Abstract
IL-10 and IL-12 are cytokines which are important in regulating immune responses. Plasma levels of IL-10 and autoantibodies against double-stranded DNA (dsDNA) often mirror disease activity in patients with SLE. IL-12 secretion from SLE patients' blood mononuclear cells also correlates with disease activity, but has an inverse relationship. The aim of this study was to measure the effect of IL-10 and of IL-12 on the production of IgG autoantibodies from patients with SLE, both cross-sectionally and longitudinally.
Peripheral blood mononuclear cells (PBMC) were cultured with IL-10 (at 20 ng/ml or 2 ng/ml) or IL-12 (at 2 ng/ml or 0·2 ng/ml) or without cytokine and the supernatanants tested for the production of double-stranded DNA antibodies (dsDNA abs), single-stranded DNA antibodies (ssDNA abs) and total IgG antibodies (IgG abs) by ELISA. The BILAG disease activity index was recorded at each patient visit (a global score of six or more is regarded as active disease).
In general, treatment with IL-10 caused PBMCs from patients with inactive disease to increase their antissDNA and dsDNA ab production (by upto 354% and 186%, respectively) while patients with active disease decreased their antibody production (by upto 91% and 97%, respectively). Overall there was a correlation between disease activity and change in antissDNA and dsDNA ab production (r = − 0·51; P = 0·03 and r = − 0·48; P = 0·042, respectively). Treatment with IL-12 at 0·2 ng/ml inhibited antissDNA and dsDNA antibody production, having the greatest effect on patients with active disease (decreasing antissDNA and dsDNA antibody production by upto 75% and 73%, respectively). This resulted in a significant correlation between disease activity and change in antissDNA antibody production (r = − 0·76; P = 0·03), but significance was not reached with antidsDNA antibody production (P = 0·06). Together these data suggest that the effect of these cytokines on antibody production by SLE PBMCs involves several factors; one of which is disease activity.
Keywords: IgG anti-double-stranded DNA antibodies, IgG anti-single-stranded DNA antibodies, interleukin-10, interleukin-12, systemic lupus erythematosus
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune rheumatic disease that is characterized by B-cell hyperactivity and the presence of various autoantibodies. IgG autoantibodies that bind to double stranded DNA (IgG antidsDNA abs) are thought to be important in SLE because they are common and may be pathogenic. Between 60 and 70% of lupus patients produce IgG antidsDNA abs and numerous lines of evidence have suggested that some of these antibodies are nephrotoxic [1–5]. Therefore a signal that leads to an elevated titre of IgG antidsDNA abs may also signal disease flare.
Cytokines regulate the immune system and may be split into two groups: T helper type 1 (Th1) cytokines which mainly activate the cellular arm of the immune system and Th2 cytokines which mainly activate the humoral arm of the immune system [6]. In accordance with the B-cell hyperactivity found in SLE there is a Th2 bias in cytokine production [7–8]. Interleukin-10 (IL-10) may be of pivotal importance in this imbalance because it inhibits the production of Th1 cytokines and correlates positively with disease activity [9–12]. The IL-10 overproduction found in SLE has an endogenous counterweight: interleukin-12 (IL-12) which encourages Th1 cytokine production and has been shown to correlate negatively with disease activity [13–15]. We therefore felt that it was important to examine the role of IL-10 and IL-12 directly on blood lymphocytes from SLE patients.
In this study the in vitro effect of IL-10 and of IL-12 on the antibody production of total peripheral blood mononuclear cells (PBMCs) from patients with SLE and healthy controls (HCs) has been measured. It was found that the change in IgG antidsDNA ab production caused by IL-10 correlated with disease activity. Patients with inactive disease tended to show an increase in IgG antidsDNA ab production whilst patients with active disease decreased their IgG antidsDNA ab production. The effect of IL-12 on IgG antissDNA ab production appeared to be similar to that seen with IL-10, there being a tendency towards a decrease in IgG anti dsDNA with increased disease activity.
METHODS
Patients and controls
Patients
Thirty-one female SLE patients were studied and each met four or more of the revised classification criteria for SLE [16]. Patients were selected at random from amongst those known to have currently raised antidsDNA antibody levels and confirmed at the time of visit to the clinic.
