Abstract
In orthotopic liver transplantation (OLT), N-acetylcysteine (NAC) reduces ischaemia/reperfusion (I/R) injury, improves liver synthesis function and prevents primary nonfunction of the graft. To further elucidate the mechanisms of these beneficial effects of NAC, we investigated influence of high-dose NAC therapy on the pattern of adhesion molecule release from liver and intestine during OLT. Nine patients receiving allograft OLT were treated with 150 mg NAC/kg during the first hour after reperfusion; 10 patients received the carrier only. One hour after reperfusion, samples of arterial, portal venous and hepatic venous plasma were taken and blood flow in the hepatic artery and the portal vein was measured. Absolute concentrations of sICAM-1, sVCAM-1, sP-selectin and sE-selectin were not markedly different. However, balance calculations showed release of selectins from NAC-treated livers as opposed to net uptake in controls (P ≤ 0·02 for sP-selectin). This shedding of selectins might be a contributing factor to the decrease in leucocyte adherence and improved haemodynamics found experimentally with NAC-treatment.
Keywords: adhesion molecules, selectins, human liver transplantation, N-acetylcysteine, ischaemia/reperfusion, intraoperative period, balance calculation
Introduction
In liver transplantation, various types of stress such as prolonged storage or ischaemia can damage grafts inducing liver dysfunction or failure in the recipient [1–3]. During graft reperfusion, injurious mechanisms involving activation of vascular endothelium as well as leucocytes with release of oxidative radicals can cause hepatocellular damage [4]. Due to liver graft donor shortage, marginally suitable organs often have to be considered for transplantation. Thus it is of paramount importance to reduce reperfusion injury.
The radical scavenging properties of N-acetylcysteine have been proven beneficial in several conditions involving oxidative damage, namely ischaemia/reperfusion injury in rat liver transplantation [5]. Consequently, NAC has been evaluated as a hepatoprotective agent in clinical OLT and has shown favourable results in a recent study [6], whereas other studies did not demonstrate clinical benefit [7,8]. More detailed investigations with NAC treatment in OLT are needed [7].
In the inflammatory cascade, expression of adhesion molecules induced by LPS, TNF alpha and IL-1 beta [9,10] is a prerequisite for neutrophil adhesion on and transmigration through activated endothelium [11–13]. These adhesion molecules can be shed from the cell surface [14,15]. The soluble forms can bind to leucocytes in the bloodstream. Thereby, firm leucocyte–endothelial interactions may be inhibited, the inflammatory response attenuated and endothelial injury limited [16].
To further elucidate effects of NAC during liver transplantation, we investigated the pattern of release of adhesion molecules from liver and intestine in NAC-treated and control transplant recipients.
Patients and methods
Patients and treatment
After approval by the ethics committee of the University of Heidelberg and informed consent, 19 consecutive patients receiving orthotopic liver transplantation were included in the study. Immunosuppressive therapy with prednisolone and either cyclosporin A or tacrolimus was initiated at reperfusion.
In an alternate fashion, the patients either received N-acetylcystein dissolved in 5% glucose solution at 150 mg/kg of body weight during 15 min after reperfusion, 50 mg/kg during the following 4 h and 100 mg/kg during the following 16 h, or received the same volume of 5% glucose without NAC to serve as controls. In addition, livers of patients in the treatment group were rinsed with 1 l Ringer's solution containing 1 g NAC/l immediately before reperfusion.
Measurements, sampling and analysis
One hour after graft reperfusion, blood flow in the hepatic artery and the portal vein was measured by Doppler sonographic flow probe [17]. At the same time, samples of arterial, portal venous and hepatic venous blood were drawn in heparinized tubes. Samples were immediately cooled on ice; plasma was separated by centrifugation within 1 h, aliquoted, frozen at −20°C and stored for awaiting further analyses.
To quantify adhesion molecule concentrations, we used commercially available enzyme-linked immunoassays with 96-well microtitre plates: sICAM-1 (Biosource, Ratingen, Germany), sVCAM-1, sP-selectin and sE-selectin (R&D Systems, Minneapolis, MN, USA).
Calculations
Individual haematocrit values at time of flow measurement were used to convert blood flow into plasma flow by multiplying by (1 – haematocrit). Mean haematocrit value was 0·28. The sum of hepatic arterial and portal venous flows ( = inflow) was taken for hepatic outflow. Portal venous flow was taken for intestinal flow.
