Abstract
Previous findings have indicated that the major surface molecule of Leishmania, lipophosphoglycan (LPG), could abrogate HIV-1-induced syncytium formation and virus replication. In the present work, we were interested in characterizing this inhibitory process. Data from a new luciferase-based semiquantitative assay for syncytium formation, relying on the coincubation of a T-cell line containing an HIV-1 LTR-driven luciferase construct with a cell line chronically infected with HIV-1, confirmed that LPG was indeed a strong inhibitor of HIV-1-dependent syncytium formation and that this inhibition was dose-dependent. As determined by flow cytometric analyses, this inhibition was not apparently due to downregulation of CD4, CXCR4 or LFA-1, three distinct surface glycoproteins known to be important in HIV-1 mediated syncytium formation. Furthermore, LPG did not seem to affect signal transduction pathways in T cells as judged by measurement of HIV-1 LTR-driven reporter gene activity upon treatment with different stimuli. However, pretreatment of either of the cell lines used in the assay with LPG led to a significant decrease of virus-mediated syncytium formation, which was further accentuated when both cell lines were pretreated. LPG inhibition of HIV-1 replication was next assessed. When measuring either infection with luciferase-encoding recombinant HIV-1 particles or multinucleated giant cell formation following an acute virus infection, we again observed that LPG was efficient at blocking HIV-1 replication. Specific assays probing different steps of viral entry demonstrated that attachment was not hindered by LPG but that viral entry was modulated, suggesting that LPG targets a postbinding step. Hence, incorporation of LPG into a target cell membrane could influence its fluidity and diminish both the virus-cell and cell-to-cell fusion processes initiated by HIV-1.
Keywords: HIV-1, syncytium formation, viral entry, lipophosphoglycan
INTRODUCTION
Human Immunodeficiency Virus Type-1 (HIV-1), the aetiological agent of AIDS, mainly infects T cells but is also targets several non-T cell types, such as monocytes and macrophages, that are known to be a potent reservoir for viral replication. Infection occurs through a tripartite interaction between the viral gp120 glycoprotein and the CD4 molecule in conjunction with a chemokine coreceptor. Determination of the tropism of the HIV-1 isolates is mainly dependent on the specific usage of the chemokine receptor; macrophage-tropic isolates use the CCR5 coreceptor while the T cell tropic isolates mainly interact with CXCR4 [1–7]. It is now thought that, following the initial gp120–CD4 interaction, a high affinity domain on gp120 resulting from a conformational change permits an efficient interaction with the appropriate coreceptor. This complex then culminates with the destabilization of the gp120 trimers, subsequent exposure of the viral gp41 fusion peptide and the ultimate fusion event between virus and cell membranes.
One of the major characteristics of HIV-1 infection is the progressive diminution in the CD4-positive T-cell count in infected individuals. Ultimately, as a result of this immune cell depletion, patients develop AIDS-related symptoms [8,9]. CD4 + T cell depletion during HIV-1 infection has been extensively investigated and several virus-dependent mechanisms have been postulated. One of the best described cytopathic phenomenon associated with HIV-1 infection remains the appearance of giant multinucleated cells (termed ‘syncytia’) resulting from multiple cell-to-cell fusion events and leading to cell death [10]. In a similar fashion to the process of HIV-1 entry, formation of syncytia depends mainly on the interaction between CD4 and the CXCR4 coreceptor on uninfected cells with gp120 on virally infected cells. Consequently, T-tropic viruses are almost exclusively responsible for syncytium formation in cell culture while macrophage-tropic viruses are generally inefficient at forming syncytia, at least when grown on cells of the monocyte lineages [3–6]. However, it must be borne in mind that many other interacting surface molecules have been demonstrated to cooperate in syncytium formation, including HLA class I [11], LFA-1 and its counter-ligands ICAM-1 [12–15], ICAM-2 and ICAM-3 [16].
Since HIV-1-induced syncytium formation and the process of HIV-1 entry bear several points in common, many researchers have investigated strategies to inhibit both syncytium formation and HIV-1 infection. In fact, many compounds such as soluble CD4 (sCD4), specific antibodies, complestatin, terpestacin, lysophosphatidylcholine and lipophosphoglycan have been reported to act as inhibitors of both syncytium formation and virus infectivity [17–23]. In one study, Easterbrook and colleagues have demonstrated that lipophosphoglycan (LPG), one of the major surface components of the protozoan parasite Leishmania, could inhibit HIV-1-mediated syncytium formation as well as virus infection of CD4+ T cells [18]. This observation is reminiscent of similar findings dealing with LPG inhibition of Sendai virus entry analysed by confocal laser scanning microscopy [24].
LPG is composed of an average of 16 repeated phosphorylated disaccharide units linked via a hexasaccharide carbohydrate core to an alkylphosphatidylinositol lipid anchor [25–28]. Many roles have been attributed to this surface molecule during the cycle of infection of the Leishmania parasite such as resistance to complement-mediated lysis [29], attachment to host macrophages [30], protection from destruction within macrophage phagolysosomes [31–33] and inhibition of protein kinase C (PKC) [34–36]. Given the potential inhibitory action of LPG on HIV-1-related processes, we were interested to determine the mechanism(s) of the inhibitory effects conferred by LPG on HIV-1-induced syncytium formation and infectivity. In our investigation, we have used a new method recently published by our group [12] to quantitatively evaluate syncytium formation. Our results suggest that inhibition of virus-mediated syncytium formation and HIV-1 infection by LPG occurs at a postbinding step, and more likely at the fusion level.
METHODS
Cells lines
All cell lines were grown in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum (Gibco BRL, Grand Island, NY), glutamine (2 mm), penicilline G (100 U/ml), and streptomycin (100 mg/ml). Sup-T1 [37] and Jurkat clone E6·1 [38] are CD4+ T cell lines. 1G5 is a Jurkat-derived cell line which harbors two copies of a stably transfected plasmid made of the luciferase reporter gene downstream of the HIV-1 long-terminal repeat region (LTR) [39]. J1·1 is a Jurkat-derived cell line that is chronically infected with the HIV-1LAV strain [40].
Reagents
Lipophosphoglycan (LPG) from stationary phase Leishmania donovani was kindly provided by Dr Salvatore J. Turco (Department of Biochemistry, University of Kentucky College of Medicine, Lexington, USA) and resuspended at a concentration of 105 mm in serum-free RPMI medium. Isolation and purification of LPG have been previously described [25]. The National Institute of Health AIDS Repository Program generously gave hybridoma that produces anti-CD4 SIM.4 antibodies. Anti-CXCR4 (clone 12G5) monoclonal antibodies were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases. The MEM-30 (anti-CD11a) anti-LFA-1 antibody was a kind gift from Dr Vaclav Horejsi (Institute of Molecular Genetics, Prague, Czech Republic). Cell activators used included PMA (Phorbol-12-myristate-13-acetate; Sigma), PHA-P (Phytohemagglutinin; Sigma), TNF-α (Sigma), anti-CD3 (clone OKT3) and anti-CD28 (clone 9·3) antibodies. Anti-CD3 and the anti-CD18 (LFA-1) TS1/18·1 hybridomas were obtained from the American Type Culture Collection (Rockville, MD, USA). Antibodies from these hybridomas were purified with mAb trap protein G affinity columns according to manufacturer's instructions (Pharmacia, LKB Biotechnology AB, Uppsala, Sweden). Purified anti-CD28 antibodies were a generous gift from Dr Jeffrey A. Ledbetter (Bristol-Myers Squibb, Seattle, USA) [41].
