Abstract
Deficiency of the innate, humoral immune component mannose-binding lectin (MBL) predisposes individuals to a variety of infections, but the importance of MBL in infection by anaerobes has not been addressed. The attachment of MBL to a wide range of anaerobic bacteria associated with human disease and colonization was surveyed. The results suggest that for the species we examined, resistance to MBL binding may be associated with organisms that are more commonly pathogenic and that MBL binding to some bacteria may be phase variable.
Keywords: anaerobes, flow cytometry, innate immunity, mannose-binding lectin
Introduction
Mannose-binding lectin (MBL) is a calcium-dependent collagenous serum lectin involved in innate immunity. It is a pattern recognition molecule which binds to mannose and N-acetyl glucosamine residues presented at the densities and orientations commonly found on microbial surfaces [1]. On binding, the protein activates the complement system independently of antibody [2] and interacts directly with phagocytic cells [3].
Deficiency of MBL in humans is caused mainly by homozygosity for one of three point mutations which result in amino acid substitutions [4–6] that disrupt the normal structure of the protein [7]. Additional variation in MBL levels is the result of polymorphisms in the promotor region of the gene which influence the serum level of the lectin [8]. MBL deficiency predisposes individuals to a generalized risk of infections [9–11] and is associated with a common defect of opsonization [12]. More recently MBL deficiency has been associated with susceptibility to a number of specific infections [13–15].
The binding of MBL to bacteria differs both between and within species, but studies have been restricted to common aerobic bacteria [16–18]. Anaerobic bacteria have not been studied despite the clinical importance of many such organisms. In this study, we surveyed MBL binding to a series of Gram-negative and Gram-positive anaerobes, including species commonly implicated in clinical disease and other species that are rare clinical isolates.
Methods
Bacterial strains
A range of Gram-negative and Gram positive strains from the National Collection of Type Cultures (NCTC) and American Type Culture Collection (ATCC) were assayed for MBL binding (Table 1). All organisms were cultured on blood agar plates at 37°C for 24 h under anaerobic conditions, except for Actinomyces israelii, Eubacterium aerofacians and E. fossor, which were cultured for 48 h to allow sufficient growth. Bacterial cultures were examined by Gram stain before use.
Table 1.
Bacterial strains
| Bacterium | Strain number | |
|---|---|---|
| Gram positive | Clostridium difficile | NCTC 11204, |
| NCTC 11223 | ||
| Clostridium perfringens Type D | NCTC 8503 | |
| Clostridium novyi Type A | NCTC 538, | |
| NCTC 277 | ||
| Clostridium novyi Type B | ATCC 27606 | |
| Clostridium tetani | ATCC 9441 | |
| Bifidobacterium bifidum | NCTC 10471 | |
| Proprionibacterium acnes | NCTC 737 | |
| Actinomyces israelii | NCTC 4860 | |
| Eubacterium aerofacians | NCTC 11838 | |
| Eubacterium fossor | NCTC 11919 | |
| Gram negative | Veillonella dispar | NCTC 11831 |
| Veillonella parvula | ATCC 10790 | |
| Fusobacterium necrogenes | NCTC 10723, ATCC 25556 | |
| Fusobacterium varium | ATCC 8501 | |
| Fusobacterium ulcerans | NCTC 12111 | |
| Fusobacterium mortiferum | ATCC 25557 | |
| Fusobacterium nucleatum ssp. nucleatum | ATCC 25586 | |
| Bacteroides vulgatus | ATCC 8482 | |
| Bacteroides ovatus | NCTC 11153 | |
| Bacteroides ureolyticus | NCTC 10941 | |
| Bacteroides fragilis | NCTC 9343 | |
| Bilophila wadsworthia | ATCC 49260 | |
| Leptotrichia buccalis | NCTC 10249 |
The influence of different growth conditions and bacterial viability on MBL binding to Fusobacterium necrogenes were determined by comparing organisms grown on blood agar for 24 h with those grown (a) for 24 h in fastidious anaerobe broth (FAB) which gave a heavy suspension of bacteria with nutrient starvation, (b) for 2 h in FAB to give a light organism suspension and (c) for 2 h in FAB and then heating at 56°C for 30 min.
