Abstract
Human membrane cofactor protein (MCP; CD46) is a widely distributed complement regulator. In the mouse, expression of MCP is largely restricted to the testis while a related, widely expressed protein (Crry) appears to perform MCP's (CD46) regulatory activity. We have developed two mouse strains transgenic for human MCP (CD46) utilizing an ∼ 400 kb YAC clone carrying the complete gene. A third mouse strain was generated using an overlapping YAC clone isolated from a second library. The expression of human MCP (CD46) in these mouse strains was characterized by immunohistochemistry, FACS, Western blotting and RT-PCR. No differences were detected in the isoform pattern or distribution among the three strains, although the expression level varied according to how many copies of the gene were integrated. The expression profile closely mimicked that observed in humans, including the same pattern of isoform expression as the donor. In addition, tissue-specific isoform expression in the kidney, salivary gland and brain paralleled that observed in man. The transgenic mice expressed low levels of MCP (CD46) on their E, in contrast to humans but in line with most other primates. These mice should be a useful tool to analyse tissue-specific expression, to establish animal models of infections and to characterize the role of MCP (CD46) in reproduction.
Keywords: complement system, membrane cofactor protein (MCP; CD46), measles virus receptor, MCP (CD46) transgenic mice
Introduction
Human membrane cofactor protein (MCP; CD46) is a cofactor for the factor I-mediated degradation of C3b and C4b deposited on self-tissue [1–3]. In addition to this complement inhibitory activity, MCP (CD46) serves as a receptor for several pathogens including the measles virus [4–6], Streptococcus pyogenes [7], Neisseria gonorrhoeae and meningitides [8] and Herpesvirus 6 [9]. It may also be involved in reproduction [10–12], being heavily expressed on placental trophoblast tissue and on the inner acrosomal membrane of spermatozoa. MCP (CD46) is expressed on most every cell lineage as four isoforms that arise by alternative splicing [13–16] (Fig. 1). MCP (CD46) of most cells migrates on SDS-PAGE as a characteristic, broad, two band pattern with ∼Mrs of 59,000–68 000 (upper band; BC1 and BC2 isoforms) and 51,000–58 000 (lower band; C1 and C2 isoforms) [13]. The relative quantity expressed of these bands is genetically controlled, being inherited in an autosomal codominant fashion: 65% of the population express predominantly the upper BC form, 29% express both forms in approximately equal amounts and 6% express the lower C form predominantly [17–19]. Interestingly, in the mouse, expression of MCP is largely restricted to the testis [20] while a related widely expressed protein (Crry) performs MCP's (CD46) complement regulatory activity.
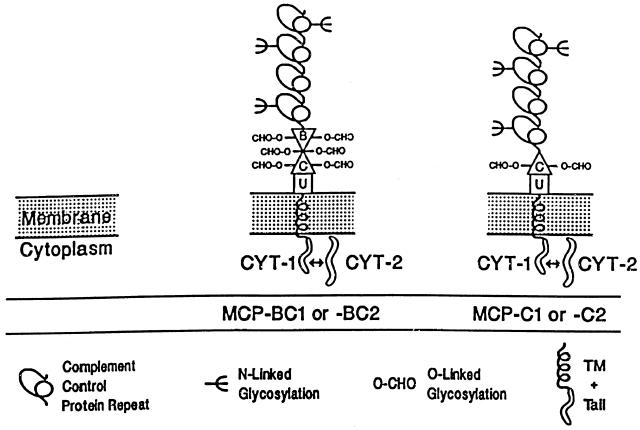
Fig. 1.
Schematic diagram of the four commonly expressed isoforms of human MCP (CD46). All isoforms contain four complement control protein repeats (CCPs) at the extramembranous amino-terminus. CCPs 1, 2 and 4 each bear one N-linked oligosaccharide. These CCPs are followed by an alternatively spliced serine, threonine and proline rich region (STP-region), designated B and C. These regions are encoded by single exons and contain O-glycosylation sites. C is expressed by all isoforms while B is alternatively spliced. Common to all isoforms is a 12-aa juxtamembranous segment of unknown function (designated as ‘U’), followed by the transmembrane region (TM) and the alternatively spliced carboxyl terminal cytoplasmic tail (CYT-1 or CYT-2). The U region and the tails arise from single exons and the TM region from two exons.
In collaboration with the groups of R. Cattaneo [21] and C. Stephensen [Stephensen, Pinkert and Atkinson, unpublished], we developed two mouse strains transgenic for human MCP (CD46) by utilizing an ∼400 kb yeast artificial chromosome (YAC) clone carrying the complete gene (Fig. 2). The YAC clone was derived from a library generated from the genomic DNA of a donor with the most commonly expressed upper band predominant MCP (CD46) phenotype [22]. An additional mouse strain was developed by a third group using an overlapping ∼420 kb clone isolated from a different YAC library [23]. Two of the mouse strains have already been utilized to establish animal models of measles virus infection [21,24]; however, only limited data regarding the human MCP (CD46) expression pattern and distribution is available. We have therefore further characterized human MCP (CD46) expression in these transgenic mouse strains.
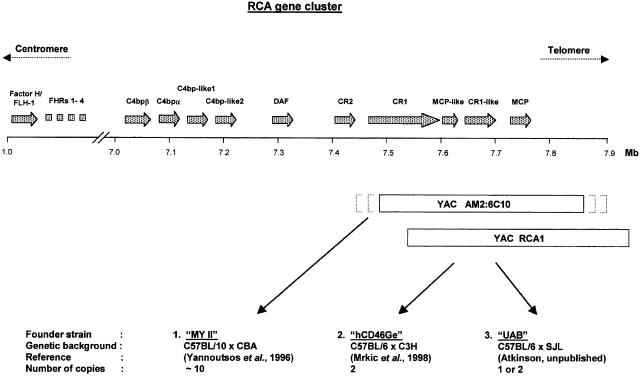
Fig. 2.
