] and described during onchocerciasis, a chronic human helminth infection [28]. The chronicity of an infection could thus induce a maturity of specific immunity characterized by this tolerance-related immune profile.
However, this profile associated to the studied antigen was gender-dependent and was predominantly observed in adult females. This difference between males and females could reflect an effect of sex steroid hormones on the specific immune response [29]. Indeed, oestradiol has a strong influence on the up-regulation of IgA [30] and increases TGF-β production at the transcriptional level [31]. Estradiol also has a direct effect on cytokine secretion by T cells, particularly in enhancing IL-10 secretion associated with the inhibition of TNFα production [32] and progesterone decreases Th1-related cytokine production [33]. In addition, it has been suggested that an endogenous metabolite of oestrogen, 2-methoxyestradiol, is able to enhance cell apoptosis [34]. These reports support the hypothesis that female steroid hormones could orientated the gender-dependent immunity and could possibility act as ≪ accelerating cofactors ≫ to establish the so-called Th3-type immune profile we observed.
The same intensity of infection and age mean were observed between both sexes but involvement of genetic background or other individual events such as previous pregnancy cannot be excluded to explain the different immunity observed in our study. Nevertheless, we demonstrated that human immune response to the Sh28GST vaccinal antigen was gender-dependent suggesting that non immune physiological parameters could play a probable role in the orientation of the acquired immune response. In contrast to Th2 profile directed towards whole parasite antigens previously described in the immunity to schistosomiasis [1,2,4], our results indicate for the first time that a particular immune profile associated with the so-called Th3 response could be part of the specific acquired immunity to Sh28GST of chronically infected females by S. haematobium.
Acknowledgments
We would like sincerely to thank the chief and the population of Ourou-Madiou village for their participation to this study; N. Milliard, M. C. Gallissot, M. M. Diakhate and E. Bassene for technical assistance; J. P. Dompnier for the field organization; J. de Bont, A. Wilcox and R.J. Pierce for critical reading of the manuscript.
This work was supported by European Economic Community contract IC18CT970240 and IC18CT960041, French Ministry of Cooperation (FAC 88/CD/91–01), the ESPOIR programme, the Region Nord-Pas-de-Calais, the Institut Pasteur de Lille and INSERM.
F. Remoué holds a fellowship of the Region Nord-Pas-de-Calais.
REFERENCES
- 1.Dunne DW, Hagan P, Abath FGC. Prospects for immunological control of schistosomiasis. Lancet. 1995;345:1488–92. doi: 10.1016/s0140-6736(95)91041-7. [DOI] [PubMed] [Google Scholar]
- 2.Couissinier-Paris P, Dessein AJ. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are TH0/2. Eur J Immunol. 1995;25:2295–302. doi: 10.1002/eji.1830250827. [DOI] [PubMed] [Google Scholar]
- 3.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth Schistosoma mansoni. J Exp Med. 1991;173:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capron A. Schistosomiasis: forty years' war on the worm. Parasitol Today. 1998;14:379–84. doi: 10.1016/s0169-4758(98)01322-2. [DOI] [PubMed] [Google Scholar]
- 5.Montenegro SML, Miranda P, Mahanty S, et al. Cytokine production in acute versus chronic human Schistosomiasis mansoni: the cross-regulatory role of interferon-γ and interleukin-10 in the responses of peripheral blood mononuclear cells and splenocytes to parasite antigens. J Infect Dis. 1999;179:1502–8. doi: 10.1086/314748. [DOI] [PubMed] [Google Scholar]
- 6.Balloul JM, Sondermeyer P, Dreyer D, et al. Molecular cloning of a protective antigen of schistosomes. Nature. 1987;326:149–53. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- 7.Auriault C, Gras-Masse H, Pierce RJ, et al. Antibody response of Schistosoma mansoni-infected human subjects to the recombinant P28 Glutathione-S-Transferase and to synthetic peptides. J Clin Microbiol. 1990;28:1918–24. doi: 10.1128/jcm.28.9.1918-1924.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grzych JM, Grezel D, Xu CB, et al. IgA antibodies to a protective antigen in human schistosomiasis mansoni. J Immunol. 1993;150:527–35. [PubMed] [Google Scholar]
