Abstract
Interleukin-12 (IL-12) is secreted from monocytes and macrophages; it exerts pleiotropic effects on T cells and natural killer (NK) cells, and stimulates interferon-γ (IFN-γ) secretion. Glutathione tripeptide regulates the intracellular redox status and other aspects of cell physiology. We examined whether IFN-γ and IL-4 affect the balance between intracellular reduced glutathione (GSH) and oxidized (GSSG) glutathione, as this may affect IL-12 production in human alveolar macrophages (AM). We used both AM from healthy non-smokers obtained by bronchoalveolar lavage and the monocytic THP-1 cell line in this study. Incubation of AM for 2 h with the GSH precursor N-acetylcysteine (NAC) increased the intracellular GSH/GSSG ratio, and enhanced lipopolysaccharide (LPS)-induced IL-12 secretion by AM. In THP-1 cells, NAC increased the GSH/GSSG ratio and the expression of LPS-induced IL-12 mRNA, whereas l-buthionine-[S,R]-sulphoximine (BSO) decreased these. NAC and BSO offset their own effects on the intracellular GSH/GSSG ratio and the expression of LPS-induced IL-12 mRNA. Furthermore, exposure of AM to the helper T cell type 1 (Th1) cytokine IFN-γ or the helper T cell type 2 (Th2) cytokine IL-4 for 72 h increased and decreased the GSH/GSSG ratio, respectively. Lipopolysaccharide (LPS)-induced secretion of IL-12 in AM was enhanced by IFN-γ but inhibited by IL-4. These results suggest that IFN-γ and IL-4 oppositely affect the GSH/GSSG balance, which may regulate IL-12 secretion from AM in response to LPS.
Keywords: human alveolar macrophages, IL-4, IFN-γ, IL-12 production, redox status
Introduction
Interleukin-12 (IL-12) is physiologically secreted by monocytes and macrophages in response to bacteria and bacterial products. IL-12 plays a pivotal role in the regulation of cell-mediated immunity, exerting pleiotropic effects on T cells and natural killer (NK) cells. IL-12 also induces helper T cell type 1 (Th1) cell development and plays an important role in maintaining the in vivo balance between Th1 and Th2 responses [1]. The development of Th1 cells from Th0 cells requires IL-12, whereas differentiation into Th2 cells requires IL-4 [2].
Glutathione (GSH) is a non-protein tripeptide that contains sulphydryl. It is abundant in virtually all cells, playing significant roles in many biological processes. Glutathione also constitutes the first line of the cellular defence mechanism against oxidative injury, and is the major intracellular redox buffer in ubiquitous cell types [3]. Accumulating evidence suggests that the intracellular redox status regulates various aspects of cellular function [4]. Although numerous reports have confirmed that the replenishment of GSH restores both depressed T cell responses and decreases in IL-2 and IFN-γ secretion by T cells [5], investigators have ignored glutathione redox potential with regard to macrophages. The glutathione level in murine antigen-presenting cells determines whether a Th1 or Th2 response predominates [6]. We have also reported that IL-12 production is regulated by the redox status of murine macrophages [7].
More than 90% of bronchoalveolar lavage (BAL) cells are alveolar macrophages in humans. They are recognized as airway scavengers and as antigen-presenting cells to T cells. Alveolar macrophages regulate T cell reactivity directly by interaction with T cells and/or indirectly by cytokine secretion [8]. Although investigators have documented IL-12 secretion by human AM [9], the mechanism that regulates this process remains unclear. The present study describes the relationship between redox status and LPS-induced IL-12 production in human AM in the presence or absence of NAC, IL-4 or IFN-γ. We also used the human monocytic cell line THP-1 instead of AM in these experiments because we were unable to obtain sufficient AM from one volunteer, and this influenced the research design. We found that IL-4 and IFN-γ affected the balance of intracellular GSH/oxidized glutathione (GSSG) and suggest that this balance regulates the amount of IL-12 secreted by lipopoplysaccharide (LPS)-stimulated human AM.
Materials and methods
Isolation and preparation of alveolar macrophages and monocytes
Human AM were harvested from healthy adult non-smokers via saline bronchoalveolar lavage [10]. Written informed consent was obtained from all volunteers to participate in the study. The collected cells consisted of >95% macrophages as determined by differential counting of Wright-stained cytocentrifuge preparations (91·8 ± 5·2% viable as determined by trypan blue dye exclusion). The lavaged cells were washed three times in Hanks's balanced salt solution (HBSS) and plated in a 24-well flat-bottomed polystyrene plate (AM density: 5·0 × 105cells/well) or 10 cm glass dishes (AM density: 5·0 × 106 cells/well). The cells were incubated for 30 min at 37°C, non-adherent cells removed by washing and adherent cells isolated by extensive vibration and five washes with HBSS. These adherent cells were then cultured in RPMI 1640 (Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS) (Equitech-Bio Inc., Ingram, TX, USA) and maintained in an atmosphere of humidified 5% CO2/95% air [11].