Disease activity was assessed using the British Isles Lupus Assessment Group (BILAG) index [17]. This index is based on the physicians intention to treat principle and divides lupus activity into eight organs or systems. The most active state is given an A grade while the absence of activity ever in that system gets an E. To convert these individual organ grades into a global score, A = 9, B = 3, C = 1 and D/E = 0. For the purposes of comparisons with antibody levels, a patient with a global score of more than or equal to 6 was regarded as active and 0–5 inactive.
All patients were tested with IL-10: three Afro-caribbeans, two Asians, 24 Caucasians and two Chinese; the mean age of this group was 35 (range 17–70); 21 of these patients had active disease at the time of testing. Twenty-five female patients with SLE were tested with IL-12: three Afro-caribbeans, two Asians, 18 Caucasians and two Chinese; the mean age was 37 (range 17–70); 16 of these patients were active at the time of testing.
Three of the patients, two inactive and one active, were on low dose steroids (< 10 mg prednisolone) all the others were taking one or more immunosuppressants (azathioprine, methotrexate or cyclophosphamide) plus prednisolone when included in this study.
Controls
Sixteen healthy female controls (HC) were used: seven Asians and nine Caucasians; mean age 26 (range 23–45). All control blood samples were tested with IL-10 and with IL-12.
Cell preparations
Twenty millilitre venous blood samples were collected in sterile, heparinized tubes. All samples were separated for culture on the day of collection. The sample was transferred to a 50-ml Falcon tube and diluted to 50 mls in RPMI. Peripheral blood mononuclear cells (PBMCs) were separated on Histopaque 1077 (Sigma). Cells were cultured at 2 × 106 cells per ml, in 1 ml cultures in 10% FCS RPMI supplemented with 1% Glutamine, 2% Non-essential amino acids, 0·2% Gentamycin, 1% Sodium Pyruvate and 1% Penicillin and streptomycin (Supplemented RPMI). After three days the supernatants were removed and tested for total IgG, IgG antisingle stranded DNA (antissDNA) and antidsDNA antibody content by ELISA. Initial time course experiments demonstrated that there are at least two phases to antibody production in vitro. A sharp increase for the initial 3–5 days followed by a slower increase to 15 days. In order to test the effect on only one phase, supernatants were taken on day 3. Total IgG production followed a similar pattern to antidsDNA but displayed a higher saturation threshold. However antidsDNA ab production was considered a more important parameter, so supernatants were taken on day 3.
Antibody production
The antidsDNA ab ELISA was performed as described elsewhere [18]. The only modification was an increase in the incubation time with the supernatant on the plate from 60 to 90 mins. Briefly, DNA (both single and double-stranded) was incubated on the plate at 500 µg/ml for 2 h at 37°C. All samples were tested undiluted and in duplicate. Anti-IgG conjugate was added at 1:1000 (Sigma) and incubated overnight at 4°C, after which the plates were developed. The ELISA used to quantify total IgG antibody levels was performed identically except for the first two hours. The Fab2 anti-IgG capture antibody (Sigma) was incubated for one hour at 37°C, the plate was then blocked for one hour at 37°C with 2% casein in PBS. The samples were tested at a 10 times greater dilution than in the anti-DNA ELISAs.
All patients were tested for total IgG, IgG antissDNA ab and antidsDNA abs. However, many patients did not produce sufficient levels of antidsDNA and/or antissDNA abs to be detectable by ELISA. For this reason there is a greater n number presented for total IgG than antidsDNA or antissDNA ab production. The sensitivity of these ELISAs was established by testing serial dilutions of a positive sample. The lower limit of the sensitive (linear) range was shown to be at an OD of 0·15.
Cytokines
Both IL-10 and IL-12 were obtained from Sigma (IL-10 EC50 = 2 ng/ml; IL-12 EC50 = 0·2 ng/ml (less than 0·1 ng/ug endotoxin)). Stock solutions were stored at −80°C at 1 µg/ml. Prior to use the stock solutions were diluted in supplemented RPMI such that 20 µl added to each cell culture would achieve the required concentration of cytokine. Once the cells had been dispensed in culture, cytokine was immediately added to all treated samples and 20 µl of supplemented 10% FCS RPMI was added to the untreated cultures as a control.