Fluorescence-activated cell sorter (FACS) analysis
Cells were incubated for 30 min on ice in 100 µl of ice-cold PBS containing saturating concentrations of monoclonal anti-CD4 (SIM.4), anti-CXCR4 (12G5) or anti-LFA-1 (MEM30 or TS1 18·1) antibodies. Cells were then washed twice with 500 µl of ice-cold PBS and incubated for another 30 min in 100 µl of ice-cold PBS containing FITC-conjugated goat antimouse IgG (Caltag Laboratories, San Francisco, USA). Cells were washed twice with ice-cold PBS and resuspended in 500 µl of PBS containing 1% (w/v) paraformaldehyde before flow cytometry analysis (EPICS XL; Coulter Corp. Miami, USA). Controls consisted of commercial isotype-matched irrevelant monoclonal antibodies (Sigma, St. Louis, USA.).
Cell activation
1G5 cells were initially either pretreated or not with increasing concentrations of LPG (5, 10 and 20 µm). Afterward, 1G5 cells (1 × 105) were aliquoted in triplicate in a final volume of 200 µl in 96-well plates and subsequently stimulated with PMA (20 ng/ml), PHA-P (3 µg/ml), TNF-α (2 ng/ml) or a combination of anti-CD3 (clone OKT3) (3 µg/ml)/anti-CD28 (clone 9·3) (1 µg/ml) antibodies. After an 8-h incubation period at 37°C, 100 µl of cell-free supernatant from each well was removed and 25 µl of 5× cell culture lysis buffer (125 mm triphosphate [pH 7·8], 10 mm dithiothreitol [DTT] 5% Triton X-100, 50% glycerol) was then added for a 30-min incubation period at room temperature. An aliquot of cell lysate was then mixed with 100 µl of luciferase assay buffer [20 mm tricine, 1·07 mm (MgCO3)4 Mg(OH)2·5H2O, 2·67 mm MgSO4, 0·1 mm EDTA, 270 µm Coenzyme A, 470 µm luciferine, 530 µm ATP, 33·3 mm DTT] and luciferase activity measured with a microplate luminometer device (MLX; Dynex Technologies, Chantilly, USA).
Syncytium assay
Syncytium formation was evaluated following a previously described luciferase-based quantitative assay [12]. Briefly, 1G5 and J1·1 cells were resuspended at 106 cells/ml and 100 µl of each cell suspension were intermixed and incubated for 12 h at 37°C in the presence or the absence of different concentrations of LPG. In some experiments, either of the cell lines used in the assay were pretreated for 1 h in the presence of LPG and washed thoroughly before starting the coculture experiment. The SIM.4 anti-CD4 antibody (20 µg/ml) was also occasionally added along with the coincubated cells as a control. Controls also consisted of 1G5 or J1·1 cells incubated alone for the same time period. After a 12-h incubation period, cells were lysed and measured for luciferase activity as described above.
Production of HIV-1 particles and infectivity assay
The production of HIV-1 particles was performed according to a previously described protocol [42]. Briefly, 293T cells were transfected by the calcium phosphate protocol with the pNL4–3 proviral DNA vector. The supernatant of transfected cells was harvested 48 h post-transfection and HIV-1 particles were quantified according to a commercially available p24 enzymatic assay (Organon Teknika, Durham, NC). The effect of LPG on HIV-1 infection was analysed by two different methods. First, Sup-T1 cells at a concentration of 106 cells/ml were incubated with 100 ng of p24 gag (NL4–3 strain) in a 24-well plate in the presence or absence of 10 µm LPG. Cells were then left at 37°C for 7 days and photographed at a 100× magnification through an inverted microscope. Second, 1G5 (1 × 105) cells were infected with 10 ng of p24 gag (NL4–3 strain) in a 96-well plate in triplicate and incubated for 48 h in the presence or absence of 5 µm LPG. It should be noted that both virus and Leishmania LPG remained throughout the duration of the experiments. Cells were then lysed and luciferase activity was determined as described above. In one set of experiments, HIV-1NL4-3 (10 ng of p24 gag) was initially incubated at 37°C for 30 min in the presence or absence of Leishmania LPG (5 µm). Excess LPG was removed by an ultracentrifugation step and the virus pellet was used to infect 1G5 cells for 48 h before monitoring luciferase activity.
Attachment and entry assays
Attachment assay was performed as follows. Jurkat cells (1 × 105) were washed once with cold PBS and incubated with HIV-1NL4-3 (10 ng of p24) in the presence or absence of 10 µm LPG for 30 min on ice to allow virus attachment only. Samples were washed three times with cold PBS and dispensed in a 96-well plate. Negative controls consisted of Jurkat cells alone. Quantification of bound viruses was performed through a commercial p24 enzymatic assay. All samples were tested in triplicate. Entry assay was performed as previously described [43,44]. Briefly, for each sample, Jurkat cells (1 × 106) were washed once with room temperature PBS and resuspended in the presence or absence of 10 µm LPG in 1 ml of complete culture medium supplemented with HIV-1NL4-3 (10 ng of p24). Cells were incubated for 2·5 h at 37°C and were subsequently washed twice with ice-cold PBS and resuspended in 1 ml of ice-cold FCS-free Dulbecco Modified Eagle Medium (DMEM) in the presence of 0·1 mg pronase (Boehringer Mannheim, Laval, Canada). Cells were then incubated for 5 min on ice and immediately washed twice with ice-cold DMEM containing 10% FCS and three times with cold PBS to remove the pronase. Cells were resuspended in 0·6% Triton-X-100-containing RPMI medium, incubated for 10 min at room temperature under constant agitation and stored at −85°C until assayed for p24 content by standard p24 enzymatic assay.