Peripheral mononuclear cells were separated from leucocyte concentrates obtained from healthy volunteers by density gradient centrifugation in lymphocyte separation medium and washed three times in HBSS. The cells consisted of about 8% monocytes and were >95% viable. Peripheral monocytes (PM) were isolated as adherent cells as described above and monocytes accounted for more than 90% of all adherent cells.
Alveolar macrophages were incubated with or without NAC (Sigma Chemical Co., St Louis, MO, USA) for 2 h. In another series of experiments, AM were incubated with or without IL-4 (R&D Systems Europe Ltd, Oxon, UK) or IFN-γ (Pepro Tech EC Ltd, London, UK) for 72 h at 37°C in an humidified atmosphere of 5% CO2/95% air.
THP-1 cells
Human monocytic THP-1 cells (American Type Culture Collection, Rockville, MD, USA) were cultured in RPMI-1640 medium with 4·5 g/l glucose, 10 mm HEPES, 1 mm sodium pyruvate and 50 µm 2-mercaptoethanol supplemented with 10% FBS and were maintained in humidified 5% CO2/95% air. For our experiments, THP-1 cells (1 × 106/500 µl) were pretreated with 1·2% DMSO for 24 h because DMSO treatment enhances the ability of human myeloid cell lines to produce IL-12 [12]. The cells were washed and incubated with or without NAC for 2 h or l-buthionine-[S,R]-sulphoximine (BSO) (Sigma Chemical Co., St Louis, MO, USA) for 24 h before the stimulation. The optimal incubation time of NAC and BSO has been reported previously [13].
IL-12 assay
The culture media of AM or PM at 5·0 × 105/well from the 24-well flat-bottom polystyrene plate were discarded after the initial incubation, and the cells were washed three times with HBSS. After preincubation with NAC, IFN-γ and IL-4, the cells were washed, stimulated with 1·0 µg/ml LPS in fresh 500 µl RPMI-10% FBS for 24 h and sedimented by centrifugation at 1500 g for 5 min. Then the IL-12 concentration of the supernatants was determined using an enzyme-linked immunosorbent assay (ELISA) kit (Quantikine™, R&D Systems Europe, Oxon, UK) that was specific for p70. The assay detected >5 pg/ml of IL-12.
Preparation of complementary RNA (cRNA) probes
A human IL-12 p40 cDNA fragment containing residues 625–1022 (numbering of residues as GeneBank accession no. M65290) [14] was amplified by PCR. For PCR, the synthesized sense and antisense primers for IL-12 p40 were 5′-GAGTCTGCCCATT GAGGTCAT-3′ and 5′-AATTTTCATCCTGGATCAGAACC-3′ (Kurabo, Osaka, Japan) [15]. The PCR products were fractionated by agarose gel electrophoresis and subsequently cloned into a pGEM-t Esay vector (Promega Corp., Madison, WI, USA). Sequencing analysis (Perkin Elmer Corp., PE Applied Biosystems, Foster City, CA, USA) confirmed the identity of the amplified DNA. A cDNA fragment of human glyceraldehyde-3-phosphate dehydrogenase (G3PDH; residues 71–1053) cDNA was cloned as described for IL-12 [16].
Northern blot analysis
The expression of IL-12 p40 mRNA in THP-1 cells has been demonstrated [17]. Total RNA was extracted from THP-1 cells using the TRIzol reagent (Gibco BRL, Life Technologies, Inc.), a modified acid guanidinium thiocyanate–phenol–chloroform method according to Chomczynski and Sacci [18]. Fifteen µg of total RNA extracted from THP-1 were fractionated per lane by electrophoresis through a 1·4% agarose gel containing 0.66 m formaldehyde and the RNA transferred overnight to Hybond-N membranes (Amersham Pharmacia Biotech, Tokyo, Japan) in 20 × SSC (1 × SSC = 150 mm sodium chloride and 15 mm trisodium citrate). The RNA was then immobilized on the membrane by UV irradiation using a UV Stratalinker (Stratagene, La Jolla, CA, USA). After prehybridization, the membrane was hybridized at 60°C overnight using hybridization buffer containing a 32P-labelled cDNA probe for human IL-12 p40. The membrane was washed and was detected by autoradiography at −70°C using Hyperfilm (Amersham Pharmacia Biotech). After IL-12 mRNA detection, the probe was stripped off and the blots rehybridized using a 32P-labelled cDNA probe for human G3PDH as a control. The mRNA level was quantified by densitometry using NIH Image Version 1·62, and the optical density of the IL-12 p40 band was corrected for G3PDH levels. RNA samples for comparison were analysed on the same blot.