Schematic representation of the genetic elements used to generate the three MCP (CD46) transgenic mouse strains. The upper part of the figure shows the part of chromosome 1, band 1q32, that contains the regulators of complement activation (RCA) gene cluster. The gene sizes and intergenic distances of the members of the RCA cluster are drawn to scale and gene orientations are designated 5′ to 3′. Below are shown the two YAC clones used to generate the three transgenic mouse strains. Both YACs contain a ∼400 kb genomic insert that includes the entire MCP (CD46) gene. While the alignment of YAC RCA1 with chromosome 1 is precise, the alignment of YAC AM2 : 6C10 is only approximate as indicated by the dotted parentheses. The insertion sites of the YACs in the mouse genomes are not known. For the designation and accession numbers of the two YAC clones see Materials and methods.
Materials and methods
Mouse strains
MY II mice were a gift of Imutran LTD (UK) and described in Yannoutsos et al. (1996) [23]. The YAC used to generate these animals contains the entire MCP gene and has the Imperial Cancer Research Fund (ICRF, London UK) reference YAC library designation number AM2: 6C10. This YAC clone is not colinear with the human genome due to triplication of a DNA region. The hCD46Ge transgenic mouse line was generated as previously described [21] using the YAC RCA1 [22] with the designation A52H9 from the library of the Center for Genetics in Medicine of the Washington University School of Medicine (St. Louis, MO). The hCD46Ge animals breed normally but tend to become obese after six months. Although the MY II animals do not develop obesity, the MY II colony is more difficult to maintain due to aggressive behaviour and frequent killing of the litters by both parents (own observation and B. Mrkic, personal communication). Mice created by the UAB Transgenic Animal/ES Cell Resource were generated using YAC RCA1 into a C57BL/6 × SJL F2 hybrid strain. These animals were then backcrossed to a C57BL/6 × C3H background. The UAB mice breed normally but show some instability regarding the YAC clone integration and occasionally converted to a negative phenotype (unpublished data). Animals were maintained in the Animal Care Facility of the Washington University School of Medicine (St. Louis, MO).
Antibodies and cell lines
The mouse monoclonal antibody TRA-2–10 was a gift of P.A. Andrews (CD46) [25]. The anti-MCP (CD46) rabbit polyclonal antiserum was produced by CytoMed (Cambridge, MA), and the mouse monoclonal control antibody MOPC-21 was purchased from Sigma (St. Louis, MO). Horseradish peroxidase linked antirabbit IgGs (Amersham, Arlington Heights, IL) and biotinylated horse antimouse IgGs (Vector Laboratories, Inc., Burlingame, CA) were used as secondary Abs for Western blot analyses. For FACS analysis, TRA-2–10 was FITC (Sigma) labelled according to Current Protocols in Molecular Biology (Wiley Interscience). PE-labelled Abs against mouse T cells (PE antimouse CD3), B-cells (PE antimouse CD45R/B220) and neutrophils (PE antimouse Ly-6G) were purchased from PharMingen (San Diego, CA). Abs against human T cells (PE antihuman CD3-RD1) and B cells (PE antihuman B4-RD1) were purchased from Coulter Corporation (Miami, FL). The Ab against human neutrophils (PE antihuman CD116) was obtained from Dako (Carpinteria, CA).
Chinese hamster ovary (CHO) cell lines transfected with cDNA coding for the BC1, BC2, C1 or C2 isoform of MCP (CD46) or the transfection vector alone have been described [26]. The cells were maintained in Ham's F12 medium supplemented with 10% FBS, 0·5 mg/ml geneticin, and 1 mm l-glutamine. The T cell leukaemia cell line, Jurkat, was obtained from the American Type Culture Collection (Manassas, VA). The cells were grown in RPMI 1640 supplemented with 10% FBS, l-glutamine and antibiotics. Tissue culture reagents were purchased from the Tissue Culture Support Center at Washington University School of Medicine.
Tissue collection and storage
Animals were sacrificed between six and 12 months of age by cervical dislocation as approved by the Washington University Animal Studies Committee and organs were dissected immediately under aseptic conditions. For protein and RNA purifications, organs were cut into 3–4 mm3 sized pieces, placed in RNase-free cryotubes and snap frozen in liquid nitrogen. For immunohistochemistry, entire organs were embedded in tissue molds (Miles, Elkhart, IN) filled with OCT-compound (Tissue-Tek, Sakura Finetek U.S.A, Inc., Torrance, CA) and snap frozen in ice cold 1-Butanol (Sigma). Human tissue samples were obtained from the Department of Pathology and Immunology of the Washington University School of Medicine (St. Louis, MO) and handled as described for the mouse samples, with the exception that 1 cm3 pieces were OCT-embedded for cryo-cutting. Samples were stored at −80°C.
RNA and protein purification
The TRIZOL reagent (GibcoBRL, Grand Island, NY) was used to purify RNA and protein fractions from tissue samples or cell lines. Frozen tissue cubes (∼20 mg) or pelleted CHO or Jurkat cells (1 × 106 cells) were placed in 1 ml TRIZOL reagent, homogenized using a portable, motorized tissue grinder (Pellet Pestle Motor, Kontes, Vineland, NJ) and RNA and protein fractions were extracted, according to manufacturer's directions.
