Abstract
Peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients with ongoing Leishmania aethiopica infection and individuals cured/under treatment from L. infantum or L. donovani infection were stimulated in vitro with LACK, the Leishmania homologue of receptors for activated C kinase. The LACK protein is conserved in related leishmanial species and is expressed both in the promastigote and amastigote stages of Leishmania. Our results show that LACK induced marked NK and some CD8+ cell proliferation in PBMC from cutaneous leishmaniasis patients with active disease. These responses were coupled with high levels of IFN-γ and IL-10 production. At the concentration tested, the proliferative responses to freeze-thawed Leishmania antigen (Ft-Leish) were higher, while the levels of IFN-γ were consistently lower than that of LACK. Although cells from individuals cured of leishmaniasis could respond to whole Leishmania lysate by proliferation and IFN-γ production, there was no evident response to LACK. Ethiopian controls tested at the same time also showed LACK induced proliferation with IFN-γ and IL-10 responses. Thus LACK reactivity in terms of proliferation and cytokine induction were present in cells from some healthy donors and most of the patients with active lesions, while this response was absent in individuals cured of L. infantum or L. donovani leishmaniasis. Since cure from leishmaniasis often results in life-long protection, and active but not cured patients showed in vitro responses to LACK stimulation, questions arose as to how this highly immunodominant molecule functions during human leishmanisasis. Some possible mechanisms are discussed.
Keywords: CD8 cells, cytokines, IFN-γ, LACK, Leishmania, natural killer cells (NK)
Introduction
Leishmania are intracellular protozoan parasites which reside exclusively within mononuclear phagocytes of the mammalian host. L. aethiopica causes cutaneous leishmaniasis in the high lands of Ethiopia [1]. Clinical disease is characterized by the most common form of the self-healing skin lesion, localized cutaneous leishmaniasis (LCL). Healing takes place within 3 months to 6 years or more resulting in apparent solid protection against re-infection. Immunity to disease following infection with Leishmania is believed to be largely mediated by CD4+ T-helper cells with IFN-γ involvement [2,3]. It has been proposed that healing of lesions involves lytic and necrotizing processes involving cytotoxic cells [4–6]. Our previous results showed that increased NK and CD8+ cells proliferate in response to Ft-Leish in individuals that have cured their infection, as well as some patients with active lesion, probably in the process of the healing stages of the infection, which could contribute to the ulcer formation associated with healing of L. aethiopica lesions [7].
Several defined parasite proteins that appear to induce beneficial human T cell responses or protection against infection in murine model systems have been identified, including the 36 kilodalton immunodominant protein, Leishmania homologue of receptors for activated C kinase (LACK). This protein has been shown to be highly conserved among Leishmania species evaluated to date and has been shown to confer protection in susceptible mice when administered together with IL-12 [8]. However, the expansion of LACK-reactive CD4+ T cells have been shown to be the focus of the early response in mouse strains susceptible to L. major infection [9]. This early response has been associated with an early burst of IL-4, which rapidly renders parasite-specific CD4+ T cell precursors unresponsive to IL-12 [10]. We have shown in another study [11] that this protein induces proliferation of cells as well as simultaneous production of IFN-γ and IL-10 in healthy blood donors. The immunodominant nature of LACK in early response in mice, which probably includes innate elements, led us to conduct the present study. Peripheral blood mononuclear cells (PBMC) from cutaneous leishmaniasis patients with ongoing infection due to L. aethiopica, cured patients from both atypical cutaneous leishmaniasis due to L. infantum [12] and visceral leishmaniasis due to L. donovani infections were analysed in the present study. Our results show that the LACK response was maintained during Leishmania infection, while this response was consistently absent in the cured individuals tested.
Materials and methods
Study group
Ten cutaneous leishmaniasis patients visiting the ALERT clinic in Addis Ababa, Ethiopia, consented to give blood for this study after the nature of the study had been explained to them. These patients had active cutaneous lesions due to Leishmania as confirmed by identification of parasites in smears and/or cultures of scrapings from the skin lesions. All patients except one had single lesions and the duration of the lesions varied from 1 month to 3 years (Table 1). In some instances a biopsy was taken for diagnosis. The histological picture observed was not predictive of the length of disease expression and biopsies of single lesions were similar to lesions of individuals with multiple lesions. L. aethiopica is the only Leishmania species causing cutaneous leishmaniasis in the catchment area where these patients came from [1]. The patients were tested in the chronological sequence in which they reported to the clinic. The results are, however, arranged according to length of infection.
Table 1.