In preliminary experiments different concentrations of IL-10 and IL-12 were tested to establish the ranges to be used. To assess the effect of IL-10 and of IL-12 on antibody production, PBMCs from each patient were cultured, in triplicate without cytokine or with IL-10 (20 and 2 ng/ml) or IL-12 (2 and 0·2 ng/ml).
Data analysis
Each treatment was tested by triplicate cell cultures. The antibody level in each of the triplicate cell cultures was quantified according to the methodology described above. This resulted in three duplicate ODs which were averaged for each treatment. The effect of IL-10 and of IL-12 in this system was measured as the change they induced in antibody production. The mean change in antibody production was calculated with the following formula:
 |
The Shapiro-Wilk test demonstrated that the antibody production was not normally distributed; therefore nonparametric tests were used. The Mann–Whitney test was used to assess whether the cytokine-induced change in antibody production varied between HCs and patients with SLE (SLE patients with active disease were compared separately from patients with inactive disease). To test whether or not the change in antibody production varied with disease activity these two parameters were correlated with the Spearman rank correlation.
RESULTS
Spontaneous IgG and specific anti ssDNA and dsDNA production
All SLE and HC lymphocytes produced measurable quantities of total IgG after 3 days of culture. HCs produced 20 ± 5·0 ng/ml of IgG spontaneously (n = 16), while patients with inactive SLE produced 138 ± 185 ng/ml of spontaneous IgG (60 ng/ml being the lowest) [n = 10] and SLE patients with active disease produced 402 ± 298 ng/ml (92 ng/ml being the lowest) [n = 21]. The differences in spontaneous IgG production between HCs and SLE patients with active or inactive disease were highly significant (P < 0·001 in both cases). However, there was no significant difference in spontaneous IgG production between SLE patients with active and inactive SLE. Lymphocytes from none of the HCs and only 20/31 (64%) and 18/31 (58%) of SLE patients produced measurable levels of antissDNA abs and antidsDNA abs, respectively. Although the antidsDNA ab production from the untreated cultures did not correlate with global score it did correlate with serum antidsDNA ab level (r = 0·7; P = 0·03). In addition no correlation between serum antidsDNA ab level and disease activity was seen.
Effect of IL-10 and IL-12 on total IgG produced by lymphocytes from SLE patients
No significant differences in response to IL-10 or IL-12 in the total IgG produced by HC or SLE lymphocytes. In addition, no correlation between the patient's disease activity and change in total IgG production, was seen using the Spearman correlation coefficient (data not shown).
Effect of IL-10 and IL-12 on anti ssDNA and anti dsDNA produced by lymphocytes from SLE patients
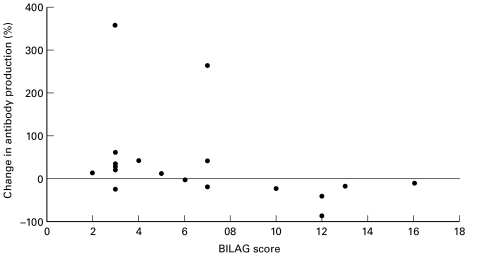
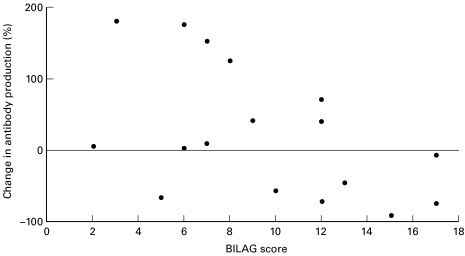
The effect of IL-10 at 20 ng/ml but not 0·2 ng/ml on antiss (Fig. 1) and dsDNA antibody production (Fig. 2) correlated with disease activity, r = − 0·51 and −0·48, P = 0·030 and 0·042, respectively; such that patients with low disease activity scores had increased antibody production whilst patients with high disease activity scores had decreased antibody production.
Fig. 1.