RT-PCT
One μg of isolated RNA was used for RT-PCR analysis using the Superscript™ One Step ™ RT-PCR Kit (Life Technologies, Rockville, MD) and the GeneAmp PCR System 9600 (Perkin-Elmer, Foster City, CA). The 5′ primer (GTGGTCAAATGTCGATTTCCAGTAGTCG) anneals to a region in CCP 4 and the 3′ primer (CAGCCACATTGCAATATTAGCTAAGCCACA) anneals to the 3′ untranslated region of the MCP transcript, allowing a discrimination between the alternatively spliced four RNAs coding for the corresponding MCP protein isoforms [13]. RT-PCR was performed in a total reaction volume of 50 μl. Reaction mixtures included 25 μl of 2X reaction mix, 1 μg RNA (1μg/μl), 1 μl of each sense and antisense primer (10 μm), 1 μl of reverse transcriptase/Taq mix and 21 μl of distilled water. RT-PCR protocol was as follows: the reaction mixture was incubated for 30 min at 51°C to allow reverse cDNA transcription; an initial denaturation step at 94°C for 2 min was followed by 40 cycles of 94°C for 15 s, 54°C for 30 s, and 71°C for 1 min. The last cycle included a final 10 min extension at 72°C. The PCR products were separated by electrophoresis in 3% agarose gels and visualized by ethidium bromide staining. To estimate the ratio of mRNA splice variants of MCP (CD46) isoforms in tissue samples, gels of control tissues were scanned utilizing the ChemiImager ™ 4400 (Alpha Innotech Corporation, San Leandro, CA) and signal intensity was determined for standards by spot densitometry with the AlphaEase ™ Stand Alone Software (Alpha Innotech Corporation). RNA ratios of tissue samples were also compared by visual inspection.
Western blotting
Total protein extract (10 μg) was separated in an 8% SDS-polyacrylamide gel under nonreducing conditions and then transferred onto nitrocellulose membranes using the MiniCell II wet blotting system from NOVEX (San Diego, CA). The membranes were incubated for 1 h at 37°C with rabbit polyclonal antiserum to MCP (CD46) diluted 1:5000 in TBS-T followed by an incubation for 1 h at room temperature with a horseradish peroxidase (HRP)-conjugated donkey antirabbit IgG secondary Ab (Amersham) diluted 1:3000 in TBS-T. The signal was developed using the enhanced chemiluminescence kit from Pierce (Rockford, IL) as per the manufacturer's suggestion. The bands were visualized by exposing the membranes to BioMax MR films (Kodak, Rochester, NY). To estimate the ratio of upper and lower MCP (CD46) isoforms in tissue samples, representative Western blot films of control tissues were scanned for standards and tissue samples were then compared by visual inspection.
Isolation of erythrocytes and white blood cells
To isolate mouse or human erythrocytes (E), 4 ml Histopaque 1119 (Sigma) was pipetted into a 15-ml centrifugation tube and overlayered with 4 ml Histopaque 1077 (Sigma). Whole heparinized mouse or human blood (500 μl) was added onto the upper gradient of the tube and the E isolated, according to manufacturer's protocol. The E samples were screened for platelet and white cell contamination with Wright stain (Accustain, Sigma). To isolate white blood cells from mouse or human blood, 200 μl of whole blood was lysed for 10 min on ice in 10 ml E lysis buffer (154 mm ammonium chloride, 10 mm potassium hydrogen carbonate and 0·1 mm EDTA). The samples were centrifuged for 5 min at 800 × g at 4°C, washed once with 10 ml E lysis buffer and then suspended in the appropriate buffer for subsequent experiments.
Flow cytometry
E (5 × 105), purified from mouse or human whole blood, were incubated on ice with 3 μg FITC-labelled TRA-2–10 diluted in 1 × PBS plus 3% FCS (PBS-F) for 30 min in a total volume of 100 μl. The cells were washed three times in PBS-F, suspended in the same buffer and analysed by FACScan. To target B cells, T cells or neutrophils, cell aliquots (5 × 105 cells) were first incubated with 2 μg of a species specific PE-labelled monoclonal antibody against cell type specific markers for 30 min on ice in 100 μl PBS-F. After washing the samples three times in PBS-F, 3 μg of FITC-TRA-2–10 were added for 30 min on ice. The cells were washed, resuspended in PBS-F and analysed by flow cytometry.
Immunohistochemistry
The protocol for indirect immunoperoxidase staining of tissue samples has been published [27].
Results
Differential mRNA splicing, protein expression and tissue localization
Differential mRNA splicing and protein expression of human MCP (CD46) in selected tissues of male and female animals were compared among the three mouse strains. The relative abundance of mRNA splice variants and the protein isoform expression patterns in multiple tissues (summarized in Table 1) were similar for all three strains (lung and kidney are shown as representative examples in Fig. 3). No differences were detected between male and female animals (Fig. 3). The pattern and quantity of the mRNA splicing products in each tissue (BC1 and BC2 were predominant for most tissues) correlated with the protein isoforms (upper band predominant) and expression levels. Immunohistochemical analysis of the tissue samples demonstrated a nearly identical pattern of MCP (CD46) expression among the three strains (see below).
Table 1.