Intracellular GSH and GSSG assays
Alveolar macrophage or PM adherents to glass plates were removed by scraping with a rubber policeman. AM, PM and THP-1 cells were washed three times in cold washing buffer (0·1 m sodium phosphate buffer, 5 mm EDTA buffer, pH 7·5), and immediately lysed with 100 µl of lysis buffer (0·1% Triton-X, 0·1 m sodium phosphate buffer, 5 mm EDTA buffer, pH 7·5). Thereafter, 15 µl of 0·1 N HCl and 15 µl of 50% sulphosalicylic acid were added. After centrifugation at 12000 g for 5 min, supernatants were collected for GSH and GSSG assays. The total cellular glutathione concentration was assayed using the GSSG-reductase-DTNB recycling procedure according to Tietze et al. [19], as modified by Buchmuller-Rouiller [13]. The GSSG concentration was assayed according to the method of Sacchetta et al. [20]. Briefly, standard solutions containing serial dilutions of GSSG in lysis buffer or portions of GSH samples were supplemented with 1–4 volumes of 0·05 m N-ethylmaleamic acid. All solutions were adjusted to pH 11 with NaOH. After 5 min neutralization with HCl, GSSG was assayed using the GSSG-reductase-DTNB recycling procedure [13].
Statistical analysis
Results are expressed as means ±s.d. Significant differences were calculated using Student's t-test. A value of P < 0·05 was considered statistically significant.
Results
Intracellular GSH and GSSG concentration and LPS-stimulated IL-12 secretion of AM and PM
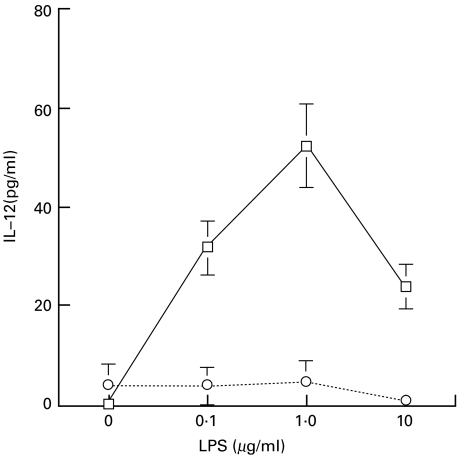
The intracellular concentrations of GSH and GSSG of AM and PM separated by the adhesion method were measured. As shown in Table 1, the concentration of GSH was higher in PM than in AM. However, the amounts of GSSG were similar, indicating a selective decrease in the GSH content of AM compared with that in PM. The ratio of GSH/GSSG was higher in PM than in AM in our experimental procedure. From these results, it seems that AM may be in a more oxidative state than PM, in terms of the polarized presence of GSSG. We then investigated the impact of LPS on IL-12 production from AM and PM. LPS induced IL-12 secretion from AM in a concentration-dependent manner, whereas LPS alone did not induce IL-12 production in PM (Fig. 1). The IL-12 concentration in the culture supernatant of AM peaked 24 h after LPS was added (data not shown).
Table 1.
Intracellular concentrations of GSH and GSSG in alveolar macrophages and peripheral monocytes
| Macrophages | GSH | GSSG | GSH/GSSG |
|---|---|---|---|
| Peripheral | 3·03 ± 0·64 | 0·64 ± 0·21 | 5·81 ± 2·15 |
| monocytes | |||
| Alveolar | 1·12 ± 0·13 | 0·66 ± 0·13 | 1·69 ± 0·81 |
| macrophages | |||
AM or PM (5·0 × 106 cells) were isolated as described in Materials and methods. Amounts of GSH (nmol/5·0 × 106 cells) and GSSG (nmol/5·0 ×106 cells) were determined by enzyme assays. Values are expressed as means ± s.d. of cells isolated from 5 participants.
Fig. 1.
IL-12 production from AM and PM stimulated with LPS. Cells (5·0 × 105/well) were incubated for 24 h with the indicated concentrations of LPS. The amount of IL-12 released into the culture medium was determined by ELISA. Values are expressed as means ± s.d. of cells isolated from 5 participants. □, Alveolar macrophages; ○, peripheral monocytes.