RESULTS
Syncytium formation is inhibited by LPG in a dose-dependent manner
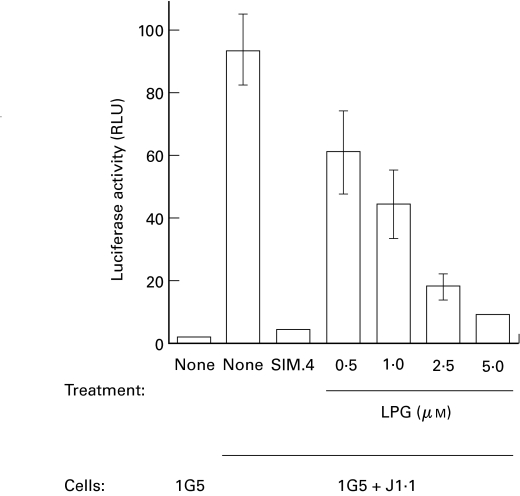
We were first interested in evaluating LPG's inhibitory potential on HIV-1-dependent syncytium formation using our previously described syncytium quantitative assay [12]. The principle of this assay is based on the use of two cell types which, upon fusion, permit free diffusion of the viral Tat protein from the chronically HIV-1 infected cell line J1·1 to the 1G5 cell line. This latter cell line stably harbors an HIV-1 LTR-driven luciferase construct and syncytium formation is measured as relative light units, correlating with transcription of the luciferase reporter gene. Increasing concentrations of LPG were added to the 1G5/J1·1 coculture and luciferase activity measured after 12 h, the optimum time point for this assay. As clearly depicted in Fig. 1, the addition of J1·1–1G5 led to a pronounced increase in luciferase activity in comparison with 1G5 cells alone. However, we observed a significant decrease in luciferase induction when LPG was added. This decrease was dose-dependent: the percentage inhibition being 35%, 52%, 81% and 90% at LPG concentrations of 0·5, 1, 2·5 and 5 µm, respectively. As a positive control, SIM.4, an anti-CD4 antibody known to be specific for the HIV-1 gp120 binding epitope on CD4 [45], was added to the cocultured cells. SIM.4 greatly diminished syncytium formation (up to 95·5%) as measured by luciferase activity. All of these results were confirmed by visual assessment of syncytium numbers in untreated versus LPG-treated coculture experiments (data not shown). Furthermore, the addition of LPG was not found to downregulate the basal level of HIV-1 LTR-dependent reporter gene expression in 1G5 cells (data not shown). Results from this syncytium quantitative assay clearly demonstrate the capacity of Leishmania LPG to abolish HIV-1-mediated syncytium formation in the context of human T cells.
Fig. 1.
LPG inhibits HIV-1-mediated syncytium formation in a dose-dependent manner. 1G5 and J1·1 cells (1 × 105 each) were mixed, in the absence or the presence of increasing concentrations of Leishmania LPG (0·5, 1, 2·5, and 5 µm), and incubated for 12 h. Controls consisted of either cocultured cell samples incubated with the anti-CD4 SIM.4 antibody (20 µg/ml) or 1G5 cells incubated alone. Cells were lysed and assayed for luciferase activity as described in Materials and Methods. Results shown are the mean ±SD of each treatment from triplicates. This is representative of two independent experiments.
Cell surface expression levels of CD4, CXCR4 and LFA-1 are not quantitatively affected by LPG
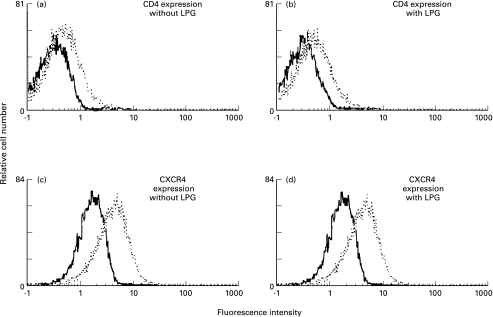
Based on the demonstration that LPG was comparable to SIM.4 in its capacity to inhibit syncytium formation, we next wanted to verify whether, through steric hindrance or other mechanisms, LPG could decrease CD4 surface expression and thereby jeopardize fusion of cellular membranes between infected and uninfected cells. By the same logic, the CXCR4 coreceptor was similarly tested. 1G5 cells were thus initially either treated or not with 5 µm LPG and analysed by FACS analysis using either SIM.4 (anti-CD4) or 12G5 (anti-CXCR4) monoclonal antibody. The level of CD4 or CXCR4 on the surface of LPG-treated Jurkat cells was not found to be quantitatively different from untreated cells (Fig. 2). Another surface molecule which was tested by FACS analysis was the LFA-1 adhesion molecule. The importance of the interaction between LFA-1 and ICAM-1, ICAM-2 and ICAM-3 for HIV-1-induced syncytium formation has previously been demonstrated [12–16,46]. In addition, LPG has been demonstrated to interact with LFA-1 [47] and so might affect syncytium formation by diminishing the amount of surface LFA-1. We thus performed FACS analysis for LFA-1 expression using the TS1 18·1 anti-LFA-1 antibody and showed that levels of LFA-1 on the surface of untreated or LPG-treated Jurkat cells did not differ (data not shown). Similar results were obtained when FACS analysis for LFA-1 expression was performed using another anti-LFA-1 antibody (clone MEM-30). These results suggest that surface expression of important cell surface molecules in syncytium formation is not altered upon LPG treatment.
Fig. 2.
IPG does not modulate levels of surface CD4 (a,b) and CXCR4 (c,d) proteins. Jurkat cells were either left untreated (a,c) or were treated with 5 µm LPG for 1 h at 37°C (b,d) before monitoring surface expression of CD4 (clone SIM.4) or CXCR4 (clone 12G5) by flow cytometry. Controls consisted of cells incubated with isotype-matched irrelevant monoclonal antibodies (—).
Intracellular signal transduction events are not altered by Leishmania LPG
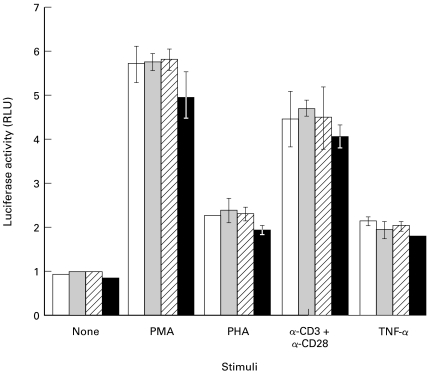
Previous experiments have reported that, depending on the state of stimulation, CD4+ T cells can be more or less sensitive to HIV-1-induced syncytium formation [48]. Since LPG has been reported to interfere with some components of signal transduction, such as calcium [31] and PKC [35,36,49], it seemed plausible that LPG could by such a mechanism affect the state of activation of the cells implicated in syncytium formation and thereby affect the extent to which syncytium formation might occur. The effect of LPG on intracellular signalling events induced by different stimulating agents was thus tested. 1G5 cells were incubated with LPG prior to treatment with different stimuli such as phorbol-12-myristate-13-acetate (PMA), phytohemagglutinin (PHA), TNF-α or the more physiological stimulus consisting of an anti-CD3/anti-CD28 antibody combination. As shown in Fig. 3, increasing concentrations of LPG (5–20 µm) did not interfere with HIV-1 LTR activity in 1G5 cells induced by any of the stimulators tested. This data set shows that LPG does not inhibit normal cell activation (at least in the 1G5 cell line and under the present experimental conditions) and thus probably does not affect syncytium formation through a change in cell activation state.
Fig. 3.
Signal transduction events are not affected by Leishmania LPG. 1G5 cells (1 × 105) were first pretreated or not (control □) with increasing concentration of LPG ( 5 µm; 10 µm; ▪ 20 µm) for 1 h at 37°C and then stimulated or not with PMA (20 ng/ml), PHA (3 µg/ml), a combination of anti-CD3 (clone OKT3 at 3 µg/ml) and anti-CD28 (clone 9·3 at 1 µg/ml) antibodies or TNF-α (2 ng/ml). After an 8-h stimulation, cells were lysed and assessed for luciferase activity as described in Materials and Methods. Results shown are the mean ±SD of each treatment from triplicates. This is representative of two independent experiments.