Summary of protein and RNA patterns of MCP in tissues of hCD46Ge mice*
| Tissue† | Protein forms (% in upper and lower forms) | RNA (% in each transcript) |
|---|---|---|
| Kidney | > 90 (BC2)‡ | BC2: >90; BC1/C1/C2: <10 |
| Liver | 80/20 | BC2:80; BC1:10; C1/C2:10 |
| Lung | 70/30 | BC2; 70; BC1:20; C1/C2:10 |
| Brain (cerebellum) | > 90 (C2)‡ | C2:80; BC1/BC2/C1:20 |
| Stomach (fundus) | > 90 (BC2)‡ | BC2: >90; BC1/C1/C2: <10 |
| Spleen | 80/20 | BC2:70; BC1:20; C1/C2:10 |
| Heart | 40/60 | All four in equal amounts |
| Testis | Two lower forms§ | BC2:70; BC1, C1, C2:30¶ |
| Ovary | 70/30 | BC2:70; BC1:20; C1/C2:10 |
| Pancreas | > 90/10 | Not determined |
| Adrenal gland | 70/30 | BC2:70; BC1 : 20; C1/C2 : 10 |
| Salivary gland | > 90 (BC2)‡ | BC2: >90; BC1/C1/C2: <10 |
| Duodenum | 90/10 | BC2: >90; BC1/C1/C2: <10 |
| Colon | 90/10 | BC2: >90; BC1/C1/C2: <10 |
| Thyroid | 70/30 | BC2 : 70; BC1 : 20; C1/C2 : 10 |
| Skeletal muscle | 70/30 | BC2 : 70; BC1 : 20; C1/C2 : 10 |
| Fat | 70/30 | BC2 : 70; BC1 : 20; C1/C2 : 10 |
| Skin | 70/30 | BC2 : 70; BC1 : 20; C1/C2 : 10 |
RNA gels and Western blot films of control tissues were scanned and signal intensity was determined for standards. Protein and RNA ratios of tissue samples were based on visual inspection. The obtained numbers were rounded off to the nearest 10%. U, Upper forms, BC1 and BC2; L, Lower forms, C1 and C2.
A minimum of two samples from two different animals was analysed.
Based on published data and RT-PCR analysis, this is the most likely protein form expressed.
Only two lower protein forms with Mrs of 41 and 37 × 103 have been detected in the testis.
A longer 5th transcript of ∼ 750 bases was also detected in the testis.
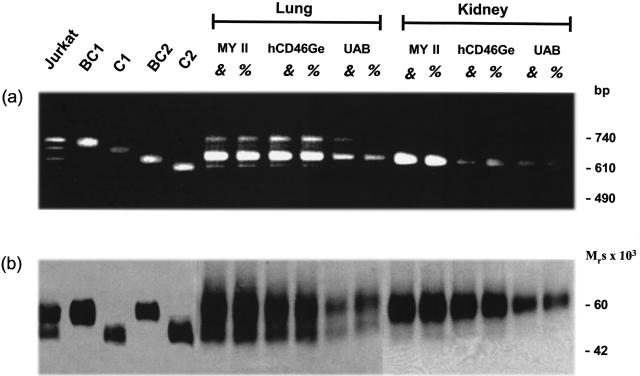
Fig. 3.
Semiquantitative comparison of the mRNA splice variants of the human MCP (CD46) gene (a) and the tissue-specific expression of the protein isoforms (b) in lung and kidney of the three transgenic mouse strains. Total protein and RNA fractions were isolated from snap frozen lung and kidney tissue of male and female animals of all three mouse strains (MY II, hCD46Ge and UAB), from Jurkat cells and from CHO cells transfected with cDNAs coding for the BC1, C1, BC2 or C2 isoform. For RNA analyses, 1 μg was used to perform RT-PCRs (a). For PCR amplification, primers annealing to CCP 4 and the 3′ (untranslated region of MCP (CD46) were used (see Materials and methods). For protein analyses 10 μg of total protein extract were separated by SDS-PAGE under nonreducing conditions and Western blotted with polyclonal anti-MCP (CD46) Ab (b). The slight differences in the Mr of the upper and lower MCP (CD46) protein forms (for example, between Jurkat and CHO cells) are commonly seen among various types of cells and reflect distinct branching patterns of the N-linked sugars [33].
The quantity of mRNAs coding for the different MCP (CD46) isoforms and the amount of expressed protein correlated with the number of inserted human MCP (CD46) genes. As determined by Southern blotting (not shown), MY II mice contain 10–12 copies in their diploid genome and showed the strongest MCP (CD46) RNA and protein expression; hCD46Ge mice possessed two copies per diploid genome [28] and demonstrated RNA and protein expression comparable to that observed in matched human tissue; while the UAB strain, having integrated one or two copies per diploid genome, was the lowest expressor (Fig. 3). Nontransgenic control animals showed no MCP (CD46) specific RNA or protein expression.
Comparison of the MCP (CD46) expression profile between the transgenic mice and humans
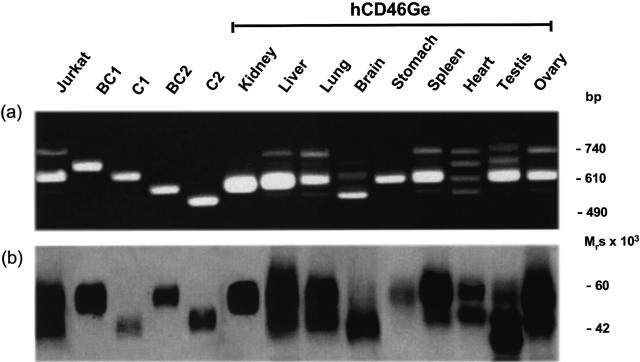
MCP (CD46) migrates on SDS-PAGE as two characteristic broad bands (i.e. upper and lower isoforms) and the relative quantity expressed is inherited in an autosomal codominant fashion [17–19]. The DNA employed to generate the MCP (CD46) containing YAC clone was obtained from an upper band predominant donor [22]. The ability of the inserted YAC fragment to confer human-like tissue-specific expression was tested by analysing the relative abundance of the mRNA splice variants and the protein expression patterns and distribution in the transgenic mouse strains in comparison to an Epstein-Barr virus transformed B lymphocyte cell line derived from the DNA donor and to matched human tissues obtained at autopsy. Most tissues of both mouse strains obtained from YAC RCA1 demonstrated the upper band predominant pattern at both the mRNA and protein levels, reflecting that of the donor's DNA. Figure 4 shows the ratio of the mRNA splicing products and corresponding protein pattern produced in the multiple tissues of hCD46Ge mice. The upper band predominant pattern was observed in liver, lung, spleen and ovary as it was in most other tissues (Table 1). In addition, tissue-specific expression of the BC2 isoform in the kidney, the C2 isoform in the brain [29], and the BC2 isoform in the salivary gland paralleled that found in humans [30,31].