Effect of NAC on intracellular GSH and GSSG levels and on secretion of LPS-induced IL-12 in AM and THP-1
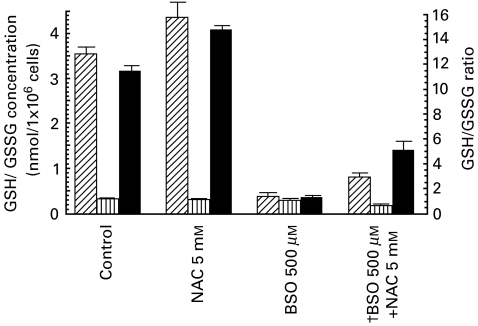
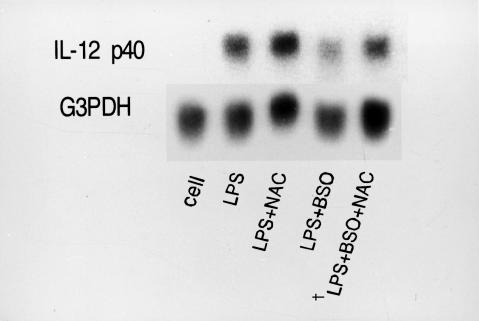
N-acetylcysteine is a precursor of intracellular glutathione and it increases intracellular GSH levels [21]. Because we were unable to obtain sufficient AM from one volunteer and were therefore limited in our human AM studies, we examined the effect of NAC on the GSH/GSSG ratio in THP-1 cells. These cells represent a relatively advanced stage of myelomonocytic differentiation, and IFN-γ can enhance LPS-induced transcription of IL-12 in them [22]. The intracellular content of GSH in THP-1 cells exposed to 0, 0·6, 2·5 and 10 mm NAC for 2 h resulted in an increased ratio of GSH/GSSG (12·2, 12·3, 16·2 and 19·1, respectively; data not shown). Next, we examined the relationship between the GSH/GSSG ratio and IL-12 p40 mRNA expression in THP-1 cells. Because THP-1 cells secrete levels of IL-12 that are below the limits of detection by ELISA, we detected IL-12 p40 mRNA expression instead of IL-12 protein. As shown in Figs 2 and 3, NAC increased the GSH/GSSG ratio and the expression of LPS-induced IL-12 p40mRNA. To examine how NAC affected IL-12 mRNA expression by modifying the GSH/GSSG ratio in THP-1 cells, we examined the effect of BSO, a specific inhibitor of γ-glutamylcysteine synthetase [23] on the GSH/GSSG ratio and the expression of LPS-induced IL-12 p40 mRNA in THP-1 cells. Figures 2 and 3 show that BSO decreased the GSH/GSSG ratio and the expression of LPS-induced IL-12 mRNA, whereas NAC increased them. NAC and BSO offset each other's effects on the intracellular GSH/GSSG ratio and the expression of LPS-induced IL-12 mRNA (Fig. 2 and Fig. 3). The mRNA level was quantified by densitometry and corrected for the level of G3PDH. The corrected IL-12 levels after NAC, BSO and NAC + BSO treatment were 125, 55 and 70% compared with the untreated control, respectively. Therefore, NAC affects LPS-induced IL-12 secretion by modulating the GSH/GSSG ratio which in turn may regulate the expression of IL-12 p40 mRNA.
Fig. 2.
Effects of NAC and BSO on the intracellular concentration of GSH and GSSG and the ratio of GSH/GSSG in THP-1 cells. Following the pretreatment of THP-1 cells (1 × 106) with 2·5 mm of NAC for 2 h or 500 µm of BSO for 24 h, GSH (nmol/1 × 106 cells) and GSSG (nmol/1 × 106 cells) were detected. †THP-1 were treated with 500 µm of BSO for 22 h, followed by 2·5 mm of NAC and 500 µm of BSO for 2 h. Then, GSH and GSSG were detected as described in Materials and methods. Values are expressed as means ± s.d. of data for cells isolated from 5 subjects.  , GSH;
, GSH;  , GSSG; ▪, GSH/GSSG ratio.
, GSSG; ▪, GSH/GSSG ratio.
Fig. 3.
Effects of NAC and BSO on the expression of IL-12 mRNA induced by LPS in THP-1 cells. Following the pretreatment of THP-1 with 2·5 mm of NAC for 2 h or 500 µm of BSO for 24 h, THP-1 were stimulated with or without LPS (1·0 µg/ml) for 6 h. Then, IL-12mRNA was detected by Northern blot analysis; 15 µg RNA were loaded on each lane. †THP-1 were treated with 500 µm of BSO for 22 h, followed by 2·5 mm of NAC and 500 µm of BSO for 2 h.
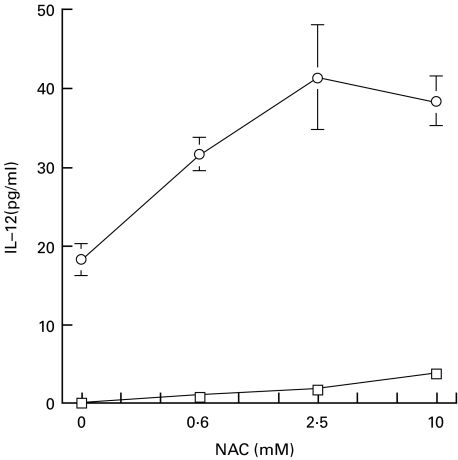
We then examined the effect of NAC on AM. AM from each study participant were divided into two dishes and incubated either with or without NAC for 2 h. NAC treatment increased the GSH level significantly (P < 0·05), but did not change the GSSG concentration, resulting in an increased ratio of GSH/GSSG (Table 2). The LPS-induced secretion of IL-12 from NAC-treated AM was higher than that of control untreated AM (Fig. 4). This suggests that IL-12 production is regulated by the GSH/GSSG ratio in AM.