Clinical data of Ethiopian L. aethiopica induced cutaneous leishmaniasis patients and healthy controls working in the hospital area; patients were tested in the order in which they attended the clinic
| Sample code | Sample ID no. | Type of lesion | Duration of lesion | Age | Sex |
|---|---|---|---|---|---|
| P1 | 1914/97 | Single | 4 weeks | 18 | Female |
| P2 | 1912/97 | Single | 5 weeks | 15 | Female |
| P3 | 0072/98 | Single | 3 months | 21 | Male |
| P4 | 1904/97 | Single | 4 months | 25 | Male |
| P5 | 1145/98 | Single | 6 months | 23 | Male |
| P6 | 1842/97 | Single | 7 months | 29 | Female |
| P7 | 1941/97 | Single | 8 months | 20 | Female |
| P8 | 1913/97 | Single | 9 months | 17 | Male |
| P9 | 1915/97 | Single | 10 months | 19 | Male |
| P10 | 1228/98 | Multiple | 3 years | 22 | Male |
| EHD1 | 0001/98 | Healthy | 36 | Male | |
| EHD2 | 0002/98 | Healthy | 35 | Male | |
| EHD3 | 0003/98 | Healthy | 38 | Male | |
| EHD4 | 0004/98 | Healthy | 46 | Male | |
| EHD5 | 0074/98 | Healthy | 48 | Female |
Results from 1 patient who has recovered from L. infantum and 10 Ethiopian leishmaniasis patients treated (n = 5) or undergoing treatment (n = 5) for L. donovani infection are also presented. It was not possible to include individuals who have cured from L. aethiopica infection for this study since, after cure, patients could not be convinced to return to the clinic for follow-up.
The cells from the Ethiopian patients (both CL and VL) were tested in Ethiopia, while cells from the L. infantum patient were tested in Sweden.
Intravenous injections of liposomal amphotericin B (AmBisome) for 14 days was the drug used to treat the L. infantum patient [12]. A complete regimen of 28 days intramascular injections of pentavalent antimony (Pentostam) were given to the Ethiopian visceral leishmaniasis patients with L. donovani infection.
Five healthy laboratory personnel from the hospital area were included as healthy controls. These individuals had no past or present history or indication of leishmaniasis.
Antigens and mitogens
Freeze-thawed whole L. aethiopica promastigote antigen (Ft-Leish Ag), prepared as described previously [13], was used at a final optimal concentration of 1·25 × 106 parasites/ml (12·5 µg/ml). LACK antigen pretreated with polymyxin B to neutralize potential endotoxin contamination during preparation [14] was used at a final concentration of 25 µg/ml after initial dose–response studies were performed (Recombinant LACK protein was a kind gift from Dr N. Glaichenhaus, Institute de Pharmacologie Moleculaire et Cellulaire, University of Nice, Valbonne, France). In initial experiments polymyxin B was shown not to have any effect on cell viability. Polymyxin B (final 25 µg/ml) treatment of LACK was carried out on ice 2 h prior to stimulation of cells. Purified protein derivative of tuberculin (PPD; Statens Serum Institute, Copenhagen, Denmark) was used as irrelevant antigen and the T cell mitogen phytohaemagglutinin (PHA; Murex, Hartford, UK) were each used at a final concentration of 12·5 µg/ml.
Preparation and stimulation of mononuclear cells
Mononuclear cells separated from defibrinated peripheral blood on Ficoll gradient [15] were washed 3 times and eventually resuspended to the appropriate concentration in RPMI containing 2 mm l-glutamine, 100 U penicillin and 100 µg/ml streptomycin (all from Gibco BRL, Paisley, Scotland, UK) supplemented with 10% heat-inactivated normal Swedish AB serum. Cells plated with appropriate controls and stimulations were cultured for 6 days, as described previously [7]. Proliferative responses are expressed as counts per minute (c.p.m.) or calculated as stimulation indices (SI = c.p.m. of cells in stimulated cultures/c.p.m. of unstimulated cultures).
Determination of IFN-γ and IL-10 levels in supernatants
Supernatants were harvested after 72 h of incubation from cells cultured as above, for analysis of IFN-γ and IL-10 protein release using commercial ELISAs (Mabtech, Stockholm, Sweden) following the manufacturers' instructions. The sensitivity of the cytokine assays for IFN-γ and IL-10 were 27 pg/ml and 9·8 U/ml, respectively.