Fig. 4.
mRNA splice variants (a) and tissue-specific protein isoform expression pattern (b) of the human MCP (CD46) gene in selected tissues of hCD46Ge mice. Methods as per Fig. 3.
During this survey, additional tissues with specific MCP (CD46) mRNA splicing and isoform expression patterns were identified: namely, all four isoforms were detected in equal amounts in heart tissue (Fig. 4); the stomach, duodenum, colon and pancreas demonstrated preferential BC2 isoform expression in higher amounts than expected (Table 1).
In human testis, MCP (CD46) displayed the characteristic four band mRNA splicing pattern with predominant BC2 and BC1 transcripts and the corresponding upper band predominant protein pattern with the major band having an Mr of 65 k and minor band of 55 k [31]. Testicular tissue of the transgenic mouse strains also expressed an upper band predominant RNA and protein pattern, but the two protein forms had lower Mrs (41 and 37 kb) (Fig. 4b). Also, in addition to the processed transcripts coding for the four usual isoforms, a fifth mRNA splice variant of approximately 750 bases was detected in the testis of the transgenic mice (Fig. 4a). These differences in splicing and protein expression between human and transgenic mouse testes are being assessed.
Histochemical analysis of multiple transgenic mouse tissues indicated that distribution of MCP (CD46) in these transgenic animals parallels the expression pattern observed in humans. MCP (CD46) was detected on most epithelial, endothelial and parenchymal cells with high levels of expression on the endothelial lining of capillaries and other blood vessels (Fig. 5). Intense staining was also observed in the glomeruli and tubular epithelial cells of the kidney (Fig. 5b) and on the epithelium in exocrine glands and ducts and on the islet cells of the pancreas (not shown). Moderate expression was detected on hepatocytes and bile duct epithelial cells (Fig. 5d) and on submucosal endothelial vessels and mucosal epithelium in the stomach (not shown). Endothelial cells and glial cells in the brain demonstrated weak MCP (CD46) expression (Fig. 5f) as did the alveoli and bronchi in the lung and lymphocytes and vascular endothelium in the spleen. Small amounts of MCP (CD46) were found in skeletal and cardiac muscle (not shown).
Fig. 5.
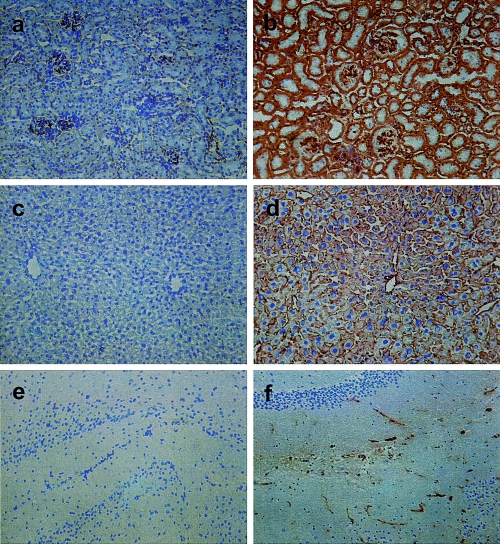
Immunohistochemistry of human MCP (CD46) in representative cryosections of kidney, liver and brain from an hCD46Ge animal. Tissue sections (7 micron) were immunostained with a monoclonal mouse antihuman-MCP (CD46) Ab (TRA-2–10). In the kidney MCP (CD46) is expressed in the glomerulus and on the tubular epithelial cells (b). In the liver MCP (CD46) was detected on the hepatocytes and the bile duct epithelial cells (d); in the brain (cerebellum) on endothelial and glial cells (f). The corresponding kidney (a), liver (c) and brain (e) sections of a nontransgenic animal demonstrated no staining for MCP (CD46). Magnification × 100.
Expression of MCP (CD46) on peripheral blood cells and erythrocytes
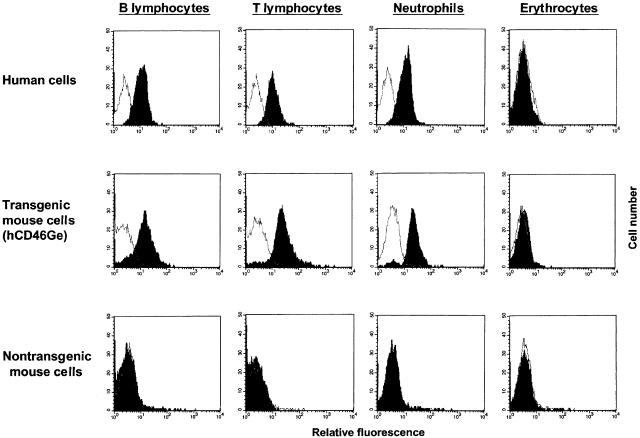
The expression of MCP (CD46) on peripheral blood cells of the three transgenic mouse strains was measured by flow cytometry. B cells, T cells and neutrophils of hCD46Ge mice (mean fluorescence, 20) reached levels comparable to those on human cells (mean fluorescence, 24) (Fig. 6). In accord with the results employing other types of tissue samples, MY II animals expressed about 10 times more MCP (CD46) on peripheral blood cells (mean fluorescence, 170), and the UAB strain showed the lowest amount (mean fluorescence, 7; data not shown).