PBMCs from SLE patients were cultured untreated or with 20 ng/ml of IL-10. The percentage change in IgG antissDNA ab production is graphed against BILAG score at the time the PBMC sample was taken. There is a significant correlation between the change in antibody production and disease activity. r = −0.51; P = 0.03.
Fig. 2.
PBMCs from SLE patients were cultured untreated or with 20 ng/ml of IL-10. The percentage change in IgG antidsDNA ab production is graphed against BILAG score at the time the PBMC sample was taken. There is a significant correlation between the change in antibody production and disease activity. r = −0.48; P = 0.042.
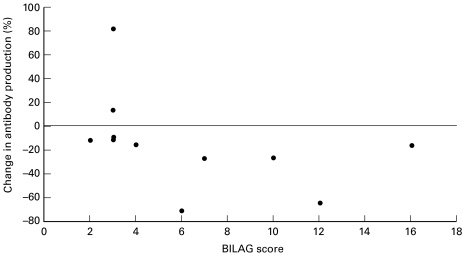
IL-12 at 0·2 ng/ml induced a change in antissDNA ab production that correlated with disease activity, r = − 0·76, P = 0·030 (Fig. 3). Treatment with IL-12 at 0·2 ng/ml tended to decrease antissDNA ab production, the decrease being more marked in patients with active disease. No correlation was seen with anti ssDNA at 2 ng/ml IL-12 and no correlation with anti dsDNA was found for either 2 or 0·2 ng/ml IL-12 (data not shown). None of these cytokine induced changes in antibody production correlated with serum antidsDNA antibody titre at the time of testing (data not shown).
Fig. 3.
PBMCs from SLE patients were cultured untreated or with 0·2 ng/ml of IL-12. The percentage change in IgG antissDNA ab production is graphed against BILAG score at the time the PBMC sample was taken. There is a significant correlation between the change in antibody production and disease activity. r = −0.73; P = 0.01.
Relationship between cytokine effects and disease activity within the same patient
In order to look more carefully at the relationship between cytokine effects and disease activity we studied individual patients at several time points. Five patients who were regularly attending the lupus clinic with active lupus and raised serum antidsDNA ab levels were selected. Each was tested on five occassions over the course of one year.
IL-10 (20 ng/ml)
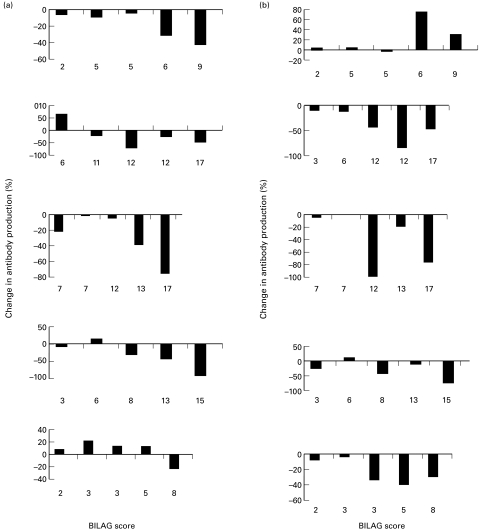
In all five patients tested at five different time points, with increasing disease activity measured as a global score, there was a trend towards a decrease in IgG antidsDNA ab production (Fig. 4a). There was also a suggestion that at lower disease activities in 3/5 of the patients there was an increase in IgG anti dsDNA ab production. This was consistent with the data obtained in the cross-sectional study and correlated with disease activity r = − 0·53; P = 0·006.
Fig. 4.
PBMCs were taken on five occasions from five different patients with SLE. Each panel represents results from a single patient. The antidsDNA ab response to either (a) IL-10 at 20 ng/ml or (b) IL-12 at 2 ng/ml are shown with the BILAG score at the time of testing.
IL-12 (2 ng/ml)
In four of the five patients tested at the different time points, there was also a tendency towards a decrease in IgG antidsDNA ab production with increased disease activity (Fig. 4b). However, there was one patient, MD who showed an enhancement of IgG anti dsDNA ab production at the higher disease activities reached in this patient. When change in antibody production was correlated with global score this data approached but did not reach significance (r = − 0·53, P = 0·06).