Phenotype analysis of proliferating cells
Cell surface marker staining for antibody combinations of CD4 and CD8 (helper and cytotoxic); CD3 and CD16/56 (pan-T and NK cells); or control IgG1 and IgG2a, double-stained with flourescein isothiocynate (FITC) and phyco-erythrin (PE), respectively, were carried out on mononuclear cells cultured for 6 days, as described previously [7]. All antibodies were obtained from Becton Dickinson (Becton Dickinson, Mountain View, CA, USA). Phenotype analysis of cells was performed on a FACScan (Becton Dickinson). An enrichment of large blast cells after 6 days' culture in response to antigenic stimulation was observed in responder PBMC. Determined by the forward light scatter, smaller cells with the least light scatter, as seen in most unstimulated cultures, were gated in R1 and the area outside this region was gated R2. In response to antigen stimulation, blast cells undergoing activation were scattered forward-most, away from the R1 cell population into the R2 region. The proportional increase (p.i.) of responding cells of a particular cell type was computed taking the total large cells in R2 into consideration using the following formula:(% large cells of total × % large CD+ of stimulated culture) + 1/(% large cells of total × % large CD+ of unstimulated culture) + 1The mathematical correction factor of +1/+1 was necessary since in some instances the percentage of large cells of a particular phenotype in the unstimulated cultures was zero.
Cytotoxic activity of LACK
NK cell-mediated cytotoxicity was measured using a 4-h 51CrO4 release assay according to standard protocols [16]. NKL (NK lymphoma cell line) were used as effectors and 721·221 as target cells (B lymphoblastoid cells) in the presence or absence of LACK antigen at effector to target cell ratios of 20:1 and 5:1.
Statistical analysis
Student's t-test was used to compare the mean responses to the different stimulations.
Results
Proliferative responses to LACK and Ft-Leish
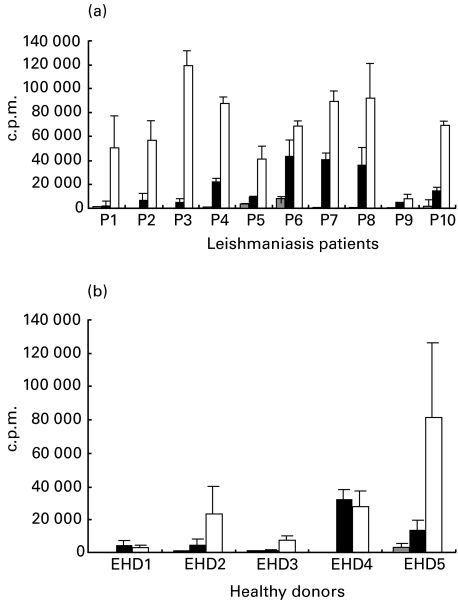
Proliferation of cells in response to LACK was evaluated and compared with freeze-thawed L. aethiopica antigen (Ft-Leish Ag) responses (Fig. 1). All PBMC from L. aethiopica patients had high levels of proliferative responses to Ft-Leish stimulation (SI range = 8–346). The proliferative response to LACK, at the concentration tested, was consistently lower than the response to Ft-Leish in all patients studied. LACK reactivity in PBMC from these patients ranged between SI of 2 and 90. Cells from the cured individual from L. infantum proliferated to Ft-Leish but not to LACK stimulation. Similarly, some of the treated L. donovani-infected individuals responded to the whole L. donovani lysate either by proliferation or by IFN-γ production (Table 2); there was, however, no such response to LACK stimulation. The PHA response in these treated individuals were variable, ranging from 2·1 to 122 stimulation indices.
Fig. 1.
Six-day proliferative responses to in vitro LACK and Ft-Leish stimulations of PBMC from Ethiopian patients (a) with active cutaneous leishmaniasis infection, and healthy (b) Ethiopian laboratory personnel. The error bars are standard deviation (s.d.) of triplicate cultures.  , RPMI; ▪, LACK; □, Leish.
, RPMI; ▪, LACK; □, Leish.
Table 2.