Fig. 6.
MCP (CD46) expression on peripheral blood cells of hCD46Ge animals. Leukocytes and erythrocytes were purified from whole blood samples of an hCD46Ge animal, a nontransgenic mouse of a similar genetic background (Swiss Webster) and a human donor. E were incubated with FITC-conjugated monoclonal mouse anti-MCP (TRA-2–10) and subjected to flow cytometric analysis. To target B lymphocytes, T-lymphocytes and neutrophils in the white cell population of mouse and human blood, aliquots of each sample were first incubated with a species specific PE-labelled monoclonal antibody against cell type specific markers (see Materials and Methods), incubated with FITC-TRA-2–10 and then stained cells were analysed by FACS. Dead cells were excluded by propidium iodide staining. Background control histograms (samples incubated only with PE-labelled cell marker specific Abs) are not filled. Results are representative of three experiments.
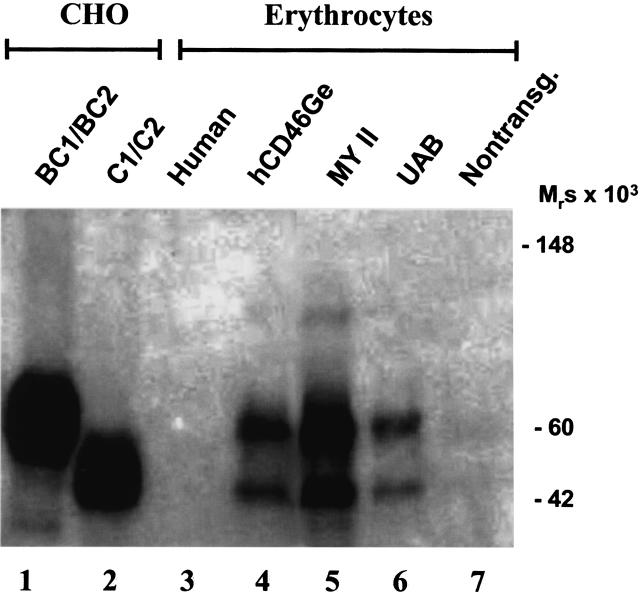
In contrast to humans but similar to many primate species [32], the transgenic mice expressed MCP (CD46) on their E (Fig. 6). The expression level was about 10% of that observed on leucocytes. E were also solubilized and subjected to Western blot analysis (Fig. 7). The isoform pattern detected on E was like most other tissues, being the upper band predominant pattern.
Fig. 7.
Detection of human MCP (CD46) on E of the three transgenic mouse strains. E were purified from whole mouse and human blood samples by centrifugation through Histopaque gradients. After hypotonic lysis, membranes were solubilized and an equivalent of 1 × 105 cells was separated by SDS-PAGE under nonreducing conditions. The samples were characterized by Western blotting using a polyclonal antihuman-MCP (CD46) Ab. Mixtures of recombinant isoforms of human MCP (CD46) (i.e. BC1/BC2 and C1/C2) expressed in CHO cells were used as controls. The relative sharp bands seen in E preparations of the three transgenic strains tested imply a distinct glycosylation pattern.
Discussion
Several groups have generated MCP (CD46) transgenic mouse strains by transferring cDNA fragments coding for one of the four isoforms with either the endogenous MCP (CD46) promotor [34] or a heterologous promotor [35–37]. In several cases, these animals showed negligible expression [36,37] while in others expression of the single isoform was observed [34,35]. In order to reproduce more closely the natural pattern of gene expression, we generated three transgenic mouse strains (hCD46Ge; MY II; UAB) by the insertion of the entire human gene. We then performed a comparative examination of MCP (CD46) expression in the three independently derived strains. This comparison did not include a fourth strain generated using a 80-kb genomic fragment [38].
We found that MCP (CD46) was expressed on all cells and tissues analysed. The ratios of the mRNA splicing variants, the protein patterns and the tissue distribution were similar among the three strains and they closely matched human control tissues. The differences in the quantity of the differentially spliced mRNA forms and protein expressed among the strains correlated well with the number of inserted genes. The hCD46Ge animals breed normally and, with two integrated genes per diploid genome, most closely mimic the human MCP (CD46) expression profile. They do though tend to become obese after six months. Although the MY II demonstrate higher protein expression, the MY II colony is more difficult to maintain. The UAB mice breed normally but show the lowest expression levels and tend to convert to the heterozygous state. In addition to the human MCP (CD46) gene, the RCA1 YAC clone encodes a carboxyl portion of the CR1 gene and the entire MCP-like and CR1-like genes, which are partial duplications [22]. No MCP-like or CR1-like proteins have been detected in any human tissues tested and the RNA transcription analysis of several tissues from hCD46Ge mice with MCP-like and CR1-like specific primers revealed no transcription of these human genes (unpublished observations). The adjacent sequences to the MCP (CD46) gene in the YAC AM2 : 6C10 have not been further characterized [23] but neither of the tested MY II mice showed RNA transcripts coding for human CR1, CR1-like or MCP-like (unpublished data).