There was a correlation between serum antidsDNA ab titre and the production of untreated PBMCs (r = 0·72, P = 0·035). There was also a correlation between serum antidsDNA antibody production and disease activity in the longitudinal patients tested with IL-10 and IL-12 (r = 0·56 and 0·52; P = 0·044 and 0·04, respectively). This may explain why the longitudinal data provided more significant results than the cross-sectional data.
The data, both longitudinal and cross-sectional, was also tested for any correlation with drug treatment. The requirement for high antidsDNA antibody production for inclusion in this study resulted in little variation in the drug treatments being taken (Nearly all the patients being on immunosuppressants). It is therefore unsurprising that no significant differences were found when correlating treatment with antibody changes. Also, when those patients not receiving immunosuppressant drugs were removed from the analysis it did not change which correlations were significant and which were not (data not shown).
DISCUSSION
In this study we have confirmed that in vitro spontaneous IgG production of PBMCs from patients with SLE is significantly higher than that produced by PBMCs from HC. However, neither IL-10 (at 2 and 20 ng/ml) nor IL-12 (at 0·2 or 2 ng/ml) significantly altered this production. A high proportion of SLE patients spontaneously made sufficient antissDNA and antidsDNA IgG, in vitro, to be able to measure the effect induced by IL-10 and IL-12. The change in antiss or dsDNA ab production correlated with disease activity at the high concentration of IL-10 (20 ng/ml) suggesting that at the higher disease activities IL-10 inhibited IgG anti-DNA antibody production. This correlation with disease activity was reflected by the five individual patients studied at five different time points. At higher disease activities the lower concentration of IL-12 (0·2 ng/ml) inhibited the patients' IgG anti ssDNA (Fig. 3). No significant correlation was found with antidsDNA ab production in response to 0·2 ng/ml of IL-12, although it did approach significance (P = 0·06).
Effect of IL-10
Several studies have shown that cultures of peripheral blood lymphocytes from SLE patients have increased spontaneous IgG and in particular antidsDNA antibody production in vitro by ELISA [18–21] and by ELISPOT [22], especially in patients with high disease activity. Since increased levels of IL-10 have been found in the serum of patients with SLE which correlate with disease activity using the SLEDAI index [10] it was important to determine the role of this cytokine in the production of spontaneous antibodies, in vitro. It has been shown that the amount of the Th2 cytokine IL-10 produced spontaneously by SLE monocytes and B cells in vitro is 33 times higher than that produced by control cells [23]. Furthermore, the PBMC from relatives and spouses of some patients also produce increased amounts of IL-10, in vitro [24–25]. Inclusion of anti IL-10 into cultures has been shown to inhibit spontaneous IgG and anti dsDNA antibodies [26] whereas inclusion of IL-10 increases IgG production independently of disease activity, in 5 day, T-cell depleted cultures [19]. It is not clear why we were unable to enhance total IgG antibody production with addition of IL-10 independently of disease activity. It is possible that had we cultured the cells for 5 days, as opposed to 3, the changes in IgG may have been more significant. However, 3 day cultures were chosen for the analysis of antidsDNA abs (see Methods).
To the authors knowledge the effect of exogenous IL-10 on in vitro anti-DNA ab production has not previously been described. There was a correlation of the effect of the higher concentration of IL-10 added to the cultures on anti ssDNA and anti dsDNA in relation to disease activity measured on a global scale (Figs 1 and 2). This was even more evident when individual patients were studied during the course of their disease and their antibody levels compared to their disease activity (Fig. 4a). This improved correlation may be due to a closer relationship between serum antidsDNA ab titre and disease activity amongst the longitudinal data than the cross-sectional data.
The enhancement effect seen at low disease activity is consistent with the enhancement of total IgG antibody production seen by Llorente et al. [19]. However, the suppressive effects of IL-10 on anti-DNA antibodies in active patients has not been described before. Llorente et al. looked at the effect of IL-10 on T depleted cultures and the inclusion of T-cells may have modulated the effects of the IL-10. In fact, the contribution that T cells have to the effects of IL-10 in these experiments are unclear. There is some evidence for a T cell contribution to the production of spontaneous autoantibodies in vitro. Early experiments in this field showed that both CD4 and CD8 T cells contributed to this production [27]. In addition, T cells expressing neither CD4 nor CD8, i.e. double negative T cells have been shown to ‘help’ the production of spontaneous autoantibodies by SLE PBMC in vitro [28–29]. In more recent experiments CD4 + RO + T cells were shown to produce IL-10 and therefore thought to contribute in this way [26]. However, data from Llorente et al. shows that SLE T cells produce little IL-10 [23]. It is clearly important to compare the effects of IL-10 with and without added T cells on both total IgG and antidsDNA ab production. These experiments are currently in progress.