Proliferation and IFN-γ responses to LACK and Leishmania antigen stimulations in PBMC from individuals who have cured from L. infantum (CP1) and L. donovani (CP2-CP11) infections
| Proliferative responses (c.p.m.) | IFN-γ response (pg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient status ID | Treatment | RPMI | LACK | Ft-Leish | PHA | RPMI | LACK | Ft-Leish | PHA |
| CP1 | Completed | 335 | 423 | 15337 | 15032 | 34 | 34 | 551 | 1342 |
| CP2 | Completed | 499 | 722 | n.d. | 15032 | 10 | 10 | n.d. | 1725 |
| CP3 | Completed | 333 | 532 | 10300 | 40756 | 0 | 29 | 1186 | 1686 |
| CP4 | Completed | 484 | 488 | 669 | 1981 | 9 | 9 | 208 | 235 |
| CP5 | Completed | 405 | 485 | 782 | 1771 | 10 | 9 | 56 | 188 |
| CP6 | 28 days | 509 | 466 | 4051 | 10499 | 17 | 41 | 379 | 1651 |
| CP7 | 16 days | 507 | 716 | 35531 | 55398 | 20 | 9 | 482 | 1686 |
| CP8 | 15 days | 588 | 478 | 574 | 3386 | 10 | 9 | 150 | 248 |
| CP9 | 15 days | 510 | 406 | 691 | 3026 | 11 | 12 | 83 | 393 |
| CP10 | 15 days | 370 | 679 | 518 | 1189 | 10 | 10 | 9 | 44 |
| CP11 | 12 days | 596 | 684 | n.d. | 1227 | 8 | 11 | n.d. | 30 |
n.d., not done.
Proliferative responses of healthy Ethiopians (EHD) to Ft-Leish and LACK stimulation were high and ranged from 8 to 109 and 6 to 130 stimulation indices, respectively (Fig. 1).
Proliferations to PHA and PPD stimulations were in the range of SI 13–363 (mean SI = 148·4 ± 116) and SI 8–219 (mean SI = 89·3 ± 82), respectively, in cells from L. aethiopica patients, while the responses ranged from SI 46 to 277 (mean SI = 155·8 ± 91·9) and SI 5 to 271 (mean SI = 153·2 ± 102·6), respectively, in cells of healthy Ethiopians.
Phenotype of responding cells
The percentage of large blast cells in the R2 gated region before and after stimulation are shown in Table 3. The percentage of large cells expressing CD4+ phenotypes in unstimulated cultures was high. This level was increased in cells from 3/10 patients in response to LACK and in 7/10 patients in response to Ft-Leish. Furthermore, higher percentages of large cells expressing CD8+ phenotype in 7/10 in response to LACK and 5/10 in response to Ft-leish were evident, while percentages in CD16/56+ cells increased in 10/10 and 4/10 patients in response to the respective antigens. However, if there is a response to stimuli, the number of cells in R2 after stimulation will be larger than the number in R2 of unstimulated cells, which means that the actual responses will not always be evident from the percentage of raw data. Thus, taking the total number of cells in R2 region into consideration, we calculated the proportional increases of the responding phenotypes of cells above the background level (materials and methods).
Table 3.
The percentage of gated large cells (in R2) expressing CD4+, CD8+ and CD16/56+ surface markers following stimulation of PBMC from active cutaneous leishmaniasis patients with LACK and Ft-Leish
| %CD4+/CD8− | %CD8+/CD4− | CD16/56+/CD3− | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | RPMI | LACK | Ft-Leish | RPMI | LACK | Ft-Leish | RPMI | LACK | Ft-Leish |
| P1 | 56·10 | 17·15 | 60·67 | 19·51 | 31·44 | 11·41 | 0·76 | 57·01 | 18·47 |
| P2 | 51·93 | 14·91 | 26·28 | 14·39 | 26·82 | 26·28 | 6·39 | 37·25 | 8·07 |
| P3 | 42·53 | 13·86 | 9·67 | 24·14 | 71·75 | 40·86 | 7·25 | 53·52 | 40·86 |