Table 2.
Effects of NAC, IL-4 and IFN-γ on intracellular concentrations of GSH and GSSG in alveolar macrophages
| Treatment | GSH | GSSG | GSH/GSSG |
|---|---|---|---|
| Control | 1·48 ± 0·27 | 0·88 ± 0·26 | 1·71 ± 0·19 |
| NAC (2·5 mm)† | 2·30 ± 0·30* | 0·93 ± 0·26 | 2·52 ± 0·38 |
| IFN-γ (100U/ml)‡ | 1·63 ± 0·27 | 0·47 ± 0·23 | 4·95 ± 0·66 |
| IL-4(400 pg/ml)‡ | 0·39 ± 0·07* | 1·31 ± 0·15** | 0·32 ± 0·10 |
After incubation of AM (5·0 × 106) with 2·5 mm of NAC for 2 h, GSH (nmol/5·0 × 106 cells) and GSSG (nmol/5·0 × 106 cells) were detected.
P < 0·05 compared with control.
After incubating AM (5× 106) with 400 pg/ml of IL-4 or 100 U/ml of IFN-γ for 72 h, GSH (nmol/5·0 × 106 cells) and GSSG (nmol/5·0 × 106 cells) were detected. Values are expressed as means ± s.d. of cells isolated from 5 participants.
*P < 0·05
P < 0·01 compared with IFN-γ treated cells.
Fig. 4.
Effect of NAC on IL-12 secretion from AM. AM (5·0 × 105/well) were incubated with the indicated concentrations of NAC for 2 h, and then stimulated with or without LPS (1·0 µg/ml) for 24 h. Values are expressed as means ± s.d. of cells isolated from 5 participants. ○, LPS (+); □, LPS (−).
Effects of IL-4 and IFN-γ on intracellular GSH and GSSG levels and upon secretion of IL-12 induced by LPS in AM
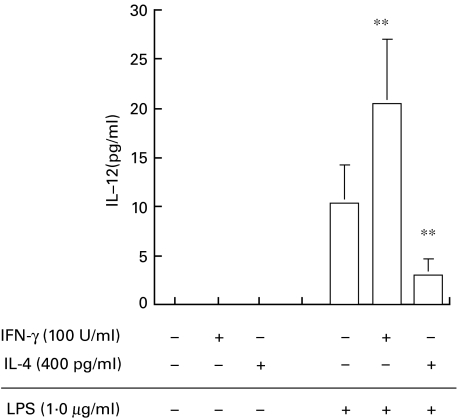
Following on from our finding that IL-4 reduces and IFN-γ augments the intracellular GSH content in peritoneal macrophages in a murine system [7], we investigated the effects of these cytokines on the intracellular concentration of GSH and GSSG in human moncytes/macrophages. We initially examined the effects of IL-4 and IFN-γ on THP-1 cells in order to determine the optimal concentrations of the cytokines for this assay. A 72-h incubation of IL-4 at concentrations 0, 4, 40 and 400 pg/ml dose-dependently decreased the GSH/GSSG ratio to 12·1 ± 0·79, 10·9 ± 0·78, 8·0 ± 1·10 and 7·3 ± 1·16, respectively (data not shown). In contrast, IFN-γ (0,1,10 and 100 U/ml) increased the GSH/GSSG ratio (10·1 ± 1·20, 10·9 ± 1·54, 14·0 ± 1·24 and 16·6 ± 0·49, respectively, data not shown). We therefore used 100 U/ml of IFN-γ and 400 pg/ml IL-4 in the study of human AM. The intracellular GSH/GSSG contents of AM incubated with IL-4 were predominantly GSSG, but those of AM incubated with IFN-γ were predominantly GSH. The differences in the intracellular GSH (P < 0·05) and GSSG (P < 0·01) contents between IL-4-and IFN-γ-treated AM was significant (Table 2). These results suggest that IL-4 reduces the GSH/GSSG ratio in AM, whereas IFN-γ increases the ratio. Greater levels of IL-12 were secreted from AM exposed to IFN-γ than from controls (P < 0·01), whereas less was secreted by AM exposed to IL-4 (P < 0·01 compared with control AM) (Fig. 5). The IL-12 content in the culture medium of AM without LPS was below the limits of detection.
Fig. 5.