Detection of MBL binding
Flow cytometry
MBL binding to the bacteria was assayed by a direct immunofluorescence method with flow cytometric detection [18,19]. Briefly, bacteria were suspended in veronal buffered saline supplemented with 5 mm CaCl2 (VBS+) at an A540 of 1. Aliquots of bacterial suspension (50 µl) were centrifuged at 2500 g for 2 min and resuspended in 50 µl of VBS+ containing MBL (5 µg/ml), purified as described previously [20], or VBS+ alone for negative controls. The suspensions were incubated at 37°C for 15 min and then, after washing, incubated with 5 µg/ml FITC-anti-MBL [20] for a further 15 min. Bacteria were then fixed in 1% paraformaldehyde before flow cytometry at low flow rate using a FACSCalibur (Becton Dickinson, Cowley, UK).
In all experiments, Neisseria meningitidis strains with well-described MBL binding [19] were included as controls (B1940 parent and isogenic cpsD-mutant) and all buffers were filtered using a 0·2-µm filter (Gellman Laboratory, Portsmouth, UK).
Determination of lectin-specific binding
To determine whether the MBL binding observed was specific and mediated by the lectin domain, the MBL preparation was incubated with known antagonists of binding (5 mm EDTA or 25 mm mannose) or a nonantagonist of binding (25 mm galactose) before addition to the bacteria.
Fluorescence microscopy
In certain experiments, the binding of MBL detected by flow cytometry was confirmed by fluorescence microscopy. Stained, fixed bacteria were allowed to settle onto plastic coverslips, which were then washed with PBS and inverted onto Vectashield (Vector Laboratories, Burlingame, CA, USA), containing 4′,6-aminido-2-phenylindole dihydrochloride to stain nucleic acid. Slides were examined at 100× magnification using a Leica DMRB microscope.
Results
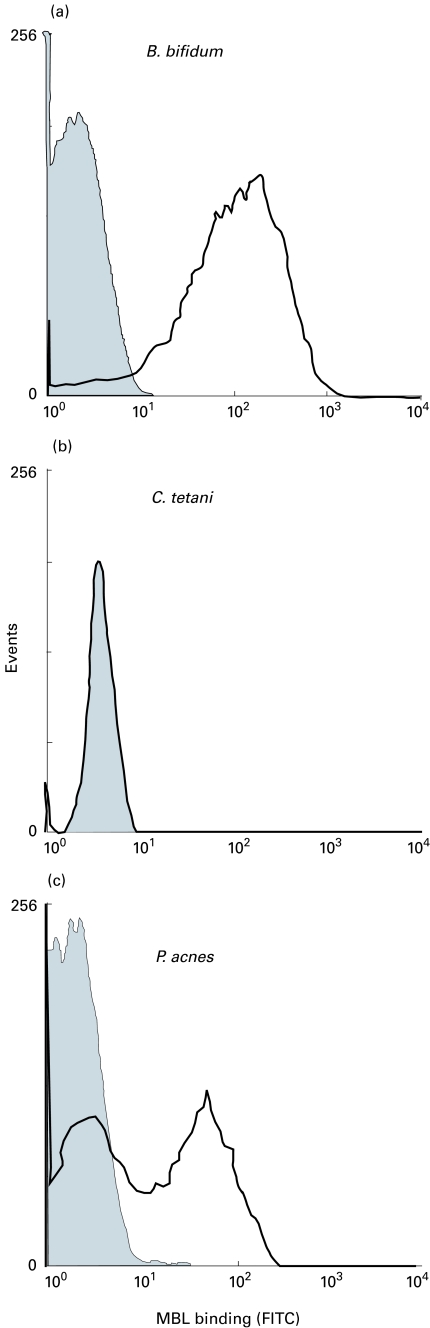
On flow cytometry, distinct populations of events were observed for all bacteria based on forward and side scatter (data not shown). Using these physical characteristics, three distinct patterns of MBL binding were observed. In certain species the entire population exhibited uniform, moderate to high levels of MBL binding (Bifidobacterium bifidum, Veillonella dispar). Other species bound low levels or no MBL as defined previously [18] (Clostridia, Bacteroides, F. mortiferum, Eubacteria). In some species, the bacterial population partitioned into cells negative for MBL binding and cells that exhibited moderate MBL binding (Proprionibacterium acnes, A. israelii, Fusobacteria, other than mortiferum, Leptotrichia buccalis) (Fig. 1). There were no differences in physical characteristics between these two populations as observed by flow cytometry. This bimodal pattern of MBL binding was further examined by fluorescence microscopy, which confirmed the partition of the population into those that did and those that did not bind MBL (data not shown). The morphology and staining characteristics of individual cells were otherwise identical.