| P4 | 51·91 | 71·34 | 72·44 | 9·16 | 10·44 | 8·71 | 4·32 | 8·73 | 4·53 |
| P5 | 35·02 | 23·08 | 78·76 | 12·73 | 28·54 | 6·34 | 0·53 | 11·42 | 1·93 |
| P6 | 31·88 | 16·23 | 27·45 | 13·04 | 36·07 | 19·24 | 15·38 | 37·81 | 38·35 |
| P7 | 18·00 | 6·33 | 25·39 | 10·00 | 64·84 | 53·48 | 11·25 | 34·01 | 13·06 |
| P8 | 51·91 | 71·34 | 72·44 | 9·16 | 10·44 | 8·71 | 4·32 | 8·73 | 4·53 |
| P9 | 51·91 | 71·34 | 72·44 | 9·16 | 10·44 | 8·71 | 4·32 | 8·73 | 4·53 |
| P10 | 54·12 | 24·96 | 65·33 | 3·61 | 21·35 | 15·19 | 1·12 | 36·28 | 8·30 |
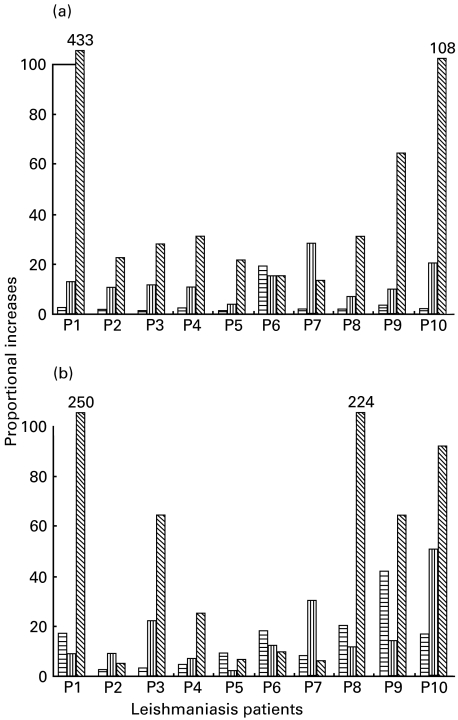
The highest proportional increase was in CD16/56+ cell population in response to both LACK (8/10) and Ft-Leish (6/10) antigen stimulation in cells from the L. aethiopica patients (Fig. 2). A strong burst of CD16/56+ proliferation in response to the two antigens were observed in 1 patient (P1). CD8+ cell increase in response to LACK and Ft-Leish antigens was moderate in cells from all patients tested, except in patient 7 (P7), whose response to both antigens was predominantly in CD8+ cell proliferation. Increases in CD4+ cell levels were more frequent in response to Ft-Leish than to LACK in these patients. Cells from the one cured individual tested had no response to LACK, while the responses to Ft-Leish stimulation involved mainly CD4+ cells (p.i. = 134) with some CD8+ (p.i. = 27) and very few CD16/56+ (p.i. = 5) cell proliferations. These experiments were not performed in the individuals treated for L. donovani due to the scarcity of cells obtained from these donors.
Fig. 2.
Phenotype of responding cells induced to proliferate in response to (a) LACK and (b) Ft-Leish antigen stimulations in PBMC of active cutaneous leishmaniasis patients. Data are presented as proportional increase of large cells above the background level.  , CD4+
, CD4+  , CD8+
, CD8+  , CD16/56+.
, CD16/56+.
In the PBMC of healthy Ethiopians, LACK mainly induced CD16/56+ (range = 4·0–21·8) and CD8+ (range = 1·4–9·2) cells to proliferate but little CD4+ cells (range = 0·5–2·5). However, moderate proportional increases in CD4+ (range = 1·4–6·1), CD8+ (range = 1·5–11·6) and CD16/56+ (range = 1·5–4·9) cells were observed in response to Ft-Leish.
Cytokine analysis
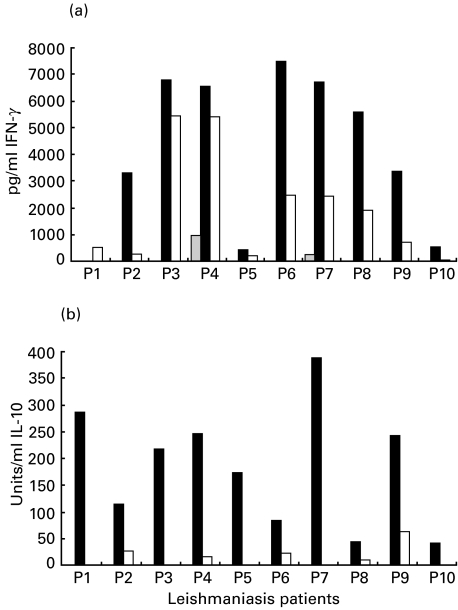
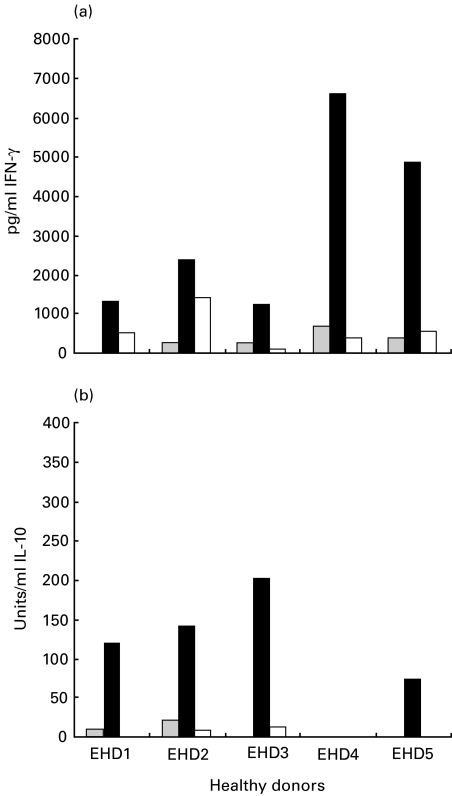
The mean levels of IFN-γ induced in response to LACK were higher than the levels measured in response to Ft-Leish in cells from the cutaneous leishmaniasis patients (P = 0·003; Fig. 3). The PHA-induced IFN-γ responses in the L. aethiopica patients ranged between 14 996 and 18 196 (mean 16 241 ± 1052). No measurable IFN-γ was detected in the cultures of the L. infantum and L. donovani cured individuals in response to LACK, although moderate to high levels were detected in response to the leishmanial antigen stimulations. High IL-10 levels were consistently induced in response to LACK stimulation in all L. aethiopica leishmaniasis patients tested. IL-10 induction by Ft-Leish was significantly lower than the LACK response (P = 0·0001). LACK induced variable levels of both IL-10 and IFN-γ secretion in PBMC of the healthy Ethiopians, the levels of which were consistently higher than that induced by Ft-Leish stimulation (Fig. 4).