Adhesion molecule balances (μg * min−1) were calculated as the arteriovenous differences multiplied by plasma flow [18]. For net hepatic balance, hepatic arterial and portal flow were considered according to their actually measured ratio.
‘Intestinal balance’ refers to the portal-draining viscera (extrahepatic splanchnic tissues), ‘splanchnic balance’ refers to portal-draining viscera plus liver. Positive values denote net uptake, negative values denote net release of substrates.
All data in the text, tables and figures are presented as mean ±s.e.m. (standard error of the mean). For statistical analysis, the Mann–Whitney U-test and Wilcoxon's two-tailed test were used.
Results
Nine patients were included in the treatment group; 10 patients served as controls. Indications for transplantation included cirrhosis for alcohol abuse, chronic hepatitis, haemochromatosis, amyloidosis, Wilson's disease, primary biliary cirrhosis and hepatocellular carcinoma. Age at time of transplantation was 54·0 ± 5·5 years in the NAC group and 47·7 ± 15·0 years in controls (mean ±s.d.; difference not significant).
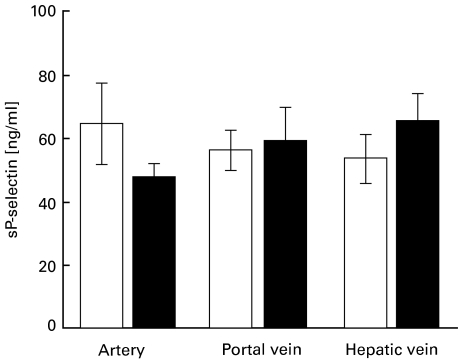
Plasma levels of sICAM-1, sVCAM-1, sP-selectin and sE-selectin were as shown in Table 1. Absolute levels in artery, portal vein and hepatic vein 1 h after reperfusion of the liver graft were not different between NAC and control groups (for absolute sP-selectin values, see Fig. 1).
Table 1.
Absolute concentrations (ng/ml) of sICAM-1, sVCAM-1, sP-selectin and sE-selectin in arterial, portal venous, and hepatic venous plasma in orthotopic liver transplantation 1 h after reperfusion. Mean ±s.e.m. NAC treatment group: n = 9; control group: n = 10. None of the differences are statistically significant
| Absolute concentrations | NAC group | Control group |
|---|---|---|
| sICAM-1 | ||
| arterial | 163·2 ± 33·0 | 204·4 ± 47·0 |
| portal venous | 244·2 ± 31·3 | 226·2 ± 48·6 |
| hepatic venous | 200·5 ± 43·5 | 253·6 ± 48·6 |
| sVCAM-1 | ||
| arterial | 558·2 ± 198·1 | 503·0 ± 89·3 |
| portal venous | 616·0 ± 184·2 | 533·1 ± 102·6 |
| hepatic venous | 595·8 ± 177·6 | 400·3 ± 66·6 |
| sP-selectin | ||
| arterial | 47·4 ± 4·5 | 64·4 ± 12·8 |
| portal venous | 59·1 ± 10·5 | 56·2 ± 6·3 |
| hepatic venous | 65·5 ± 8·6 | 53·6 ± 7·8 |
| sE-selectin | ||
| arterial | 29·4 ± 6·0 | 47·7 ± 13·1 |
| portal venous | 39·3 ± 11·0 | 46·4 ± 10·7 |
| hepatic venous | 38·5 ± 7·3 | 40·0 ± 10·5 |
Fig. 1.
Absolute concentrations (ng/ml) of sP-selectin in arterial, portal venous and hepatic venous plasma 1 h after reperfusion of the liver graft. The data show the mean values ±s.e.m. of the NAC-treated (n = 9) and control patients (n = 10). The differences are not significant. □, Control group (n = 10); ▪, NAC group (n = 9).