Fig. 1.
Representative MBL binding to anaerobic bacteria determined by flow cytometry. Organisms grown overnight on blood agar were incubated with MBL (5 µg/ml) or buffer alone for 15 min before washing and resuspension in FITC-anti-MBL for 15 min. (a) MBL binding to B. bifidum. The shaded histogram is the control sample incubated without MBL, with the thick line representing MBL binding. (b) MBL binding to C. tetani. The control and experimental histograms overlie each other indicating little or no MBL binding to this organism. (c)MBL binding to P. acnes. Several of the species analysed in this investigation showed a similar, biphasic pattern of MBL binding. A certain proportion of the bacterial population showed little or no binding of MBL, while another group within the same population exhibited clear MBL binding.
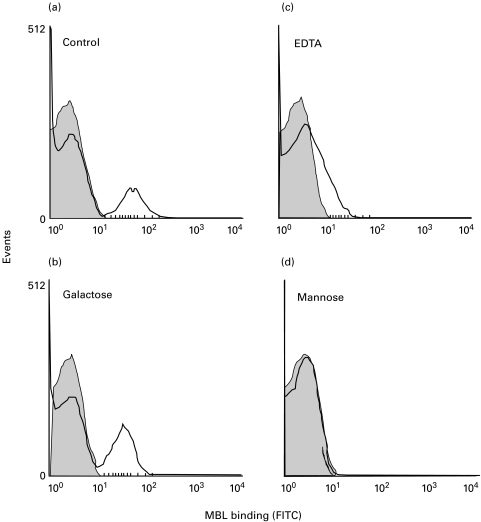
To determine whether the MBL binding observed was specific and mediated by the lectin domain, the MBL preparation was incubated with known antagonists of binding (5 mm EDTA or 25 mm mannose) or a non-antagonist of binding (25 mm galactose) before addition to the bacteria. As expected for lectin-mediated attachment [21], EDTA or mannose inhibited the observed binding, but galactose did not. This was observed for strains in which the whole population bound MBL, such as B. bifidum, for which the median fluorescence intensity (MFI) was inhibited by 73%, 65% and 3·5% by EDTA, mannose and galactose, respectively. Specificity was also shown for those strains where only a proportion of the population had bound MBL (Fig. 2). MBL binding data are summarized in Fig. 3.
Fig. 2.
Calcium and monosaccharide specificity of MBL binding. The specificity of the observed MBL binding was determined by incubation of the MBL preparation with either (a) VBS+ buffer, (b) galactose (25 mm), (c) EDTA (5 mm) or (d) mannose (25 mm) prior to incubation with F. necrogenes. MBL binding was then assayed as described in the text.
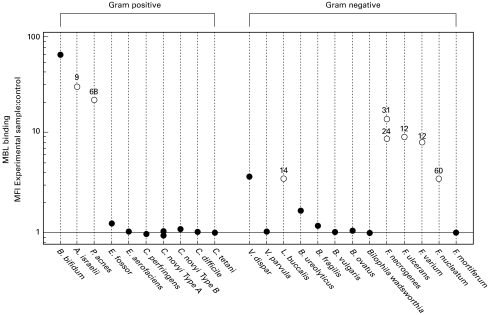
Fig. 3.
Summarized binding of MBL to anaerobes. MBL binding was determined after growth on blood agar plates for 24 or 48 h depending on rate of growth. The MBL binding ratio has been calculated by dividing the median fluorescence intensity (MFI) of MBL binding of the test sample by the control MFI as described previously [18]. For organisms with biphasic binding as in Fig. 1c, the MFI of the positive population has been calculated. Each data point represents the mean of two separate experiments with open symbols representing organisms with biphasic binding and closed symbols representing all others. The number above the open symbols is the mean percentage of organisms positive for MBL binding. The reference line shows a ratio of one, i.e. no binding.