Fig. 3.
Levels of measurable (a) IFN-γ and (b) IL-10 in 72 h culture supernatant in response to LACK and Ft-Leish stimulation in cells from active cutaneous leishmaniasis patients.  , RPMI; ▪, LACK; □, Leish.
, RPMI; ▪, LACK; □, Leish.
Fig. 4.
Levels of measurable (a) IFN-γ and (b) IL-10 in 72 h culture supernatant in response to LACK and Ft-Leish stimulation in cells from uninfected healthy Ethiopians.  ;, RPMI; ▪, LACK; □, Leish.
;, RPMI; ▪, LACK; □, Leish.
LACK-induced cytotoxicity
Cytotoxic assays were performed on NK cell clones in order to test whether, as well as activation of NK cells to proliferate and secrete IFN-γ, LACK could also induce enhanced non-specific cytotoxic activity in these cells. Addition of LACK to NKL (a transformed NK cell clone) did not induce enhanced cytotoxicity over controls at an effector to target ratio of 20 : 1 (45% versus 48%) nor 5 : 1 ratio (23% versus 29%).
Discussion
The in vitro immune reactivity to Leishmania antigens noted previously in non-Leishmania exposed blood donors [17], includes a significant response to the Leishmania homologue of receptors for activated C kinase (LACK), which is well conserved in Leishmania species. LACK response in the highly L. major-susceptible BALB/c mouse induces an early induction of IL-4 secreting cells [10], which are responsible for subsequently driving the immune response towards the highly susceptible Th2 phenotype seen in this mouse model [9]. In our earlier studies IL-4 was not found to be a consistent feature of human leishmaniasis due to L. aethiopica, while an association of IL-10 and disease development has been observed [18].
The presence of LACK responses in unexposed donors [11], coupled with the immunodominant nature of LACK in the early response in mice, as well as the anecdotal reports indicating the absence of LACK activity by proliferation or cytokine induction in cured leishmaniasis patients, led us to explore the possibility that the initial (innate) LACK response may be abrogated in the healing process from human leishmaniasis. When we collated the present data in healthy Ethiopian donors and our results from studies using other healthy blood donors from Sweden and Sudan [11], the cumulative data show that while some uninfected donors (70%) have strong proliferative responses to LACK, some do not. During an active leishmaniasis infection response to LACK was evident in 90% of the patients. However, none of the cured individuals tested showed LACK reactivity. It has been demonstrated previously that patients with active visceral leishmaniasis tend to be generally immunosuppresed and do not respond to in vivo or in vitro leishmanial antigen stimulations. There is no response to the T cell mitogen PHA. Upon successful treatment, cellular response to Leishmania antigens and PHA returns [19]. The individual recovered from L. infantum infection had a strong CD4 response to a crude preparation of a heterologous Leishmania promastigote antigen. Cells from some of the individuals treated for L. donovani infection also responded by proliferation and/or by IFN-γ production to the whole L. donovani lysate but showed no response to LACK stimulation. Cells from those individuals that had high proliferative responses to PHA also responded to the L. donovani antigen stimulation. Not all of the treated patients had responses to L. donovani or to the PHA stimulations. Unresponsiveness to Pentostam is not uncommon in patients with L. donovani infection. The low PHA response in cells from the 2 individuals (CP4 and CP5) who have completed Pentostam treatment suggests their inadequate response to this therapy. Improvement in cellular responses even after 15 days of treatment was evident in some. None of the individuals with adequate responses to PHA and Ft-Leish responded to LACK.