5 µm; 10 µm; ▪ 20 µm) for 1 h at 37°C and then stimulated or not with PMA (20 ng/ml), PHA (3 µg/ml), a combination of anti-CD3 (clone OKT3 at 3 µg/ml) and anti-CD28 (clone 9·3 at 1 µg/ml) antibodies or TNF-α (2 ng/ml). After an 8-h stimulation, cells were lysed and assessed for luciferase activity as described in Materials and Methods. Results shown are the mean ±SD of each treatment from triplicates. This is representative of two independent experiments.
LPG acts on both viral envelope-expressing cells and the uninfected cellular fusion partner
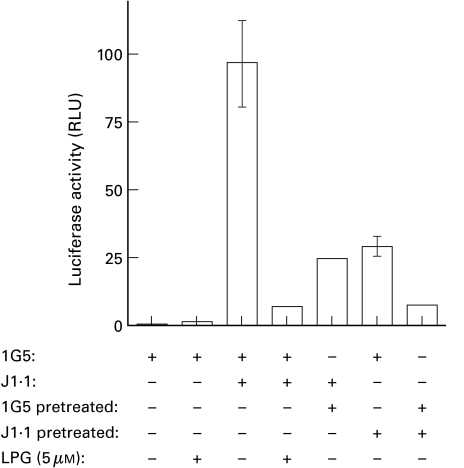
LPG has been proposed to influence the membrane fluidity of cells by its insertion in the cell membrane [24]. Such a mechanism might contribute to the LPG-mediated decrease of syncytium formation and would suggest that LPG inhibition is not specific for either cell line involved in syncytium formation. Thus, we next investigated the cell specificity of LPG inhibition. 1G5 or J1·1 cell lines were pretreated with 5 µm LPG and, after washing both untreated or pretreated cell lines, the cells were coincubated for 12 h and assessed for HIV-1 LTR-driven luciferase activity. Results show that, regardless of the cell line, pretreatment of only one of the cell fusion partners (i.e. 1G5 or J1·1) led to a significant and equal diminution in luciferase activity (Fig. 4). In addition, independent pretreatment of both cell lines and subsequent coculture resulted in comparable inhibition of syncytium formation as compared with direct LPG treatment of coincubated cells. All of these experiments were confirmed visually by inverted microscopy (data not shown). These experiments demonstrate that LPG inhibits syncytium formation through an interaction with either the infected or uninfected cell line and that pretreatment with LPG is sufficient for inhibition, suggesting a required strong interaction of LPG with either cell line.
Fig. 4.
LPG inhibits syncytium formation through both uninfected and HIV-1-infected cells. 1G5 and J1·1 cells were first pretreated individually or not for 1 h at 37°C with 5 µm of LPG. Pretreated or untreated cells (1 × 105) were then coincubated in equal numbers. Co-incubation experiments were also performed with 1G5 and J1·1 cells to which LPG was directly added. As controls, LPG-pretreated or untreated 1G5 cells were incubated alone. After a 12-h incubation period, cells were lysed and assayed for luciferase activity as described in Materials and Methods. Results shown are the mean ±SD of each treatment from triplicates. This is representative of two independent experiments.
Early events in the replicative cycle of HIV-1 are affected by LPG
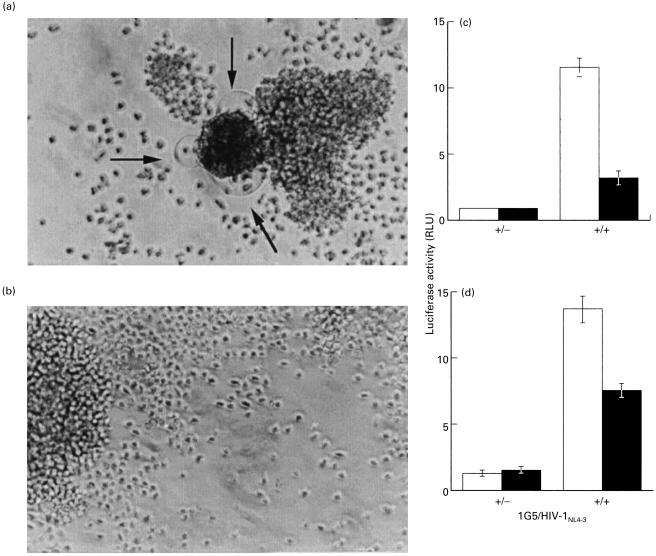
We next wanted to investigate the effect of LPG on the process of virus replication and initially decided to look at this in two different systems. First, the CD4-positive T-lymphoid cell line Sup-T1 was infected for 7 days with the T-tropic NL4–3 strain of HIV-1 in the presence or absence of 5 µm LPG. For each day of the incubation period, infection was qualitatively monitored by the presence of syncytia and photographed (Fig. 5). Fig. 5a shows typical syncytia in virally infected Sup-T1 cells in the absence of LPG. However, the presence of LPG caused an important diminution in the number of observed syncytia (Fig. 5b). To further confirm these results, we tested HIV-1 infectivity using a previously described protocol whereby HIV-1 entry and integration leads to Tat production and subsequent activation of a luciferase reporter gene driven by the HIV-1 LTR [42]. Thus, 1G5 cells were infected with HIV-1NL4-3 in the presence or absence of 5 µm LPG and incubated for 48 h before measuring luciferase activity. In Fig. 5c, results show that the addition of LPG to cultured 1G5 cells resulted in an inhibition of up to 70% of virus-encoded luciferase activity. Altogether, these results demonstrate that, in two different systems, LPG inhibited HIV-1 replication. Furthermore, because of the short incubation time between 1G5 cells and viruses (i.e. 48 h) allowing little or no reinfection, this suggests that LPG most likely targeted an early point in the HIV-1 life cycle. Additional experiments revealed that Leishmania LPG exerted a direct effect on the virus alone (Fig. 5d). However, the degree of inhibition was somewhat lower than that of LPG on the virus/cell mixture (Fig. 5c) suggesting that LPG has an effect on both entities (i.e. the virus and target cells).
Fig. 5.
LPG inhibits HIV-1 replication. Sup-T1 cells (1 × 106) were infected with HIV-1NL4–3 (100 ng of p24 gag) in the absence (a) or presence (b) of LPG (10 µm). After an incubation period of 7 days, cells were photographed at a magnification of 100× with an inverted microscope. Arrows indicate the presence of syncytia. 1G5 cells (1 × 105) were infected with HIV-1NL4-3 (10 ng of p24 gag) in the presence (▪) or absence (□) of Leishmania LPG (5 µm) for 48 h (c). In some experiments, HIVNL4-3 (10 ng of p24) was first incubated in the presence or the absence of Leishmania LPG (5 µm) before inoculation of 1G5 cells (1 × 105) for 48 h (d). Finally, cells were lysed and assayed for luciferase activity as described in Materials and Methods. Results shown are the mean ±SD of each treatment from triplicates. This is representative of two independent experiments.