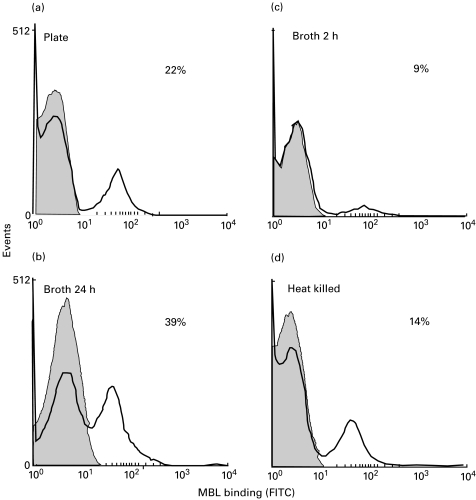
We investigated whether the biphasic MBL binding exhibited by certain species might be due to growth conditions and/or bacterial cell death by growing F. necrogenes under different conditions. Growth for 24 h in broth increased the proportion of organisms positive for MBL, but growth for 2 h in broth decreased positivity compared to organisms grown on solid media (Fig. 4). Heat-killed bacteria exhibited only a small increase in binding over viable organisms.
Fig. 4.
The influence of growth conditions on MBL binding to F. necrogenes. Organims were grown under various conditions and the binding of MBL was then assayed. (a) Overnight growth on blood agar (control). (b) Growth for 24 h in fastidious anaerobe broth (FAB). (c) Growth for 2 h in FAB. (d) Growth for 2 h in FAB, then heat killed at 56°C for 30 min. The percentage of organisms positive for MBL binding is identified in each panel.
Discussion
We surveyed the attachment of human mannose-binding lectin, a protein involved in first-line host defence, to a range of anaerobic bacteria. As with aerobic bacteria [17,18], we have detected large differences in the binding of MBL to different anaerobes. Of the species that we studied, those that are most commonly implicated in clinical disease (Bacteroides and Clostridium [22]) bound little or no MBL. Organisms such as Fusobacterium, which are more rarely isolated but still capable of causing severe invasive disease, bound measurable amounts of MBL. Organisms which very rarely cause significant infections such as B. bifidum, P. acnes, L. buccalis and V. dispar bound MBL. In contrast, the only Veillonella species that causes any appreciable disease, V. parvula [23], bound little or no MBL. This suggests that there may be an inverse relationship between pathogenicity and the level of MBL binding.
We noted an unusual pattern of MBL binding for certain species in which the bacterial population segregated into bacteria which bound little or no MBL and those which bound moderate amounts of the lectin. The results were reproducible and did not appear to be associated with contamination of the bacterial population, as determined by Gram stain, or with changes in the physical characteristics of the organisms, as assessed by flow cytometry or by microscopy. Some bacterial components are subject to phase variation in which expression varies according to growth phase. We found that F. necrogenes bound less MBL during the log phase than they did during nutrient restriction. This may be due to phase differences either in the expression of surface structures or cell morphology. The carbohydrate content of Fusobacterium is invariant with growth phase [24], which suggests that alteration in the disposition of sugar groups may be responsible for the observed phase variation.
All the Fusobacterium species that were assayed showed segregation into populations of bacteria which bound MBL and populations that did not bind, except for F. mortiferum to which no MBL binding was detected. F. mortiferum differs from the other strains studied in that it lacks mannose [24], which could explain the lack of MBL binding compared to other Fusobacterium species. However, it has been noted previously that simple carbohydrate composition cannot be relied upon to predict MBL binding [20], and in this study C. difficile, which does contain mannose [25], was found not to bind MBL. This suggests that the pattern in which carbohydrates are displayed is as important as the composition.
There have been no specific investigations of MBL deficiency in association with soft tissue infections or systemic infections that may be caused by anaerobic bacteria. To the best of our knowledge, the only data that exists are from an evaluation of consecutive admissions to a paediatric department [9]. In this study MBL deficiency was associated with a generalized risk of infections, and one of the disease groups identified was cellulitis and abscess. Anaerobic organisms often cause infections such as these, but specific pathogens were not identified in this previous study. The data presented here indicate the need for further investigation into the role of MBL deficiency in susceptibility to infection by certain anaerobes.