Unfortunately, in this hospital-based study it was not possible to obtain patients who had been cured from L. aethiopica infection since such individuals did not report to the clinic after cure. Also, the study design and circumstances did not allow the active follow-up of individual patients. However, taken together with published and unpublished data and the results obtained from the L. infantum and L. donovani patients, it would appear that the LACK response is not evident after cure of full-blown leishmaniasis. A LACK response was, however, evident in a laboratory-infected donor who never showed disease expression but had leishmanin skin test conversion after accidental infection (data not shown). The significance of this is as yet unclear.
Similar to our previous observation in unexposed healthy blood donors [20] and later from a field study in patients [7], the Ft-Leish stimulation of cells from L. aethiopica patients involved proliferation of NK and CD8+ cells. In the latter study we concluded that the ability to mount NK cell responses, as seen in some of the patients, could be a marker for the process of healing from the infection. In this context patients P8–P10, who showed large proportional increases in CD16/56+ cells, could have been in the process of healing their lesion. These donors also had strong proportional increase in CD4+ cells. It is not uncommon for L. aethiopica lesions to take several months and sometimes years before healing.
We also have data to suggest that NK cells may act not only in terms of their capacity to produce IFN-γ but also in their capacity to present antigen to T cells via MHC class II mechanisms [11] and [21,22]. Unlike the findings in healthy, unexposed donors, CD4+ cells proliferated more frequently in PBMC of L. aethiopica patients, and more so towards the crude Ft-Leish promastigote antigen than to LACK. In terms of proliferation, the LACK responses were weaker than Ft-Leish antigen-induced responses. However, the IFN-γ and IL-10 induction were consistently higher in the LACK-stimulated cells with prominent NK and some CD8 cell proliferation.
Although no patients with cured L. aethiopica infection were tested in this study, it would seem from the results obtained from the L. infantum and L. donovani cured individuals that the LACK response in human leishmaniasis, which may be present prior to infection, seem to be absent after cure. Of course, it could be argued that the absence of a LACK response in L. donovani patients, even after treatment, could be because this infection is associated with severe immunosuppression and that the patients may slowly and successively gain their immune response to different antigens of Leishmania. In such a scenario the response to LACK may be regained later than that of the mixed Ft-Leish antigen.
Leishmania of different species have unique features, including different antigens and virulence factors that may determine the mechanisms of resistance to each of these unique parasites. However, all Leishmania tested to date express LACK. Furthermore, many ‘negative’ and thus unpublished results testing LACK in patients recovered from cutaneous leishmaniasis indicate that following cutaneous leishmaniasis, LACK responses in vitro are low or absent. If the elimination of LACK reactive cells do occur, this could have been through the enhanced stimulation of NK cells with cytotoxic activity. However, our results indicated that LACK stimulation of NK cells did not enhance non-specific cytotoxic activity. This does not exclude the possibility that more specific cytotoxicity may occur in vivo. An alternative mechanism of elimination of LACK reactive cells could be through activation-induced apoptosis, a hypothesis we are in the process of testing. The feasibility of LACK in a human antileishmaniasis vaccine would then be in directing the LACK response away from a Th2 towards a Th1 response. Vaccine strategies to date are directed at stimulating memory cells that are recalled during natural infection. An alternative strategy in the control of leishmaniasis is for dominant immunoreactive Th2 cells to be abrogated in order to obtain an appropriate protective Th1-type response, an approach apparently utilized during the natural immune response. One experimental approach in mice has been to tolerize the host to the Th2 inducing LACK antigen [9]. Patients who have recovered from leishmaniasis are resistant to subsequent infection, thus one could speculate that these individuals are made ‘tolerant’ to LACK through the elimination of LACK reactive clones. In the absence of inducing a full infection, the strategy of preventing a Th2 response through IL-12 has been shown to be a feasible one in mice and could also be attainable in humans, especially if this results in the eventual elimination of the LACK reactive clones which can not be reactivated following subsequent infection.
Acknowledgments
This work was supported by funds from the Swedish International Development Agency (SIDA/SAREC) and the Swedish Medical Research Fund (MFR). The authors would like to thank Dr Farrokh Modabber for reading and commenting on the manuscript. The data from this manuscript has in part been presented in a PhD thesis of Kerima Maasho.