LPG does not affect viral attachment but inhibits the fusion step in the HIV-1 entry process
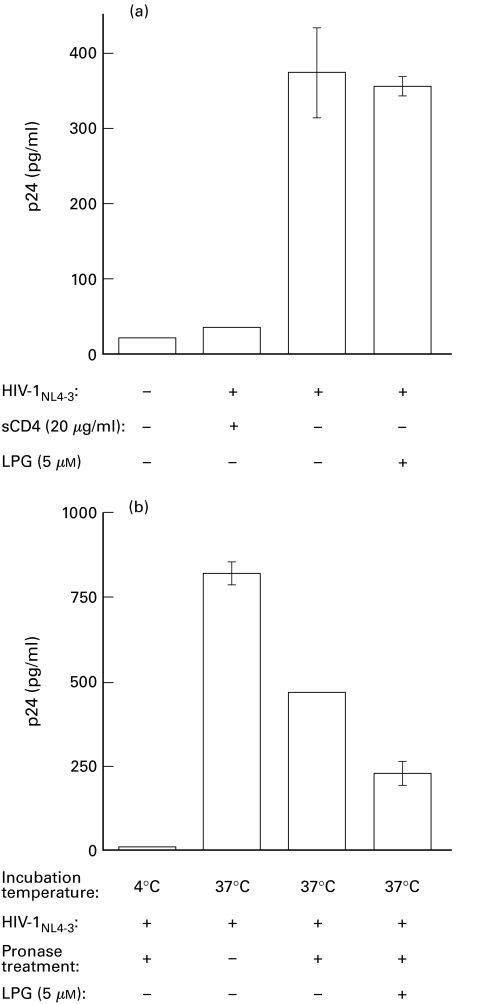
Since most of the early steps in the virus life cycle occur in a similar fashion to syncytium formation, we next wanted to see whether LPG inhibition of both HIV-mediated syncytium formation and virus replication involved blockade of either the binding or the fusion step. To do so, we used two different assays. We first initiated an attachment assay based on the notion that HIV-1 binding is not energy-dependent and so can be achieved at 4°C unlike the fusion event which requires a 37°C incubation period (see the negative control of Fig. 6, panel B). Bound viruses can then directly be quantified by a simple p24 enzymatic assay using washed cells. As presented in Fig. 6a, LPG did not modulate the binding of the virus to the target cells, as viral p24 levels in LPG-treated cells were only minimally reduced compared with the levels in untreated cells. As a control, sCD4 was shown to abolish the binding of virions to target cells. Second, to test whether fusion of the viral and cell membranes might instead be the affected entry point in LPG-treated cells, cells were incubated at 37°C with HIV-1NL4-3 for 2·5 h in the absence or presence of LPG. The cells were then treated with pronase to remove any bound viruses that had not fused with the cell membrane and this was followed by several washes. Assessment of viral p24 levels in these cells permitted us to selectively determine the extent to which viruses fused and penetrated the target cells. As a positive control, Jurkat cells incubated with the virus in the absence of pronase treatment gave, as expected, the highest p24 values resulting from the presence of both fused and bound viruses (Fig. 6b). A negative control consisting of pronase-treated cells incubated with the same number of HIV-1 particles at 4°C showed nearly basal level values of p24, demonstrating the need for the 37°C incubation period for fusion to occur. However, when internal p24 was measured in pronase-treated cells incubated at 37°C, a significant reduction in p24 levels was noticed when cells were pretreated with LPG. We thus suggest that the fusion event during HIV-1 infection is affected by LPG treatment of target cells and most likely parallels the LPG-mediated inhibition observed in virus-dependent syncytium formation.
Fig. 6.
LPG acts at a postbinding step on the HIV-1 replicative cycle. (a) LPG-treated or untreated Jurkat cells (1 × 105) were incubated in the presence of HIV-1NL4-3 (10 ng of p24 gag) at 4°C for 30 min. After extensive washing, cells were lysed and p24 quantification was performed. Controls consisted of either uninfected or Jurkat cells treated with sCD4 before inoculation with viruses. (b) LPG-treated or untreated Jurkat cells (1 × 105) were incubated in the presence of HIV-1NL4-3 (100 ng of p24 gag) at 37°C for 2·5 h. and then treated with pronase (0·1 mg/ml) for 5 min. Controls consisted of samples not treated with pronase or samples which were incubated with the virus at 4°C prior to the addition of pronase. Standard p24 quantification was subsequently performed after cell lysis. Results shown represent the mean ±SD for each sample carried out in triplicate. These data are representative of three independent experiments.
DISCUSSION
Investigating the mechanisms by which specific and nonspecific inhibitors alter the primary steps and the cytopathic effects of HIV-1 infection has always been important for understanding AIDS. On the basis of a previous report [18], we were interested to specifically analyse the inhibitory effect of the LPG surface molecule of Leishmania on HIV-1-induced syncytium formation and viral entry. In the current study, we provide evidence suggesting that Leishmania LPG blocks HIV-1 viral entry at a postbinding step. Based on data from the present and previous studies involving LPG inhibition of Sendai virus entry [24,50], we propose that the inhibitory process of LPG on both HIV-1-mediated syncytium formation and viral entry occurs at the fusion step.
We first tested the Leishmania LPG inhibitory potential on multinucleated giant cell formation with our new syncytium quantitative assay. Our results permitted us to corroborate the inhibitory potential previously observed by Easterbrook and coworkers [18]. Our quantitative method measured a dose-dependent inhibition of up to 90% with 5 µm LPG. A similar dose-dependent inhibition of syncytia formation by LPG was also observed by this group but with a maximal inhibition of no greater than 76% (20 µm LPG). We believe that our quantitative assay based on viral Tat-dependent activation of luciferase reporter gene transcription which further relies on cell-to-cell fusion events, might be more accurate for assessing syncytium formation. Simple visual counting underestimates the extent of syncytium formation, especially when high cell numbers are involved in each syncytia, whereas such underestimation is likely to be minimized in our syncytium quantitative assay.
HIV-1 usually binds to target cells by attaching to the cell surface CD4 molecule [51–54] followed by an interaction with either the CXCR4 or CCR5 coreceptors before fusion of viral and cellular membranes (reviewed in [55–57]). Consequently, soluble CD4, SDF-1 (stromal cell-derived factor 1), which is the natural ligand of CXCR4, as well as monoclonal antibodies against CD4 or CXCR4 have been observed to inhibit the process of virus infection and HIV-1-mediated syncytium formation [17,20,23,58–63]. Unlike these latter demonstrations, LPG inhibition of syncytium formation is suggested, by our results, to be independent of the gp120/CD4/CXCR4 trimeric complex, as determined by FACS analysis using anti-CD4 (SIM-4) and anti-CXCR4 (12G5) monoclonal antibodies and also by virus binding studies. It should be pointed out that such FACS analysis has also been performed using several other antibodies against epitopes different to those targeted by SIM-4 and 12G5 (data not shown). In all cases, LPG treatment was ineffective at altering the accessibility of CXCR4 and CD4 cell surface molecules, thus suggesting that LPG does not modulate the detection of these cell surface receptors in an epitope-specific fashion. Although we cannot totally eliminate the possibility that LPG abrogates HIV-1-mediated syncytium formation via steric hindrance, the observation that HIV-1 attachment is unaffected by LPG while the entry step is inhibited is clearly suggestive of a LPG-mediated effect at the level of fusion (see below).