In conclusion, these results suggest that among the anaerobes that were studied resistance to MBL binding may be associated with bacteria which are more commonly pathogenic. Wider studies will be required to confirm whether this relationship occurs in other anaerobic bacteria. However, the clinical consequences of these findings deserve further investigation.
Acknowledgments
This work was funded by Wellcome Trust grant number 059847. The technical assistance of Tim Clarke, Department of Microbiology, Royal Hallamshire Hospital, Sheffield is gratefully acknowledged. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive.
References
- 1.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 2.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 3.Tenner AJ, Robinson SL, Ezekowitz RAB. Mannose-binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 126, 000 M (r) component of the C1q receptor. Immunity. 1995;3:485–93. doi: 10.1016/1074-7613(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 4.Sumiya M, Super M, Tabona P, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 5.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene [published erratum appears in Hum Mol Genet 1993; 2: 342] Hum Mol Genet. 1992;1:709–15. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Madsen HO, Garred P, Kurtzhals JA, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 7.Wallis R, Cheng JT. Molecular defects in variant forms of mannose-binding protein associated with immunodeficiency. J Immunol. 1999;163:4953–9. [PubMed] [Google Scholar]
- 8.Madsen HO, Garred P, et al. Interplay between promotor and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 9.Summerfield JA, Sumiya M, Levin M, Turner MW. Mannose-binding protein gene mutations are associated with childhood infections in a consecutive hospital series. Br Med J. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–3. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 11.Summerfield JA, Ryder S, Sumiya M, et al. Mannose-binding protein gene-mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–9. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 12.Super M, Thiel S, Lu J, Levinsky RJ, Turner MW. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;ii:1236–9. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 13.Garred P, Madsen HO, Balsev U, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–40. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 14.Luty AJF, Kun JFJ, Kremsner PG. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J Infect Dis. 1998;178:1221–4. doi: 10.1086/515690. [DOI] [PubMed] [Google Scholar]
- 15.Kelly P, Jack DL, Mandanda B, et al. Mannose binding lectin is a component of innate mucosal defence against Cryptosporidium parvum in AIDS. Gastroenterology. 2000;119:1236–42. doi: 10.1053/gast.2000.19573. [DOI] [PubMed] [Google Scholar]
- 16.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–45. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Emmerik LC, Kuijper EJ, Fijen CA, Dankert J, Thiel S. Binding of mannan-binding protein to various bacterial pathogens of meningitis. Clin Exp Immunol. 1994;97:411–6. doi: 10.1111/j.1365-2249.1994.tb06103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun. 2000;68:688–93. doi: 10.1128/iai.68.2.688-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack DL, Dodds AW, Anwar N, et al. Activation of complement by mannose-binding lectin on isogenic mutants of Neisseria meningitidis serogroup B. J Immunol. 1998;160:1346–53. [PubMed] [Google Scholar]
- 20.Devyatyarova-Johnson M, Rees IH, Robertson BD, Turner MW, Klein NJ, Jack DL. The lipopolysaccharide structures of Salmonella enterica serovar typhimurium and Neisseria gonorrhoeae determine the attachment of human mannose-binding lectin to intact organisms. Infect Immun. 2000;68:3894–9. doi: 10.1128/iai.68.7.3894-3899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defence system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein EC. Clinical anaerobic infections. Anaerobe. 1999;5:347–50. [Google Scholar]
- 23.Rosenblatt JE. Manual of clinical microbiology. 4. Washington, DC: American Society for Microbiology; 1985. Anaerobic cocci; pp. 445–9. [Google Scholar]
- 24.Heiske A, Mutters R. Cytochemical characterization of members of the genus Fusobacterium by cellular carbohydrate fingerprints and selected phenotypic features. Anaerobe. 1996;2:47–56. [Google Scholar]
- 25.Poxton IR, Cartmill TD. Immunochemistry of the cell-surface carbohydrate antigens of Clostridium difficile. J Gen Microbiol. 1982;128:1365–70. doi: 10.1099/00221287-128-6-1365. [DOI] [PubMed] [Google Scholar]