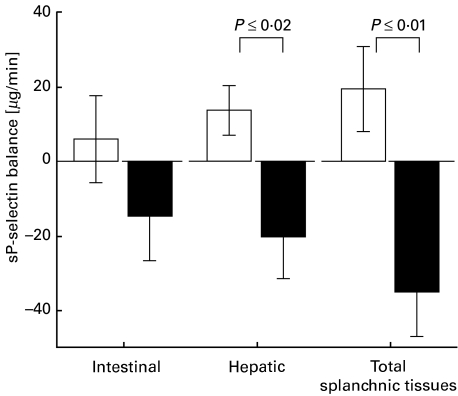
NAC treatment caused intraoperative release of selectins as opposed to net uptake in the control group (Table 2). For sP-selectin (Fig. 2), the differences in hepatic and total splanchnic balance (i.e. intestinal and hepatic balance combined) were significant (P ≤ 0·02 and P ≤ 0·01, respectively). There was a trend towards increased splanchnic release of sE-selectin in the NAC group. Balance values of sICAM-1 and sVCAM-1 showed high interpatient variability and were not statistically different between the groups (Table 2).
Table 2.
Balance values (µg/min) of sICAM-1, sVCAM-1, sP-selectin and sE-selectin across the intestinal organs, the liver, and the total of both ( = splanchnic balance) in orthotopic liver transplantation 1 h after reperfusion. Mean values ±s.e.m. NAC treatment group: n = 9; control group: n = 10. P-values are shown in parentheses. NS, not significant
| Balance values | NAC group | Control group |
|---|---|---|
| sICAM-1 | ||
| intestinal balance | − 120·9 ± 47·1 | − 22·2 ± 29·0 (NS) |
| hepatic balance | 52·4 ± 64·4 | − 67·5 ± 32·0 (NS) |
| total splanchnic balance | − 68·3 ± 54·1 | − 89·8 ± 38·9 (NS) |
| sVCAM-1 | ||
| intestinal balance | − 51·3 ± 176·4 | − 115·3 ± 111·8 (NS) |
| hepatic balance | 80·9 ± 333·3 | 284·4 ± 108·3 (NS) |
| total splanchnic balance | 29·7 ± 406·8 | 169·2 ± 107·6 (NS) |
| sP-selectin | ||
| intestinal balance | − 14·6 ± 11·8 | 5·9 ± 11·6 (NS) |
| hepatic balance | − 20·1 ± 11·4 | 13·7 ± 6·6 (P ≤ 0·02) |
| total splanchnic balance | − 34·7 ± 12·2 | 19·6 ± 11·3 (P ≤ 0·01) |
| sE-selectin | ||
| intestinal balance | − 10·5 ± 10·6 | − 2·5 ± 7·7 (NS) |
| hepatic balance | − 7·4 ± 9·4 | 14·5 ± 5·0 (NS) |
| total splanchnic balance | − 17·8 ± 9·8 | 12·0 ± 10·0 (NS) |
Fig. 2.
Balance values of sP-selectin (µg/min) across the intestinal organs, the liver and the total of both ( = splanchnic balance), 1 h after reperfusion of the liver graft. Mean values ±s.e.m. Positive values indicate an uptake, negative values indicate the release of sP-selectin. □, Control group (n = 10); ▪, NAC group (n = 9).
Discussion
High-dose therapy with N-acetylcysteine has become part of the standard treatment for patients with acute liver failure. Primarily, the regimen was developed for liver failure due to paracetamol (acetaminophen) poisoning [19]. In the treatment of acute liver failure from other causes, NAC has also proven beneficial [20].
In animal models, the use of NAC caused an improvement in hepatic macro- and microcirculation after reperfusion following cold and warm liver ischaemia [21,22]. In rat liver transplantation, NAC treatment reduced early phase reperfusion injury [5] and decreased leucocyte adherence to sinusoidal endothelium [23]. In a model of hypothermic I/R injury of the steatotic rat liver, NAC given before cold storage for 24 h diminished sinusoidal microcirculatory injury and lowered enzyme release after reperfusion [24]. In an endotoxin sepsis model of the rat, NAC treatment given before as well as after induction of sepsis decreased leucocyte adherence in mesentery vessels and improved haemodynamics [25,26].
In clinical use of NAC during liver transplantation, two studies failed to show improved clinical outcome. In the study by Bromley et al. [8] modest intermittent haemodynamic improvement was seen initially during the loading dose. Whether this advantage is methodologically valid remains controversial [27]. Steib et al. used higher maintenance doses of NAC but found no differences at all between the groups [7]. The most recently published study by Thies et al., however, demonstrated attenuated reperfusion injury as histologically graded, significantly increased flow in the portal vein, improved liver synthesis function, and prevention of primary graft non-function in NAC-treated liver graft recipients [6]. Since the dosing regimen of the two latter studies differs only in an additional rinsing of the graft with NAC containing solution (Thies et al.), the divergence of the results may be attributable to the high interpatient variability in orthotopic liver transplantation. Larger multicentre studies would be needed to finally answer the question of whether NAC is useful in this setting. On the other hand, we believe that investigating immunological questions in lower scale studies may contribute to this ongoing discussion.