Effects of IL-4 or IFN-γ on LPS-induced secretion of IL-12 from AM. AM (5·0 × 105/well) were incubated with 400 pg/ml of IL-4 or 100 U/ml of IFN-γ for 72 h, then stimulated with LPS (1·0 µg/ml) for 24 h. Values are expressed as means ± s.d. of cells isolated from 6 participants. ** P < 0·01compared with LPS alone.
Discussion
The key findings of this study are that Th1 and Th2 cytokines counterregulate the GSH/GSSG balance, and that LPS-induced IL-12 secretion from human AM correlates positively with the intracellular GSH/GSSG ratio.
The cellular glutathione contents and the GSH/GSSG ratio vary among organs. The contents of GSH per unit weight are very low and the GSSG/total glutathione ratio is very high in the lung compared with other organs [24,25]. The GSH content in the rat lung is rapidly reduced by smoking, in association with increased GSSG levels [26]. This demonstrates that the lung is exposed to widely polarized oxidation statuses and that environmental oxidative stress may easily affect the lung GSH/GSSG ratio. Although PM are believed to be the primary source of AM, it is the latter that are always exposed to and activated by inhaled environmental agents. It is therefore not surprising that the GSH/GSSG ratio may be lower in AM than in PM (Table 1). However, we should remember that the GSH contents and GSH/GSSG ratio are easily altered by a variety of stimuli and we recognize the limitations of this study imposed by the experimental procedures employed. Phagocytosis decreases GSH content [27] and only cold stimulation (4°C, 30 min) increases GSSG [28]. Furthermore, we cannot exclude the possibility that the AM were exposed to oxidative stress during bronchoalveolar lavage, which could have accounted for the lower GSH contents in AM than PM. Because we can obtain viable human AM only by bronchoalveolar lavage, it remains problematic to control for every factor capable of modulating the GSH/GSSG ratio during this procedure.
The GSH contents and the GSH/GSSG ratio in both AM and PM were markedly lower than those in THP-1 cells. Attachment to a plate is one method to stimulate phagocytosis. Therefore, the GSH contents in AM and PM may have been lowered by attachment in this study. We used such a method because we wanted to examine the relationship between the intracellular GSH/GSSG ratio and IL-12 secretion in AM, and AM attachment was required for this. We were unable to compare GSH and GSSG contents in AM separated by another isolation procedure because of the limitations of AM availability in this study. Therefore, GSH and GSSG levels in human AM in vivo require clarification and future methodological advances may help to achieve this.
The level of IL-12 secreted from monocytes or macrophages varies depending on their origin. Human PM produce very low levels of IL-12, and LPS alone does not increase secretion [29]; however, costimulation with IFN-γ and LPS enhances cellular IL-12 production [12,30]. In contrast, some IL-12 was released without stimulation, and LPS induced high levels of IL-12 secretion without IFN-γ by AM [9]. Our data (Fig. 1) were consistent with those published by other investigators.
The relationship between the GSH/GSSG ratio in AM and PM and IL-12 production seems to be discordant in our results. Human AM are significantly more deficient in AP-1 DNA binding activity than PM due to a lack of REF-1; differentiation of PM into AM is associated with the loss of REF-1 and AP-1 activities [31]. In addition, a lack of the transcriptional factor c-fos in AM alters the post-transcriptional modification factor complex, thereby changing the AP-1 DNA binding characteristics and the transcriptional performance with respect to IL-12 gene expression in macrophages [32]. These factors may explain some of the functional differences between AM and PM. Thus, the relationship between the GSH/GSSG ratio and IL-12 secretion in AM and PM cannot be compared directly. The present study therefore compared the effect of the GSH/GSSG ratio on LPS-induced IL-12 production between oxidative and reduced AM. Myelomonocytic THP-1 cells have a high GSH/GSSG ratio but cannot secrete a detectable level of IL-12. However, reduced THP-1 express more LPS-induced IL-12 p40mRNA than oxidative THP-1 (Figs 2 and 3)
Inducible expression of IL-12 has been documented in macrophages, dendritic cells and THP-1 after stimulation by microbial antigen or via CD-40–CD40L interactions [33–35]. The expression of the IL-12 p40 subunit is highly inducible and is regulated primarily at the transcription level [17,36]. Expression of IL-12 p35 is also subject to similar regulation, although to a much lesser extent than p40 [17,35,37,38]. As production of IL-12 protein in THP-1 cells was below the detection level, we only examined the effect of redox status on IL-12 p40 mRNA production in these cells.