It is well known that the LPG molecule on the surface of the Leishmania parasite is partially responsible for its tropism towards macrophages. Some of the macrophage surface molecules acting as potential cellular receptors are members of the CD18 complex of the integrin family [47]. More specifically, it has been demonstrated that antibodies against CD11b (CR3) can cause strong inhibition of infection of macrophages by the Leishmania parasite while anti-CD11c antibodies (p150,95) cause only a moderate inhibition of infection. In addition, anti-CD11a (LFA-1) antibodies were only able to slightly inhibit Leishmania entry into macrophages. Since others and we have demonstrated that the LFA-1/ICAM−1 interaction is important for HIV-1-dependent syncytium formation [12–16,46], we looked at the surface levels of LFA-1 and found that they were not altered by LPG treatment. This probably further suggests that the LPG/LFA−1 interaction might be too weak to have any consequence on the higher affinity LFA-1/ICAM interactions.
Another issue that we have found to be of importance in the analysis of LPG inhibition of syncytium formation is related to T cell activation. Mohagheghpour and coworkers have previously demonstrated that the activation of receptive CD4+ T cells with immunological stimuli resulted in more rapid induction of syncytium formation when coincubated with HIV-1 envelope-expressing cells [48]. This effect was partly attributed to PKC activation. It has been well documented that LPG modulates intracellular functions of host cells such as hydrolytic enzymatic actions [31,64], calcium chelation [26,65], c-fos gene expression [26,66] and PKC activity [35,36,49]. The initial contact of CD4 molecules with gp120 of HIV-1-infected cells might itself induce signalling events which could influence syncytium formation. Indeed, several intracellular events have been suggested to be initiated by the gp120/CD4 or the gp120/CXCR4 interaction such as activation of MAP-kinase, PI 3-kinase, PI 4-kinase and Pyk2, calcium mobilization, and induction of hydrolysed phosphatidylinositol and protein tyrosine phosphorylation [67–73]. It is plausible that LPG, in a certain way, might inhibit such signalling events which could potentially downregulate syncytium formation. However, the use of different stimuli on Jurkat cells, including the more physiological anti-CD3/anti-CD28 combination, showed that LPG did not seem to have an impact on cell signalling in this cell line. It follows that any potential signalling events initiated via a gp120–dependent interaction (being either beneficial or not for syncytia formation) are likely to be unaffected by the addition of Leishmania LPG.
Our analysis has also focused on the postulate made by Easterbrook et al. [18] that LPG could inhibit HIV-1 entry. We have tested this hypothesis by looking at both syncytium number after an acute HIV-1 infection and luciferase activity after infection of 1G5 cells by HIV-1NL4-3. Through both attachment and entry assays, we have demonstrated that LPG inhibition of HIV-1 replication occurred during viral entry at a postbinding step. Mechanistically speaking, it appears that HIV-1-mediated syncytium formation and virus entry are two processes which are inhibited by LPG in a similar fashion, likely to be at the level of the fusion process. This inhibition of fusion most likely results from a change in membrane fluidity caused by LPG insertion into the cell and/or virus membrane. Although the inhibitory processes of LPG on both HIV-1-mediated syncytium formation and virus entry are likely to be identical, HIV-1 viral entry was affected to a lesser extent than syncytium formation by LPG treatment. This might be due to the fact that syncytium formation is a more complex process than virus-cell fusion and the former is thus more prone to inhibition by various means [74].
Overall, it seems contradictory that LPG would inhibit HIV-1-mediated syncytium formation and viral entry when dual infection with HIV-1 and Leishmania accelerates the progression of HIV-1-related diseases. In fact, Leishmania is suggested to be a potent cofactor for HIV-1 replication and AIDS progression (reviewed in [75–77]). The reason for such a discrepancy probably stems from the fact that Leishmania/HIV−1 interactions are very complex, in part through their sharing of similar targets, namely cells of the mononuclear phagocyte series (i.e. monocytes/macrophages). This interaction is believed to be very beneficial for the replication of both the virus and the parasite. In fact, our group has demonstrated that Leishmania donovani and its derived LPG molecules are efficient at directly activating the HIV-1 LTR [78,79]. These various mechanisms induced by the Leishmania parasite might override the inhibitory potential of LPG on HIV-1-related processes and so account for the increasing number of Leishmania-infected individuals afflicted with AIDS.
Since the action of LPG permits blockage of HIV-1 entry, this agent might be efficient at slowing down AIDS progression by reducing reinfection events. In addition, the positive modulation of the HIV-1 LTR by purified LPG might concomitantly help in purging the previously described HIV-1 latent reservoir which is resistant to actual anti-HIV-1 therapies [80]. We are presently attempting to evaluate the modulatory role of Leishmania LPG on virus replication in primary human mononuclear cells bearing in mind that the same molecule can exert a dichotomous effect as exemplified by the observation that LPG can activate HIV-1 transcription and virus replication in both human T lymphoid and monocytoid cells [78,79], but it can also, as shown by us in the present study and others [18], inhibit HIV-1-mediated syncytium formation and virus entry.
Acknowledgments
Please note that N.G. and B.B. contributed equally to this work. We are grateful to Dr Maurice Dufour for technical assistance in flow cytometry studies. This work was performed by N.G. in partial fulfillment of the M.Sc. degree at the Faculty of Graduate Studies, Department of Medical Biology, Faculty of Medicine, Laval University. This work was financially supported by a grant from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program to M.J.T. and M.O. (grant no. #MOP-37781). M.J.T. is the recipient of a CIHR Investigator (HIV/AIDS) Award. M.O. holds a Junior 2 Scholarship Award from the Fonds de la Recherche en Santé du Québec and is a Burroughs Wellcome Fund Awardee in Molecular Parasitology.