The inserted YAC fragments confer human-like expression of MCP (CD46) in these mice including the donor's upper band predominant protein pattern on most cells and tissues. Also, the tissue-specific expression of isoforms in selected tissues was faithfully reproduced in multiple tissues. In the fetal heart, however, the lower band predominant pattern is expressed, independent of the MCP (CD46) genotype [39]. As demonstrated herein, the isoform pattern in the adult heart also differs from the MCP (CD46) phenotype of other organs. The functional consequences of these findings are not known yet. However, accumulating data suggest differences in function among isoforms, i.e. the ability to trigger distinct signalling pathways [40,41], and the quantitative altered phenotypic expression of MCP (CD46) in the heart may therefore be of considerable importance. Another interesting variation in the MCP (CD46) mRNA splicing and protein expression pattern occurred between human and transgenic mouse testes. Since the murine MCP (CD46) homologue is only abundantly expressed in the testis [42], the transcription or translation of the endogenous mouse MCP (CD46) gene may interfere with the expression of the human gene. We are currently further characterizing MCP (CD46) expression in the testis.
The hCD46Ge strain, with two copies of the MCP (CD46) gene, expressed similar levels of MCP (CD46) on peripheral blood cells as observed in humans. Surprisingly though, low levels were detected on the E of all three mouse strains. A recent publication by Oldstone et al. [24] also noted MCP (CD46) on the E of MY II animals. In humans, E are the only peripheral blood cell type that do not express MCP (CD46). However, Old and New World monkeys express MCP (CD46) on their E [32]. The expression of MCP (CD46) on the E of the MCP transgenic mice may occur because regulatory elements which silence expression on human E are not located on the YAC clones or because of a species-specific incompatibility between the human regulatory sequences and the mouse transcriptional and/or translational cell apparatus which prevents E-specific MCP (CD46) gene silencing.
The data obtained in this study indicate that most elements necessary for the tissue-specific expression of the human MCP (CD46) gene were transferred to the mice and that the expression profile in these animals closely mimics that observed in humans. These mice have already been [21,24,43] and will continue to be a useful tool to further analyse the significance of tissue-specific isoform expression patterns and to generate additional suitable small animal models for infections with pathogens that use MCP (CD46) as a receptor. We also plan to utilize these mice for the characterization of MCP (CD46) expression during embryonic development. In particular, by crossing MCP (CD46) transgenic mice with Crry knock out mice (the Crry−/– animals have an embryonic lethal phenotype due to the lack of protection of the placenta against complement attack [44]), we hope to further demonstrate a critical role for complement regulatory proteins in protecting reproductive tissue.
Acknowledgments
We thank Dr Jeff Saffitz from the Department of Pathology and Immunology of the Washington University School of Medicine for his help in providing us with human autopsy tissues; Andreas Gnirke from Mercator Genetics (Menlo Park, CA) for help in generating the UAB mouse strain; and Madonna Bogacki and Lorraine Whiteley for secretarial support.
This work was supported in part by funding from the National Institute of Health (RO1 AI37618) and by a scholarship from the DAAD (Deutscher Akademischer Austauschdienst) (Hochschulsonderprogramm III), Germany. Production of UAB mice was supported in part by NCI grant CA13148.
References
- 1.Cole J, Hously GA, Dykman TR, et al. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci USA. 1985;82:859–63. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seya T, Atkinson JP. Functional properties of membrane cofactor protein of complement. Biochem J. 1989;264:581–8. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oglesby TJ, Allen CJ, Liszewski MK, et al. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992;175:1547–51. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naniche D, Varior-Krishnan G, Cervoni F, et al. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–32. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonton strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 6.Manchester M, Liszewski MK, Atkinson JP, Oldstone MBA. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–5. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada N, Liszewski MK, Atkinson JP, Caparon M. Membrane cofactor protein (MCP; CD46) is a keratinocyte receptor for the M protein of group A streptococcus. Proc Natl Acad Sci USA. 1995;92:2489–93. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallström H, Liszewski MK, Atkinson JP, Jonsson AB. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–47. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 9.Santoro F, Kennedy PE, Locatelli G, et al. CD46 is a cellular receptor for human Herpesvirus 6. Cell. 1999;99:817–27. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 10.Rooney IA, Oglesby TJ, Atkinson JP. Complement in human reproduction: activation and control. Immunol Res. 1993;12:276–94. doi: 10.1007/BF02918258. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DJ, Abbott AF, Jack RM. The role of complement component C3b and its receptors in sperm–oocyte interaction. Proc Natl Acad Sci USA. 1993;90:10051–5. doi: 10.1073/pnas.90.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitamura M, Matsumiya M, Yamanaka M, et al. Possible association of infertility with sperm-specific abnormality of CD46. J Reprod Immunol. 1997;33:83–8. doi: 10.1016/s0165-0378(97)01017-6. [DOI] [PubMed] [Google Scholar]