Our principal finding is that in NAC-treated liver transplant recipients P-selectin is released from the graft 1 h after reperfusion, as opposed to control patients. It has been shown that P-selectin expression is high 30 and 60 min after resuscitation in murine [28] and rat [29] liver tissue, respectively, in I/R models. Therefore we assume that NAC enhances shedding of these adhesion molecules from liver endothelial cells. Soluble P-selectin has shown to inhibit binding of activated neurophils to endothelium in vitro [30] and was postulated to play an important protective role in murine glomerulonephritis [16].
If shedding reduces the amount of endothelial P-selectin, this may further decrease neutrophil rolling and adhesion, which possibly is of therapeutic benefit in NAC treated liver transplant recipients. A study on rat liver microvasculature could not demonstrate an involvement of endothelial selectins in the recruitment of leucocytes [31]. However, a clear dependency of leucocyte–endothelial interaction on functioning P-selectin molecules has been proven in murine hepatic [32,33] and splanchnic [34] I/R models. Similarly, in a murine renal I/R model, P-selectin deficiency as well as blocking P-selectin by antibodies resulted in less neutrophil infiltration and in better organ function [35]. The role of selectins in the human liver has yet to be further clarified.
Apart from this hypothesis, possible clinical benefits of NAC-treatment might also be due to unspecific attenuation of oxidant stress and subsequent inflammatory injury in endothelium and hepatocytes [36]. Advantages of NAC treatment could also be attributed to its vaso-relaxant properties [37], most probably via formation of S-nitrosocysteine [38].
For the short amount of time passed since the beginning of the NAC-treatment (60 min), modulations of the expression of selectins [39] at the transcriptional level by NAC as an inhibitor of NFκB-activation [40–42] are unlikely to explain our findings.
In our study, we found no difference in the absolute plasma concentrations of sICAM-1, sVCAM-1, sP-selectin, and sE-selectin 1 h after reperfusion. Only by calculating the organ balances [18] was the shedding of selectins discovered. We used the unique opportunity of direct and easy access to vessels of liver inflow and outflow presenting only intraoperatively. In the study by Müller et al. [43], hepatic venous concentrations of adhesion molecules were taken into account in addition to systemic concentrations, but balances were not determined.
Our study reflects only the intraoperative situation 1 h after reperfusion, giving some limited insight into early NAC effects. With the small number of patients studied here it was not intended to show differences in clinical outcome between the groups. To better understand the mechanisms involved as well as the long-term consequences of NAC treatment in OLT, further studies including more patients are needed.
In conclusion, by calculating intraoperative net organ balances we could demonstrate early shedding of selectins in NAC treated liver grafts. This is in line with experimental findings of decreased leucocyte adherence and improved microhaemodynamics and might help to explain favourable clinical results found recently with the use of NAC in orthotopic liver transplantation.