REFERENCES
- 1.Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–58. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 2.Choe H, Farzan M, Sun Y, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 3.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–6. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Broder CC, Kennedey PE, Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 5.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 6.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedey PE, Murphy PM, Berger EACC. CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–9. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 7.Clapham PR, Weiss RA. Spoilt of choice of co-receptors. Nature. 1997;388:230–1. doi: 10.1038/40758. [DOI] [PubMed] [Google Scholar]
- 8.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantaleo G, Graziosi C, Fauci AS. The immunopathogenesis of human immunodeficiency virus infection. New Engl J Med. 1993;328:327–35. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 10.Lifson JD, Feinberg MB, Reyes GR, et al. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986;323:725–8. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- 11.de Santis C, Robbioni P, Longhi R, Carrow E, Siccardi AG, Beretta A. Role of HLA class I in HIV type 1-induced syncytium formation. AIDS Res Hum Retroviruses. 1996;12:1031–40. doi: 10.1089/aid.1996.12.1031. [DOI] [PubMed] [Google Scholar]
- 12.Barbeau B, Fortin J-F, Genois N, Tremblay MJ. Modulation of human immunodeficiency virus type 1-induced syncytium formation by the conformational state of LFA-1 determined by a new luciferase-based syncytium quantitative assay. J Virol. 1998;72:7125–36. doi: 10.1128/jvi.72.9.7125-7136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber MF, Webb DSA, Gerrard TL, Mostowski HS, Vujcic L, Golding H. Re-evaluation of the involvement of the adhesion molecules ICAM/LFA-1 in syncytia formation of HIV-infected subclones of a CEM T-cell leukemic line. AIDS Res Human Retrovir. 1991;7:45–53. doi: 10.1089/aid.1991.7.45. [DOI] [PubMed] [Google Scholar]
- 14.Hildreth JEK, Orentas RJ. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–8. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 15.Valentin A, Lundin K, Pararroyo M, Asjö B. The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells. J Immunol. 1990;144:934–7. [PubMed] [Google Scholar]
- 16.Butini L, De Fougerolles AR, Vaccarezza M, Cohen DI, Montroni M, Springer TA, Pantaleo G, Fauci AS. Intercellular adhesion molecules (ICAM) -1 ICAM-2 and ICAM-3 function as counter-receptors for lymphocyte function-associated molecule 1 in human immunodeficiency virus-mediated syncytia formation. Eur J Immunol. 1994;24:2191–5. doi: 10.1002/eji.1830240939. [DOI] [PubMed] [Google Scholar]
- 17.Clapham PR, Weber JN, Whitby D, et al. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989;227:368–70. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- 18.Easterbrook MD, Levy MH, Gomez AM, Turco SJ, Epand RM, Rosenthal KL. Inhibition of HIV-1-induced syncytia formation and infectivity by lipophosphoglycan from Leishmania. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:496–505. [PubMed] [Google Scholar]
- 19.Gunther-Ausborn S, Stegmann T. How lysophosphatidylcholine inhibits cell-cell fusion mediated by the envelope glycoprotein of human immunodeficiency virus. Virology. 1997;235:201–8. doi: 10.1006/viro.1997.8699. [DOI] [PubMed] [Google Scholar]
- 20.McKnight A, Wilkinson D, Simmons G, et al. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–6. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momota K, Kaneko I, Kimura S, Mitamura K, Shimada K. Inhibition of human immunodeficiency virus type-1-induced syncytium formation and cytopathicity by complestatin. Biochem Biophys Res Commun. 1991;179:243–50. doi: 10.1016/0006-291x(91)91361-f. [DOI] [PubMed] [Google Scholar]
- 22.Oka M, Iimura S, Tenmyo O, et al. Terpestacin, a new syncytium formation inhibitor from Arthrinium sp. J Antibiot. 1993;46:367–73. doi: 10.7164/antibiotics.46.367. [DOI] [PubMed] [Google Scholar]
- 23.Rieber EP, Federle C, Reiter C, Krauss S, Gürtler L, Eberle J, Deinhardt F, Riethmuller G. The monoclonal CD4 antibody M-T413 inhibits cellular infection with human immunodeficiency virus after viral attachment to the cell membrane. An approach to postexposure prohylaxis. Proc Natl Acad Sci USA. 1993;89:10792–6. doi: 10.1073/pnas.89.22.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmusson BJ, Flanagan TD, Turco SJ, Epand RM. Petersen no. Fusion of Sendai virus and individual host cells and inhibition of fusion by lipophosphoglycan measured with image correlation spectroscopy. Biochim Biophys Acta. 1998;1404:338–52. doi: 10.1016/s0167-4889(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 25.Orlandi PA, Turco SJ. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987;262:10384–91. [PubMed] [Google Scholar]
- 26.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 27.Turco SJ, Hull SR, Orlandi Pa, Jr, Shepherd SD, Homans SW, Dwek RA, Rademacher TW. Structure of the major carbohydrate fragment of the Leishmania donovani lipophosphoglycan. Biochemistry. 1987;26:6233–8. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- 28.Turco SJ, Orlandi Pa, Jr, Homans SW, Ferguson MA, Dwek RA, Rademacher TW. Structure of the phosphosaccharide-inositol core of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1989;264:6711–5. [PubMed] [Google Scholar]
- 29.Puentes SM, Sacks DL, da Silva RP, Joiner KA. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988;167:887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell DG, Talama-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989;10:328–33. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- 31.Eilam Y, El-On J, Spira DT. Leishmania major: excreted factor, calcium ions, and the survival of amastigotes. Exp Parasitol. 1985;59:161–8. doi: 10.1016/0014-4894(85)90068-2. [DOI] [PubMed] [Google Scholar]
- 32.Handman E, Schnur LF, Spithill TW, Mitchell GF. Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J Immunol. 1986;137:3608–13. [PubMed] [Google Scholar]
- 33.McNeely TB, Turco SJ. Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol. 1990;144:2745–50. [PubMed] [Google Scholar]
- 34.Descoteaux A, Turco SJ, Sacks DL, Matlashewski G. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J Immunol. 1991;146:2747–53. [PubMed] [Google Scholar]
- 35.McNeely TB, Rosen G, Londner MV, Turco SJ. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem J. 1989;259:601–4. doi: 10.1042/bj2590601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeely TB, Turco SJ. Inhibition of protein kinase C activity by the Leishmania donovani lipophosphoglycan. Biochem Biophys Res Commun. 1987;148:653–7. doi: 10.1016/0006-291x(87)90926-0. [DOI] [PubMed] [Google Scholar]
- 37.Smith SD, Shatsky Cohen MPS, Warnke R, Link MP, Glader BE. Monoclonal antibody and enzymatic profiles of human malignant T lymphoid cells and derived cell lines. Cancer Res. 1984;44:5657–60. [PubMed] [Google Scholar]
- 38.Weiss A, Imboden J, Shoback D, Strobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci USA. 1984;81:4169–73. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilar-Cordova E, Chinen J, Donehower L, Lewis DE, Belmont JW. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res Human Retrovir. 1994;10:295–301. doi: 10.1089/aid.1994.10.295. [DOI] [PubMed] [Google Scholar]
- 40.Perez VL, Rowe T, Justement JS, Butera ST, June CH, Folks TM. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J Immunol. 1991;147:3145–8. [PubMed] [Google Scholar]
- 41.Martin PJ, Ledbetter JA, Morishita Y, June CH, Beatty PG, Hansen JAA. 44 kilodalton cell surface homodimer regulates interleukin 2 production by activated human T lymphocytes. J Immunol. 1986;136:3282–7. [PubMed] [Google Scholar]