References
- 1.Bray RS, Ashford RW, Bray MA. The parasite causing cutaneous leishmaniasis in Ethiopia. Trans Roy Soc Trop Med Hyg. 1973;67:345–8. doi: 10.1016/0035-9203(73)90111-9. [DOI] [PubMed] [Google Scholar]
- 2.Melby PC, Sacks DL. Identification of antigens recognized by T cells in human leishmaniasis: analysis of T-cell clones by immunoblotting. Infect Immun. 1989;57:2971–6. doi: 10.1128/iai.57.10.2971-2976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott P, Caspar P, Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990;144:1075–9. [PubMed] [Google Scholar]
- 4.el Hassan AM, Gaafar A, Theander TG. Antigen-presenting cells in human cutaneous leishmaniasis due to Leishmania major. Clin Exp Immunol. 1994;99:445–53. doi: 10.1111/j.1365-2249.1995.tb05571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaafar A, el Kadaro AY, Theander TG, et al. The pathology of cutaneous leishmaniasis due to Leishmania major in Sudan. Am J Trop Med Hyg. 1995;52:438–42. doi: 10.4269/ajtmh.1995.52.438. [DOI] [PubMed] [Google Scholar]
- 6.Muller I, Pedrazzini T, Kropf P, et al. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite-specific CD8+ T cells. Int Immunol. 1991;3:587–97. doi: 10.1093/intimm/3.6.587. [DOI] [PubMed] [Google Scholar]
- 7.Maasho K, Sanchez F, Schurr E, et al. Indication of the protective role of natural killer cells in human cutaneous leishmaniasis in an area of endemicity. Infect Immun. 1998;66:2698–704. doi: 10.1128/iai.66.6.2698-2704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mougneau E, Altare F, Wakil AE, et al. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–6. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 9.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421–3. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 10.Launois P, Conciecao-Silva F, Himmerlich H, et al. Setting in motion the immune mechanisms underlying geneticlly determined resistance and susceptibility to infection with Leishmania major. Parasite Immunol. 1998;20:223–30. doi: 10.1046/j.1365-3024.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- 11.Maasho K, Satti I, Nylen S, et al. A leishmania homologue of receptors for activated C-kinase (LACK) induces both interferon-gamma and interleukin-10 in natural killer cells of healthy blood donors. J Infect Dis. 2000;182:570–8. doi: 10.1086/315725. [DOI] [PubMed] [Google Scholar]
- 12.Montelius S, Maasho K, Pratlong F, et al. Skin rash for 15 years. Lancet. 1998;352:1438. doi: 10.1016/S0140-6736(98)06498-8. [DOI] [PubMed] [Google Scholar]
- 13.Maasho K, Akuffo HO. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol. 1992;36:179–84. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 14.Duff GW, Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982;52:333–40. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- 15.Bøyum A. Separation of lymphocytes from blood and bone marrow. Scand J Clin Lab Invest. 1969;21(Suppl.):77–89. [Google Scholar]
- 16.Gronberg A, Ferm M, Tsai L, et al. Interferon is able to reduce tumor cell susceptibility to human lymphokine-activated killer (LAK) cells. Cell Immunol. 1989;118:10–21. doi: 10.1016/0008-8749(89)90353-5. [DOI] [PubMed] [Google Scholar]
- 17.Akuffo HO, Britton SFF. Contribution of non-Leishmania-specific immunity to resistance to Leishmania infection in humans. Clin Exp Immunol. 1992;87:58–64. doi: 10.1111/j.1365-2249.1992.tb06413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akuffo H, Maasho K, Blostedt M, et al. Leishmania aethiopica derived from diffuse leishmaniasis patients preferentially induce mRNA for IL-10 while those from localized leishmaniasis patients induce IFN-γ. J Infect Dis. 1997;175:737–41. doi: 10.1093/infdis/175.3.737. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho EM, Bacellar O, Barral A, et al. Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. Clin Invest. 1989;83:860–4. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akuffo H, Maasho K, Howe R. Natural and acquired resistance to Leishmania: cellular activation of Leishmania aethiopica of mononuclear cells from unexposed individuals is through the stimulation of NK cells. Clin Exp Immunol. 1993;94:516–21. doi: 10.1111/j.1365-2249.1993.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roncarolo MG, Bigler M, Haanen JB, et al. Natural killer cell clones can efficiently process and present protein antigens. J Immunol. 1991;147:781–7. [PubMed] [Google Scholar]
- 22.D'Orazio JA, Stein-Streilein J. Human natural killer (NK) cells present staphylococcal enterotoxin B (SEB) to T-lymphocytes. Clin Exp Immunol. 1996;104:366–73. doi: 10.1046/j.1365-2249.1996.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]