A more trivial explanation of the inhibition at higher concentrations could be due to toxicity and in particular induction of apoptosis in the T-cells from patients with active disease [30]. This might remove enough T-cell help to reduce antibody production as compared to untreated cultures; this possibiltiy is being investigated. The existing literature suggests that IL-10 does not induce apoptosis in B-cells from SLE patients [31].
It was not surprising that no effects on anti DNA antibodies were seen at the lower dose of IL-10 (2 ng/ml) since this was below the levels of spontaneously produced IL-10 by SLE PBMC in culture [24].
IL-6 is also increased in PBMC cultures of SLE patients compared with controls and RA patients [32] and probably plays a role in spontaneous IgG production in vitro. However, there appears to be a defect in SLE PBMC in the inhibitory effect that IL-10 normally has on IL-6 production [32] suggesting that IL-6 mediated overproduction is not affected by IL-10. Although other researchers have not been able to reproduce these findings [33].
Monocytes are thought to be the major source of IL-10 in SLE [23]. IL-10 has been shown capable of inhibiting the release of many cytokines from monocytes including IL-10 itself [34]. IL-10 is also thought to be capable of inhibiting T-cell activity [35–36]. These experiments however, suggest that IL-10 can have an inhibitory effect on B cell function at high doses. This inhibitory action may work directly on B-cells, may be a function of IL-10 s inhibitory action on other cell types or some combination of both of these factors.
Effect of IL-12
PBMCs from patients with SLE have been shown to produce less spontaneous IL-12, in vitro, than healthy controls [13, 37], this appears to be due to increased IL-10 output which inhibits production of IL-12 by monocytes [14]. In our study we have added exogenous IL-12 with the aim of reversing the balance of a predominantly Th2 related cytokine IL-10 to that of a Th1 predominant cytokine IL-12. Addition of 0·2 ng/ml of IL-12 had no overall effect on IgG production but did suppress antissDNA ab production, particularly at higher disease activities (Fig. 3).
A similar finding was made in another study where addition of 2 ng/ml of IL-12 to PBMC cultures from SLE patients decreased the number of anti-DNA antibody producing B-cells as measured by ELISPOT [22]. The mechanism by which this occurs is unclear. IL-12 receptors have been described on human B cells activated by Staphylococcus aureus Cowan strain [38]. Since B cells from SLE patients show an activated phenotype it is not inconceivable that IL-12 directly binds to those cells particularly active in making anti DNA antibodies resulting in inhibition of antibody synthesis.
Surprisingly, treatment with IL-12 at 2 ng/ml increased the antidsDNA ab production of some patients in our study (Fig. 4b). Contrary to the antibody responses to IL-10 and the lower concentration of IL-12, disease activity did not seem to be a consistent governing factor.
Preliminary experiments using PBMC from three patients have shown an increase of IL-10 in culture following addition of IL-12. Up to 167 pg/ml of IL-10 has been detected following culture with IL-12 at 2 ng/ml, none was detected in response to IL-12 at 0·2 ng/ml. Indeed IL-12 has been shown to induce IL-10 production from T-cell clones [39]. This difference in the ability of these two concentrations of IL-12 to induce IL-10 may explain their contrasting effects on antibody production. An effect on antibody production that is reliant on the induction of other cytokines may take some time to develop. This may explain why Houssiau et al. [22] did not detect it in their 16 h cultures.
Studies on the direct effects of IL-10 and IL-12 on human anti dsDNA producing clones are currently under way.
In conclusion, the relationship between IL-10, IL-12 and antibody production are likely to be complex since autoantibody production is the result of interaction with different cytokines acting on populations of cells at different stages of activation depending on the disease activity and treatment with cytokine modulating drugs.