- 9.Gryseels B. Human resistance to Schistosoma infections: age or experience? Parasitol Today. 1994;10:380–5. doi: 10.1016/0169-4758(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 10.Fulford AJC, Webster M, Ouma JH, Kimani G, Dunne DW. Puberty and age-related changes in susceptibility to schistosome infection. Parasitol Today. 1998;14:23–9. doi: 10.1016/s0169-4758(97)01168-x. [DOI] [PubMed] [Google Scholar]
- 11.Webster M, Libranda-Ramirez BDL, Aligui GD, et al. The influence of sex and age on antibody isotype responses to Schistosoma mansoni and Schistosoma japonicum in human populations in Kenya and the Philippines. Parasitology. 1997;114:383–93. doi: 10.1017/s003118209600858x. [DOI] [PubMed] [Google Scholar]
- 12.Remoué F, Rogerie F, Gallissot MC, et al. Sex-dependent neutralizing humoral response to Schistosoma mansoni 28GST antigen in infected human population. J Infect Dis. 2000;181:1855–9. doi: 10.1086/315454. [DOI] [PubMed] [Google Scholar]
- 13.Shaw DJ, Vercruysse J, Picquet M, Sambou B, Ly A. The effect of different treatment regimens on the epidemiology of seasonally transmitted Schistosoma haematobium infections in four villages in the Senegal River Basin. Senegal Trans Roy Soc Trop Med. 1999;93:1–9. doi: 10.1016/s0035-9203(99)90288-2. [DOI] [PubMed] [Google Scholar]
- 14.Deelder AM, Qian ZL, Kremsner PG, et al. Quantitative diagnosis of Schistosoma infections by measurements of circulating antigens in serum and urine. Trop Geo Med. 1994;46:233–8. [PubMed] [Google Scholar]
- 15.Miller TE, Beausang LA, Meneghini M, Lidgard G. Apoptosis II The Molecular Basis of Apoptosis in Disease. Coldspring Harbor: Coldspring Harbor Laboratory Press; 1994. Cell death and nuclear matrix proteins; pp. 357–76. [Google Scholar]
- 16.Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nature Med. 1999;5:423. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 17.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Hensin PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chantry D, Turner M, Abney E, Feldman M. Modulation of cytokine production by transforming growth factor-β. J Immunol. 1989;142:4295–300. [PubMed] [Google Scholar]
- 20.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and Transforming Growth Factor β cooperate to induce anti-CD40-activated naive human B cells to secrete Immunoglobulin A. J Exp Med. 1992;175:671–82. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 23.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating Myelin Basic Protein and Proteolipid Protein-specific transforming growth factor-β1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–7. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–43. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 25.Ruegemer JJ, Ho SN, Augustine JA, et al. Regulatory effects of transforming growth factor-β on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol. 1990;144:1767–76. [PubMed] [Google Scholar]
- 26.De Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–26. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkins NS, Lindo JF, Lee MG, et al. Humoral responses in human strongyloidiasis: correlations with infection chronicity. Trans Roy Soc Trop Med Hyg. 1997;91:609–13. doi: 10.1016/s0035-9203(97)90049-3. [DOI] [PubMed] [Google Scholar]
- 28.Doetze A, Satoguina J, Burchard G, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and transforming growth factor-β but not by a Th1 to Th2 shift. Intern Immunol. 2000;12:623–30. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 29.Schuurs AHWM, Verheul HAM. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990;35:157–72. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 30.Gomez E, Ortiz V, Saint-Martin B, Boeck L, Diaz-Sanchez V, Bourges H. Hormonal regulation of the secretory IgA (sIgA) system: estradiol- and progesterone-induced changes in sIgA in parotid saliva along the menstrual cycle. Am J Reprod Immunol. 1993;29:219–23. doi: 10.1111/j.1600-0897.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 31.Ashroft GS, Dodsworth J, van Boxtel E, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-β1 levels. Nature Med. 1997;3:1209–15. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 32.Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from Multiple Sclerosis patients and normal control subjects. J Immunol. 1997;158:446–51. [PubMed] [Google Scholar]
- 33.Piccini MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]
- 34.Tsukamoto A, Kaneko Y, Yoshida T, Han K, Ichinose M, Kimura S. 2-methoxyestradiol, an endogenous metabolite of estrogen, enhances apoptosis and β-galactosidase expression in vascular endothelial cells. Biochem Biophys Res Commun. 1998;248:9–12. doi: 10.1006/bbrc.1998.8902. [DOI] [PubMed] [Google Scholar]