- 42.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–96. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz O, Maréchal V, Danos O, Heard J-M. Human immunodeficiency virus type 1 nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–9. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paquette JS, Fortin JF, Blanchard L, Tremblay MJ. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J Virol. 1998;72:9329–36. doi: 10.1128/jvi.72.11.9329-9336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCallus DE, Ugen KE, Sato AI, Williams WV, Weiner DB. Construction of a recombinant bacterial human CD4 expression system producing a bioactive CD4 molecule. Viral Immunol. 1992;5:163–72. doi: 10.1089/vim.1992.5.163. [DOI] [PubMed] [Google Scholar]
- 46.Pantaleo G, Butini L, Graziosi C, Poli G, Schnittman SM, Greenhouse JJ, Gallin JI, Fauci AS. Human immunodeficiency virus (HIV) infection in CD4+ T lymphocytes genetically deficient in LFA-1: LFA-1 is required for HIV-mediated cell fusion but not for viral transmission. J Exp Med. 1991;173:511–4. doi: 10.1084/jem.173.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talamas-Rohana P, Wright SD, Lennartz MR, Russell DG. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA−1 family of leukocyte integrins. JImmunol. 1990;144:4817–24. [PubMed] [Google Scholar]
- 48.Mohagheghpour N, Chakrabarti R, Stein BS, Gowda SD, Engleman EG. Early activation events render T cells susceptible of HIV-1-induced syncytia formation. J Biol Chem. 1991;266:7233–8. [PubMed] [Google Scholar]
- 49.Giorgione JR, Turco SJ, Epand RM. Transbilayer inhibition of protein kinase C by the lipophosphoglycan from Leishmania donovani. Proc Natl Acad Sci U S A. 1996;93:11634–9. doi: 10.1073/pnas.93.21.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miao L, Stafford A, Nir S, Turco SJ, Flanagan TD, Epand RM. Potent inhibition of viral fusion by the lipophosphoglycan of Leishmania donovani. Biochemistry. 1995;34:4676–83. doi: 10.1021/bi00014a022. [DOI] [PubMed] [Google Scholar]
- 51.Dalgleish AG, Beverly PCL, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–7. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 52.Eiden LE, Lifson JDHIV. interactions with CD4: a continuum of conformations and consequences. Immunol Today. 1992;13:201–6. doi: 10.1016/0167-5699(92)90154-Y. [DOI] [PubMed] [Google Scholar]
- 53.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–8. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 54.Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The CD4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–48. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 55.Broder CC, Collman RG. Chemokine receptors and HIV. J Leukoc Biol. 1997;62:20–9. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 56.Moore JP. Coreceptors: Implications for HIV pathogenesis and therapy. Science. 1997;276:51–2. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- 57.Doms RW, Peiper SC. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–90. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 58.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 59.Healey D, Dianda L, Moore JP, et al. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990;17:1233–42. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klasse PJ, McKeating JA. Soluble CD4 and CD4 immunoglobulin-selected HIV-1 variants: a phenotypic characterization. AIDS Res Hum Retroviruses. 1993;9:595–604. doi: 10.1089/aid.1993.9.595. [DOI] [PubMed] [Google Scholar]
- 61.Klasse PJ, Sattentau QJ. Altered CD4 interactions of HIV type 1 LAI variants selected for the capacity to induce membrane fusion in the presence of a monoclonal antibody to domain 2 of CD4. AIDS Res Hum Retroviruses. 1996;12:1015–21. doi: 10.1089/aid.1996.12.1015. [DOI] [PubMed] [Google Scholar]
- 62.Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992;66:4784–93. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–5. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 64.El-On J, Bradley DJ, Freeman JC. Leishmania donovani: action of excreted factor on hydrolytic enzyme activity of macrophages from mice with genetically different resistance to infection. Exp Parasitol. 1980;49:167–74. doi: 10.1016/0014-4894(80)90114-9. [DOI] [PubMed] [Google Scholar]
- 65.Homans SW, Mehlert A, Turco SJ. Solution structure of the lipophosphoglycan of Leishmania donovani. Biochemistry. 1992;31:654–61. doi: 10.1021/bi00118a004. [DOI] [PubMed] [Google Scholar]
- 66.Descoteaux A, Matlashewski G. c-fos and tumor necrosis factor gene expression in L. donovani infected macrophages. Mol Cell Biol. 1989;9:5223–7. doi: 10.1128/mcb.9.11.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briand G, Barbeau B, Tremblay M. Binding of HIV-1 to its receptor induces tyrosine phosphorylation of several CD4-associated proteins, including the phosphatidylinositol 3-kinase. Virology. 1997;228:171–9. doi: 10.1006/viro.1996.8399. [DOI] [PubMed] [Google Scholar]
- 68.Chirmurle N, Goonewardena H, Pahwa S, Pasieka R, Kalyanaraman VS, Pahwa S. HIV-1 envelope glycoproteins induce activation of activated protein-1 in CD4+ T cells. J Biol Chem. 1995;270:19364–9. doi: 10.1074/jbc.270.33.19364. [DOI] [PubMed] [Google Scholar]
- 69.Cruikshank WW, Center DM, Pyle SW, Kornfeld H. Biologic activities of HIV-1 envelope glycoprotein: the effects of crosslinking. Biomed Pharmacother. 1990;44:5–11. doi: 10.1016/0753-3322(90)90062-e. [DOI] [PubMed] [Google Scholar]
- 70.Davis CB, Dikic I, Unutmaz D, et al. Signal transduction due to HIV−1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–8. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hivroz C, Mazerolles F, Soula M, Fagard R, Gratton S, Meloche S, Sékaly RP, Fischer A. Human immunodeficiency virus gp120 and derived peptides activate protein tyrosine kinase p56lck in human CD4 T lymphocytes. Eur J Immunol. 1993;23:600–7. doi: 10.1002/eji.1830230303. [DOI] [PubMed] [Google Scholar]
- 72.Kornfeld H, Cruikshank WW, Pyle SW, Berman JS, Center DM. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988;335:445–8. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- 73.Schmid-Antomarchi H, Benkirane M, Breittmayer V, Husson H, Ticchioni M, Devaux C, Rossi B. HIV induces activation of phosphatidylinositol 4-kinase and mitogen-activated protein kinase by interacting with T cell CD4 surface molecules. Eur J Immunol. 1996;26:717–20. doi: 10.1002/eji.1830260331. [DOI] [PubMed] [Google Scholar]
- 74.Hernandez LD, Hoffman LR, Wolfsberg TG, White JM. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–61. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 75.Alvar J, Verdejo J, Osuna A, Najera R. Visceral leishmaniasis in a patient seropositive for HIV. Eur J Clin Microbiol. 1987;6:604–6. doi: 10.1007/BF02014266. [DOI] [PubMed] [Google Scholar]
- 76.Tremblay M, Olivier M, Bernier R. Leishmania and the pathogenesis of HIV infection. Parasitol Today. 1996;12:257–61. doi: 10.1016/0169-4758(96)10021-1. [DOI] [PubMed] [Google Scholar]
- 77.Wolday D, Berhe N, Akuffo H, Britton S. Leishmania–HIV interactions: Immunopathogenic mechanisms. Parasitol Today. 1999;15:182–7. doi: 10.1016/s0169-4758(99)01431-3. [DOI] [PubMed] [Google Scholar]
- 78.Bernier R, Barbeau B, Tremblay MJ, Olivier M. The lipophosphoglycan of Leishmania donovani up-regulates HIV-1 transcription in T cells through the NF-κB elements. J Immunol. 1998;160:2881–8. [PubMed] [Google Scholar]
- 79.Bernier R, Turco SJ, Olivier M, Tremblay M. Activation of human immunodeficiency virus type 1 in monocytoid cells by the protozoan parasite Leishmania donovani. J Virol. 1995;69:7282–5. doi: 10.1128/jvi.69.11.7282-7285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chun TW, Fauci AS. Latent reservoirs of HIV. Obstacles to the eradication of virus. Proc Natl Acad Sci U S A. 1999;96:10958–61. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]