Acknowledgments
The authors would like to thank Myer Salaman for his help in setting up the ELISAs used in this study and Esther Crawley for her statistical advice. This research was funded by the Emily de Rossignol fund, a studentship from University College London.
REFERENCES
- 1.Quismorio FP. Clinical application of serological abnormalities in SLE. In: Wallace DJ, Hahn BH, editors. Dubois' Lupus Erythematosus. Baltimore: Lea and Febiger; 1993. p. 462. [Google Scholar]
- 2.Williams CR, Malone C, Blood B, Sivestri F. Anti-DNA and anti-nucleosome antibody affinity – A mirror image of lupus nephritis? J Rheumatol. 1999;26:331–46. [PubMed] [Google Scholar]
- 3.Ehrenstein MR, Katz DR, Griffiths MH, et al. Human IgG DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–11. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 4.Ravirajan CT, Rahman MA, Papadaki L, et al. Genetic, structural and functional properties of an IgG DNA-binding monoclonal antibody from a lupus patient with nephritis. Eur J Immunol. 1998;28:339–50. doi: 10.1002/(SICI)1521-4141(199801)28:01<339::AID-IMMU339>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.D'Andrea DM, Coupaye Gerard B, Kleyman TR, Foster MH, Madaio MP. Lupus autoantibodies interact directly with distinct glomerular and vascular cell surface antigens. Kidney Int. 1996;49:1214–21. doi: 10.1038/ki.1996.175. [DOI] [PubMed] [Google Scholar]
- 6.Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- 7.Viallard JF, Pellegrin JL, Ranchin V, et al. Th1 (IL-2, interferon-gamma (IFN gamma) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;115:189–95. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funauchi M, Ikoma S, Enomoto H, Horiuchi A. Decreased Th1 and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheumatol. 1998;27:219–24. doi: 10.1080/030097498440859. [DOI] [PubMed] [Google Scholar]
- 9.De Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, De Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 10.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–8. [PubMed] [Google Scholar]
- 11.Hagiwara E, Gourley MF, Lee S, Klinman DK. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin 10: interferon gamma secreting cells in peripheral blood. Arthritis Rheum. 1996;39:379–85. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 12.Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer JP, Renauld JC. Serum interleukin-10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–5. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 13.Lui TF, Jones BM. Impaired production of IL-12 in systemic lupus erythematosus. II. IL-12 production in vitro I correlated negatively with serum IL-10, positively with serum IFN gamma and negatively with disease activity in SLE. Cytokine. 1998;10:148–53. doi: 10.1006/cyto.1997.0269. [DOI] [PubMed] [Google Scholar]
- 14.Lui TF, Jones BM. Impaired production of IL-12 in sytemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes. Cytokine. 1998;10:140–7. doi: 10.1006/cyto.1997.0268. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–78. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17.Hay EM, Bacon PA, Gordon C, et al. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86:447–58. [PubMed] [Google Scholar]
- 18.Dar O, Salaman MR, Seifert MH, Isenberg DA. B lymphocyte activation in sytemic lupus erythematosus: spontaneous production of IgG antibodies to DNA and environmental antigens in cultures of blood mononuclear cells. Clin Exp Immunol. 1988;73:430–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Llorente L, Zou W, Levy Y, et al. Role of interleukin-10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spronk PE, van der Gun BT, Limburg PC, Kallenberg CG. B-cell activation in clinically quiescent systemic lupus erythematosus (SLE) is related to immunoglobulin levels, but not to anti-dsDNA, nor to concurrent T-cell activation. Clin Exp Immunol. 1993;93:39–44. doi: 10.1111/j.1365-2249.1993.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spronk PE, Horst G, van der Gun BT, Limburg PC, Kallenberg CG. Anti-dsDNA production coincides with concurrent B and T-cell activation during development of active disease in systemic lupus erythematosus (SLE) Clin Exp Immunol. 1996;104:446–53. doi: 10.1046/j.1365-2249.1996.44754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houssiau FA, Mascart Lemone F, Stevens M, et al. IL-12 inhibits in vitro immunoglobulin production by human lupus peripheral blood mononuclear cells (PBMC) Clin Exp Immunol. 1997;108:375–80. doi: 10.1046/j.1365-2249.1997.d01-1009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llorente L, Richaud Patin Y, Wijdnes J, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupis erythematosus. Eur Cytokine Netw: 1993;4:421–7. [PubMed] [Google Scholar]