Intracellular GSH may be important for macrophage activation, because low intracellular GSH levels in antigen-presenting cells (APCs) are correlated with defective antigen processing [39]. Moreover, GSH depletion in murine APCs decreases the secretion of IL-12 and leads to polarization from the typical Th1 cytokine profile towards Th2 response patterns, suggesting that GSH levels in APCs play a central role in determining whether a Th1 or Th2 cytokine response predominates [6]. We have also reported that IL-12 production is regulated by the redox potential of murine peritoneal macrophages. Reductive macrophages with elevated GSH levels induced by glutathione monoethylester produced IL-12 upon IFN-γ and LPS stimulation in vitro, whereas oxidative macrophages with a reduced level of GSH induced by maleic acid diethylester did not [7,40].
The effects of IL-4 and IFN-γ on human AM have been reported. IL-4 causes significant suppression of the LPS-induced elevation of COX-2 expression and PG production [10] and IFN-γ augments LPS induced TNF-α and IL-6 cytokine release in AM [41]. We have reported that incubation with IFN-γ for 72 h increases the intracellular GSH level and the ratio of GSH/GSSG in mouse peritoneal macrophages, while IL-4 decreased them [7]. We also reported that IL-4 depresses IL-12 production in murine peritoneal macrophages costimulated with IFN-γ and LPS [7]. Similarly, LPS-induced production of IL-12 in AM was also enhanced by IFN-γ, but suppressed by IL-4 (Fig. 5). Because 5·0 × 106 AM were necessary to detect the intracellular GSSG in our recycling assay system but only about 1 × 107 AM were usually obtained from one participant, we divided AM into only two dishes and could not include a control for the study of IL-4 and IFN-γ.
We have demonstrated that IFN-γ and IL-4 counterregulate the GSH/GSSG balance and that the GSH/GSSG ratio plays a pivotal role in the production of IL-12 in AM. Although further extensive investigation will be required to clarify the underlying mechanism governing whether a Th1 or Th2-related disease predominates in a given environment, the findings presented here should provide new insights into immunological disorders accompanied by Th1 and Th2 imbalances in lung diseases.
Acknowledgments
We thank H. Tsukagoshi and T. Ishizuka for their helpful comments. This work was supported in part by a grant (no. 09670466) from the Ministry of Education, Science and Culture, Japan.
References
- 1.Chehimi J, Trinchieri G. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol. 1994;14:149–61. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 2.Karpus WJ, Swanborg RH. CD4+ suppressor cells differentially affect the production of IFN-γ by effector cells of experimental autoimmune encephalomyelitis. J Immunol. 1989;143:3492–7. [PubMed] [Google Scholar]
- 3.Meister A. Glutathione centennial; molecular properties and clinical applications. New York: Academic Press; 1989. [Google Scholar]
- 4.Nakamura H, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 5.Maurice MM, Nakamura H, van der Voort EAM, et al. Evidence for the role of an altered redox state in hyporesponsiveness of synovial T cells in rheumatoid arthritis. J Immunol. 1997;158:1458–65. [PubMed] [Google Scholar]
- 6.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–6. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamuro J, Murata Y, Suzuki M. The triggering and healing of tumor stromal inflammatory reactions regulated by oxidative and reductive macrophages. Gann Monograph Cancer Res. 1999;48:153–64. [Google Scholar]
- 8.Poulter L. Macrophages and allergic lung disease. Immunobiology. 1996;195:574–87. doi: 10.1016/S0171-2985(96)80023-4. [DOI] [PubMed] [Google Scholar]
- 9.Denis M, Ghadirian E. Dysregulation of interleukin 8,interleukin 10,and interleukin 12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS. 1994;10:1619–27. doi: 10.1089/aid.1994.10.1619. [DOI] [PubMed] [Google Scholar]
- 10.Endo T, Ogushi F, Kawano T, Sone S. Comparison of the regulations by Th2-type cytokines of the arachidonic-acid metabolic pathway in human alveolar macrophages and monocytes. Am J Respir Cell Mol Biol. 1998;19(2):300–7. doi: 10.1165/ajrcmb.19.2.2915. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro SD, Campbell EJ, Kobayashi DK, Welgus HG. Immune modulation of metalloproteinase production in human macrophages. Selective pretranslational suppression of interstitial collagenase and stromelysin biosynthesis by interferon-gamma. J Clin Invest. 1990;86:1204–10. doi: 10.1172/JCI114826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubin M, Chow JM GT. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor-α, and IL-1β, production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;7:1847–85. [PubMed] [Google Scholar]
- 13.Buchmuller-Rouiller Y, Corradin S, Smith J, et al. Role of glutathione in macrophage activation. Effect of cellular gluthatione depletion on nitrite production and leishmanicidal activity. Cell Immunol. 1995;164:73–80. doi: 10.1006/cimm.1995.1144. [DOI] [PubMed] [Google Scholar]