- 13.Post TW, Liszewski MK, Adams EM, et al. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms which correlate with protein phenotype. J Exp Med. 1991;174:93–102. doi: 10.1084/jem.174.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorig RE, Marcil A, Richardson CD. CD46, a primate-specific receptor for measles virus. Trends Microbiol. 1994;2:312–8. doi: 10.1016/0966-842x(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 15.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of the complement activation gene cluster. Ann Rev Immunol. 1991;9:431–55. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 16.Seya T, Hirano A, Matsumoto M, et al. Human membrane cofactor protein (MCP, CD46): multiple isoforms and functions. Int J Biochem Cell Biol. 1999;31:1255–60. doi: 10.1016/s1357-2725(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 17.Ballard L, Seya T, Teckman J, et al. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45–70) J Immunol. 1987;138:3850–5. [PubMed] [Google Scholar]
- 18.Bora NS, Post TW, Atkinson JP. Membrane cofactor protein (MCP) of the complement system: a Hind III RFLP that correlates with expression polymorphism. J Immunol. 1991;146:2821–5. [PubMed] [Google Scholar]
- 19.Wilton AN, Johnstone RW, McKenzie IFC, et al. Strong associations between RFLP and protein polymorphism for CD46. Immunogenetics. 1992;36:79–85. doi: 10.1007/BF00215283. [DOI] [PubMed] [Google Scholar]
- 20.Holers VM, Kinoshita T, Molina H. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunol Today. 1992;13:231–6. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 21.Mrkic B, Pavlovic J, Rülicke T, et al. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hourcade D, Garcia AD, Post TW, et al. Analysis of the human regulators of complement activation (RCA) gene cluster with yeast artificial chromosomes (YACs) Genomics. 1992;12:289–300. doi: 10.1016/0888-7543(92)90376-4. [DOI] [PubMed] [Google Scholar]
- 23.Yannoutsos N, Ijzermans JNM, Harkes C, et al. A membrane cofactor protein transgenic mouse model for the study of discordant xenograft rejection. Genes Cell. 1996;1:409–19. doi: 10.1046/j.1365-2443.1996.d01-244.x. [DOI] [PubMed] [Google Scholar]
- 24.Oldstone MBA, Lewicki H, Thomas D, et al. Measles virus infection in a transgenic model: virus-induced immunosuppression and central nervous system disease. Cell. 1999;98:629–40. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 25.Andrews PW, Knowles BB, Parkar M, et al. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on human chromosome 1. Ann Hum Genet. 1985;49:31–9. doi: 10.1111/j.1469-1809.1985.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 26.Liszewski MK, Atkinson JP. Membrane cofactor protein (MCP; CD46): isoforms differ in protection against the classical pathway of complement. J Immunol. 1996;156:4415–21. [PubMed] [Google Scholar]
- 27.Oglesby TJ, Longwith JE, Huettner PC, et al. Human complement regulator expression by the normal female reproductive tract. Anat Rec. 1996;246:78–86. doi: 10.1002/(SICI)1097-0185(199609)246:1<78::AID-AR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Volpe P. University of Zurich. Zurich, Switzerland: 1997. MSc Thesis. [Google Scholar]
- 29.Buchholz CJ, Gerlier D, Hu A, Cathomen T, Liszewski MK, Atkinson JP, Cattaneo R. Selective expression of a subset of measles virus receptor-competent CD46 isoforms in human brain. Virology. 1996;217:349–55. [Google Scholar]
- 30.Johnstone RW, Loveland BE, McKenzie IFC. Identification and quantification of complement regulator CD46 on normal human tissue. Immunology. 1993;79:341–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Johnstone RW, Russel SM, Loveland BE, et al. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231–41. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 32.Nickells MW, Atkinson JP. Characterization of CR1 and membrane cofactor protein-like proteins of two primates. J Immunol. 1990;144:4262–8. [PubMed] [Google Scholar]
- 33.Liszewski MK, Leung MK, Atkinson JP. Membrane cofactor protein: importance of N- and O-glycosylation for complement regulatory function. J Immunol. 1998;161:3711–8. [PubMed] [Google Scholar]
- 34.Thorley BR, Milland J, Christiansen D, et al. Transgenic expression of a CD46 (membrane cofactor protein) minigene: studies of xenotransplantation and measles virus infection. Eur J Immunol. 1997;27:726–34. doi: 10.1002/eji.1830270322. [DOI] [PubMed] [Google Scholar]
- 35.Horvat B, Rivailler B, Varior-Krishnan G, et al. Transgenic mice expressing human measles virus receptor (CD46) provide cell exhibiting different permissivities to MV infection. J Virol. 1997;70:6673–81. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyagawa S, Mikata S, Tanaka H, et al. The regulation of membrane cofactor protein (CD46) expression by the 3 (untranslated region in transgenic mice. Biochem Biophys Res Commun. 1997;233:829–33. doi: 10.1006/bbrc.1997.6556. [DOI] [PubMed] [Google Scholar]
- 37.Rall GF, Manchester M, Daniels LR, et al. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci USA. 1997;94:4659–63. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blixenkrone-Moller M, Bernard A, Bencsik A, et al. Role of CD46 in measles virus infection in CD46 transgenic mice. Virology. 1998;249:238–48. doi: 10.1006/viro.1998.9301. [DOI] [PubMed] [Google Scholar]
- 39.Gorelick A, Oglesby T, Rashbaum W, et al. Ontogeny of membrane cofactor protein: phenotypic divergence in the fetal heart. Lupus. 1995;4:293–6. doi: 10.1177/096120339500400410. [DOI] [PubMed] [Google Scholar]
- 40.Kallström H, Islam MS, Berggren PO, Jonsson AB. Cell signaling by the type IV pili of pathogenic Neisseria. J Biol Chem. 1998;273:21777–82. doi: 10.1074/jbc.273.34.21777. [DOI] [PubMed] [Google Scholar]
- 41.Lozahic S, Christiansen D, Manie S, et al. CD46 (membrane cofactor protein) associates with multiple β1 integrins and tetraspans. Eur J Immunol. 2000;30:900–7. doi: 10.1002/1521-4141(200003)30:3<900::AID-IMMU900>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.Tsujimura A, Shida K, Kitamura M, et al. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330:163–8. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mrkic B, Odermatt B, Klein MA, Billeter MA, Pavlovic J, Cattaneo R. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J Virol. 2000;74:1364–72. doi: 10.1128/jvi.74.3.1364-1372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu C, Mao D, Holers VM, Palanca B, et al. A critical role for the murine complement regulator Crry in fetomaternal tolerance. Science. 2000;287:498–50145. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]