References
- 1.Thurman RG, Marzi I, Seitz G, Thies J, Lemasters JJ, Zimmerman F. Hepatic reperfusion injury following orthotopic liver transplantation in the rat. Transplantation. 1988;46:502–6. doi: 10.1097/00007890-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Hellinger A, Fiegen R, Lange R, et al. Preservation of pig liver allografts after warm ischemia: normothermic perfusion versus cold storage. Langenbecks Arch Chir. 1997;382:175–84. doi: 10.1007/BF02391863. [DOI] [PubMed] [Google Scholar]
- 3.Schon MR, Hunt CJ, Pegg DE, Wight DG. The possibility of resuscitating livers after warm ischemic injury. Transplantation. 1993;56:24–31. doi: 10.1097/00007890-199307000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Thiel M, Imendorffer S, Chouker A, et al. Expression of adhesion molecules on circulating polymorphonuclear leukocytes during orthotopic liver transplantation. Hepatology. 1998;28:1538–50. doi: 10.1002/hep.510280614. [DOI] [PubMed] [Google Scholar]
- 5.Nakano H, Boudjema K, Alexandre E, et al. Protective effects of N-acetylcysteine on hypothermic ischemia-reperfusion injury of rat liver. Hepatology. 1995;22:539–45. [PubMed] [Google Scholar]
- 6.Thies JC, Teklote J, Clauer U, et al. The efficacy of N-acetylcysteine as a hepatoprotective agent in liver transplantation. Transpl Int. 1998;11(Suppl. 1):S390–2. doi: 10.1007/s001470050505. [DOI] [PubMed] [Google Scholar]
- 7.Steib A, Freys G, Collin F, Launoy A, Mark G, Boudjema K. Does N-acetylcysteine improve hemodynamics and graft function in liver transplantation? Liver Transpl Surg. 1998;4:152–7. doi: 10.1002/lt.500040204. [DOI] [PubMed] [Google Scholar]
- 8.Bromley PN, Cottam SJ, Hilmi I, et al. Effects of intraoperative N-acetylcysteine in orthotopic liver transplantation. Br J Anaesth. 1995;75:352–4. doi: 10.1093/bja/75.3.352. [DOI] [PubMed] [Google Scholar]
- 9.Wyble CW, Desai TR, Clark ET, Hynes KL, Gewertz BL. Physiologic concentrations of TNFalpha and IL-1beta released from reperfused human intestine upregulate E-selectin and ICAM-1. J Surg Res. 1996;63:333–8. doi: 10.1006/jsre.1996.0271. [DOI] [PubMed] [Google Scholar]
- 10.Fassbender K, Kaptur S, Becker P, Groschl J, Hennerici M. Adhesion molecules in tissue injury: kinetics of expression and shedding and association with cytokine release in humans. Clin Immunol Immunopathol. 1998;89:54–60. doi: 10.1006/clin.1998.4583. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–73. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 12.Ley K, Gaehtgens P, Fennie C, Singer MS, Lasky LA, Rosen SD. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77:2553–5. [PubMed] [Google Scholar]
- 13.Ramos CL, Kunkel EJ, Lawrence MB, et al. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood. 1997;89:3009–18. [PubMed] [Google Scholar]
- 14.Wyble CW, Hynes KL, Kuchibhotla J, Marcus BC, Hallahan D, Gewertz BL. TNF-alpha and IL-1 upregulate membrane-bound and soluble E-selectin through a common pathway. J Surg Res. 1997;73:107–12. doi: 10.1006/jsre.1997.5207. [DOI] [PubMed] [Google Scholar]
- 15.Pigott R, Dillon LP, Hemingway IH, Gearing AJ. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992;187:584–9. doi: 10.1016/0006-291x(92)91234-h. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz AR, Mendrick DL, Cotran RS, Mayadas TN. P-selectin deficiency exacerbates experimental glomerulonephritis: a protective role for endothelial P-selectin in inflammation. J Clin Invest. 1999;103:649–59. doi: 10.1172/JCI5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laustsen J, Pedersen EM, Terp K, et al. Validation of a new transit time ultrasound flowmeter in man. Eur J Vasc Endovasc Surg. 1996;12:91–6. doi: 10.1016/s1078-5884(96)80282-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller BM, Cersosimo E, McRae J, Williams PE, Lacy WW, Abumrad NN. Interorgan relationships of alanine and glutamine during fasting in the conscious dog. J Surg Res. 1983;35:310–8. doi: 10.1016/0022-4804(83)90006-9. [DOI] [PubMed] [Google Scholar]
- 19.Keays R, Harrison PM, Wendon JA, et al. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303:1026–9. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med. 1991;324:1852–7. doi: 10.1056/NEJM199106273242604. [DOI] [PubMed] [Google Scholar]
- 21.Dunne JB, Davenport M, Williams R, Tredger JM. Evidence that S-adenosylmethionine and N-acetylcysteine reduce injury from sequential cold and warm ischemia in the isolated perfused rat liver. Transplantation. 1994;57:1161–8. doi: 10.1097/00007890-199404270-00004. [DOI] [PubMed] [Google Scholar]