- 14.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 15.Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of Gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–12. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arcari P, Martinelli R, Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA specie. Nucleic Acids Res. 1984;12:9179–89. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X, Chow JM, Gri G, et al. The interleukin-12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–57. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Tietze F. Enzymic method for quantitative determination of nanogram amounts of ttotal and oxidized glutathione: application to mammalian blood and other tissue. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 20.Sacchetta P, Dicola D, Federici G. Alkaline hydrolysis of N-ethylmaleimide allows a rapid assay of glutathione disulfide in biological samples. Anal Biochem. 1986;154:205–8. doi: 10.1016/0003-2697(86)90516-6. [DOI] [PubMed] [Google Scholar]
- 21.Yim CY, Hibbs Jb, Jr, McGregor JR, Galinsky RE, Samlowski WE. Use of N-acetyl cysteine to increase intracellular glutathione during the induction of antitumor responses by IL-2. J Immunol. 1994;152:5796–805. [PubMed] [Google Scholar]
- 22.Li C, Goodrich JM, Yang X. Interferon-gamma (IFN-γ) regulates production of IL-10 and IL-12 in human herpesvirus-6 (HHV-6) -infected monocytes/macrophage lineage. Clin Exp Immunol. 1997;109:421–5. doi: 10.1046/j.1365-2249.1997.4661362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–73. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 24.Chang J, Jaeschke H, Randerath K. Effect of Ni (II) on tissue hydrogen peroxide content in mice as inferred from glutathione and glutathione disulfide measurements. Life Sci. 55:1789–96. doi: 10.1016/0024-3205(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 25.Sen CK, Atalay M, Hanninen O. Exercise-induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol. 1994;77(5):2177–87. doi: 10.1152/jappl.1994.77.5.2177. [DOI] [PubMed] [Google Scholar]
- 26.Li XY, Rahman I, Donaldson K, MacNee W. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax. 1996;51:465–71. doi: 10.1136/thx.51.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouzer CA, Scott WA, Griffith OW, Hamill AL, Cohn ZA. Glutathione metabolism in resting and phagocytizing peritoneal macrophages. J Biol Chem. 1982;257:2002–8. [PubMed] [Google Scholar]
- 28.Wataha JC, Lewis JBPEL, Rakich DR. Effect of dental metal ions on glutathione level in THP-1 human monocytes. J Oral Rehab. 2000;27:508–16. doi: 10.1046/j.1365-2842.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 29.D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes MP, Wang JH, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-γ of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–50. [PubMed] [Google Scholar]
- 31.Monik M, Carter B, Hunninghake G. Human alveolar macrophages are markedly deficient in REF-1 and AP-1 DNA binding activity. J Biol Chem. 1999;274:18075–80. doi: 10.1074/jbc.274.25.18075. [DOI] [PubMed] [Google Scholar]
- 32.Roy S, Charboneau R, Cain K, DeTurris S, Melnyk D, Barke R. Deficiency of the transcription factor c-fos increases lipopolysaccharide-induced macrophage interleukin 12 production. Surgery. 1999;126:239–47. [PubMed] [Google Scholar]
- 33.Cella M, Scheidegger D, Palmer-Lehman K, Lane P, Lanzavecchia A GA. Ligation of CD40 on human dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity. T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch F, Stanzl U, Jennewein K, et al. High level of IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Ambrosio D, Cippitelli M, Cocciolo MG, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3; involvement of NF-kB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Neurath G, Gri G, Trinchieri G. Identification and characterization of novel est-2-related nuclear complex implicated in the activation of the human IL-12 p40 gene promoter. J Biol Chem. 1997;272:10389–95. doi: 10.1074/jbc.272.16.10389. [DOI] [PubMed] [Google Scholar]
- 37.Tone Y, Thompson SA, Babik KF, et al. Structure and chromosomal location of mouse interleukin-12 p35 and p40 subunit genes. Eur J Immunol. 1996;26:1222–7. doi: 10.1002/eji.1830260606. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto T, Kojima K, Funakosi Y, Endo Y, Fujita T, Nariuchi H. Molecular cloning and characterization of murine IL-12 genes. J Immunol. 1996;156:1082–8. [PubMed] [Google Scholar]
- 39.Short S, Merkel BJ, Caffrey R, McCoy KL. Defective antigen processing correlates with a low level of intracellular glutathione. Eur J Immunol. 1996;26:3015–20. doi: 10.1002/eji.1830261229. [DOI] [PubMed] [Google Scholar]
- 40.Hamuro J. Lentinan regulates the local inflammatory cellular reaction at tumor tissue — its relation with antitumor effect. Biotherapy. 1996;10:581–8. [Google Scholar]
- 41.Maus U, Rosseuau S, Knies U, Seeger W, Lohmeyer J. Expression of proinflammatory cytokines by flow-sorted alveolar macrophages in severe pneumonia. Eur Respir J. 1998;11:534–41. [PubMed] [Google Scholar]