- 24.Llorente L, Richaud Patin Y, Couderc J, et al. Dysregulation of interleukin-10 production in relatives of patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1429–35. doi: 10.1002/art.1780400810. [DOI] [PubMed] [Google Scholar]
- 25.Grondal G, Kristjansdottir H, Gunnlaugsdottir B, et al. Increased number of interleukin-10 producing cells in systemic lupus erythematosus patients and their first degree relatives and spouses in Icelandic multicase families. Arthritis Rheum. 1999;42:1649–54. doi: 10.1002/1529-0131(199908)42:8<1649::AID-ANR13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.al Janadi M, al-Dalaan A, al-Balla S, al-Humaidi M, Razuiddin S. Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45+RO+ T cell-B cell antibody synthesis. J Clin Immunol. 1996;16:198–207. doi: 10.1007/BF01541225. [DOI] [PubMed] [Google Scholar]
- 27.Linker Israeli M, Quismorio FP, Horwitz DA. CD8+ lymphocytes from patients with systemic lupus eryhthematosus sustain rather than suppress spontaneous polyclonal IgG production and synergize with CD4+ cells to support autoantibody synthesis. Arthritis Rheumatism. 1990;33:1216–25. doi: 10.1002/art.1780330823. [DOI] [PubMed] [Google Scholar]
- 28.Datta SK. Production of pathogenic antibodies: cognate interactions between autoimmune T and B cells. Lupus. 1998;7:591–6. doi: 10.1191/096120398678920703. [DOI] [PubMed] [Google Scholar]
- 29.Datta SK, Kaliyaperumal A, Mohan C, Desai Mehta A. T-helper cells driving pathogenic autoantibody production in lupus: nucleosomal epitopes and CD40 Ligand signals. Lupus. 1997;6:333–6. doi: 10.1177/096120339700600330. [DOI] [PubMed] [Google Scholar]
- 30.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation induced cell death of SLE lymphocytes mediated by Fas ligand. J Clin Invest. 1997;100:2622–33. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toubi E, Telford W, Elkon KB. An apoptosis defect in lupus B cells revealed by B cell receptor or IL-4 rescue from activation induced cell death. Arthritis Rheumatism. 1998;41(9) Suppl.:abstract222. [Google Scholar]
- 32.Mongan RE, Ramdahin S, Warrington RJ. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand J Immunol. 1997;46:406–12. doi: 10.1046/j.1365-3083.1997.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 33.Linker Israeli M, Honda M, Nand R, et al. Exogenous IL-10 and IL-4 down-regulate IL-6 production by SLE derived PBMC. Clin Immunol. 1999;91:6–16. doi: 10.1006/clim.1998.4680. [DOI] [PubMed] [Google Scholar]
- 34.De Waal Malefyt R, Abrams J, Bennet B, Fidgor CG, De Vries JE. Interleukin 10 (IL-10) Inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akdis CA, Blaser K. IL 10-induced anergy in peripheral T-cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999;13:603–9. doi: 10.1096/fasebj.13.6.603. [DOI] [PubMed] [Google Scholar]
- 36.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin-10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwitz DA, Gray JD, Behrendsen SC, et al. Decreased production of interleukin-12 and other Th1-Type cytokines in patients wih recent onset systemic lupus erythematosus. Arthritis Rheumatism. 1997;41:838–44. doi: 10.1002/1529-0131(199805)41:5<838::AID-ART10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Vogel LA, Showe LC, Lester TL, McNutt RM, Van Cleave VH, Metzger DW. Direct binding of IL-12 to human and murine B lymphocytes. Int Immunol. 1996;8:1955–62. doi: 10.1093/intimm/8.12.1955. [DOI] [PubMed] [Google Scholar]
- 39.Gerosa F, Paganin C, Peritt D, et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon gamma and interleukin-10. J Exp Med. 1996;183:2559–69. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]