- 22.Fukuzawa K, Emre S, Senyuz O, Acarli K, Schwartz ME, Miller CM. N-acetylcysteine ameliorates reperfusion injury after warm hepatic ischemia [see comments] Transplantation. 1995;59:6–9. doi: 10.1097/00007890-199501150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Koeppel TA, Lehmann TG, Thies JC, et al. Impact of N-acetylcysteine on the hepatic microcirculation after orthotopic liver transplantation. Transplantation. 1996;61:1397–402. doi: 10.1097/00007890-199605150-00020. [DOI] [PubMed] [Google Scholar]
- 24.Nakano H, Nagasaki H, Barama A, et al. The effects of N-acetylcysteine and anti-intercellular adhesion molecule-1 monoclonal antibody against ischemia-reperfusion injury of the rat steatotic liver produced by a choline-methionine-deficient diet. Hepatology. 1997;26:670–8. doi: 10.1053/jhep.1997.v26.pm0009303498. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt H, Schmidt W, Muller T, Bohrer H, Gebhard MM, Martin E. N-acetylcysteine attenuates endotoxin-induced leukocyte-endothelial cell adhesion and macromolecular leakage in vivo. Crit Care Med. 1997;25:858–63. doi: 10.1097/00003246-199705000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt W, Walther A, Gebhard MM, Martin E, Schmidt H. Influence of N-acetylcysteine treatment on endotoxin-induced microcirculatory disturbances. Intensive Care Med. 1998;24:967–72. doi: 10.1007/s001340050697. [DOI] [PubMed] [Google Scholar]
- 27.Walsh TS, Hopton P, Philips BJ, Mackenzie SJ, Lee A. The effect of N-acetylcysteine on oxygen transport and uptake in patients with fulminant hepatic failure. Hepatology. 1998;27:1332–40. doi: 10.1002/hep.510270520. [DOI] [PubMed] [Google Scholar]
- 28.Akgur FM, Zibari GB, McDonald JC, Granger DN, Brown MF. Kinetics of P-selectin expression in regional vascular beds after resuscitation of hemorrhagic shock: a clue to the mechanism of multiple system organ failure. Shock. 2000;13:140–4. doi: 10.1097/00024382-200013020-00008. [DOI] [PubMed] [Google Scholar]
- 29.Basile J, Wang L, Tarcsafalvi A, Han R, Boros P, Miller CM. Expression of GMP-140 (P-selectin) correlates with graft viability in cold-preserved rat livers. Transplantation. 2000;69:2440–2. doi: 10.1097/00007890-200006150-00039. [DOI] [PubMed] [Google Scholar]
- 30.Gamble JR, Skinner MP, Berndt MC, Vadas MA. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP140. Science. 1990;249:414–7. doi: 10.1126/science.1696029. [DOI] [PubMed] [Google Scholar]
- 31.Wong J, Johnston B, Lee SS, et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–90. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawaya De, Jr, Zibari GB, Minardi A, et al. P-selectin contributes to the initial recruitment of rolling and adherent leukocytes in hepatic venules after ischemia/reperfusion [see comments] Shock. 1999;12:227–32. doi: 10.1097/00024382-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494–502. doi: 10.1002/hep.510290505. [DOI] [PubMed] [Google Scholar]
- 34.Scalia R, Armstead VE, Minchenko AG, Lefer AM. Essential role of P-selectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J Exp Med. 1999;189:931–8. doi: 10.1084/jem.189.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. Faseb J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- 36.Jaeschke H, Ho YS, Fisher MA, Lawson JA, Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–50. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- 37.Koeppel TA, Thies JC, Lehmann T, et al. Improvement of hepatic microhemodynamics by N-acetylcysteine after warm ischemia. Eur Surg Res. 1996;28:270–7. doi: 10.1159/000129466. [DOI] [PubMed] [Google Scholar]
- 38.Stamler J, Mendelsohn ME, Amarante P, et al. N-acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ Res. 1989;65:789–95. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- 39.Boyle Em, Jr, Sato TT, Noel Rf, Jr, Verrier ED, Pohlman TH. Transcriptional arrest of the human e-selectin gene. J Surg Res. 1999;82:194–200. doi: 10.1006/jsre.1998.5536. [DOI] [PubMed] [Google Scholar]
- 40.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–7. [PubMed] [Google Scholar]
- 42.Tsuji F, Miyake Y, Aono H, Kawashima Y, Mita S. Effects of bucillamine and N-acetyl-l-cysteine on cytokine production and collagen-induced arthritis (CIA) Clin Exp Immunol. 1999;115:26–31. doi: 10.1046/j.1365-2249.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller AR, Platz KP, Haak M, et al. The release of cytokines, adhesion molecules, and extracellular matrix parameters during and after reperfusion in human liver transplantation. Transplantation. 1996;62:1118–26. doi: 10.1097/00007890-199610270-00017. [DOI] [PubMed] [Google Scholar]