Abstract
Although most chemotherapeutic agents are known to cause primarily reduction or suppression of immune responses, surprisingly little is known about the influence of cytostatic agents on lymphoid tissue compartments such as the splenic marginal zone. The marginal zone plays an important role in the defence against encapsulated bacteria, which are potential candidates for postchemotherapeutic infections. We studied the effect of three different cytostatic agents (cisplatin, methotrexate, and cyclophosphamide) on B cell subpopulations in a rat model. Rats received a single dose of a single cytostatic agent and were sacrificed at different time points after treatment. Bone marrow, blood, mesenteric lymph nodes and spleens were analysed by flow-cytometry and immunohistochemistry. All three cytostatic agents showed severe bone marrow depression. CP and MTX showed only mild reduction of cell populations in the spleen. CyPh showed a severe reduction of recirculating follicular B (RF-B) cells and marginal zone B (MZ-B) cells. At day 24 most populations were already recovered, but RF-B cells and MZ-B cells were still reduced. The reduction of the marginal zone and late recovery may imply that, beside the overall increased infection risk due to neutropenia, patients treated with chemotherapy are at risk for developing infections from encapsulated bacteria for a considerable period of time after treatment, extending beyond the period of bone marrow depression.
Keywords: B cells, marginal zone, rat, TI-2 antigens, spleen
Introduction
The use of chemotherapeutic agents in the treatment of cancer has, beside its beneficial effect, severe unwanted side-effects. Suppression of the immune system is a major drawback which can lead to the development of infections with a high mortality risk [1,2]. In patients treated for cancer an impressive part of the morbidity and mortality during the periods they receive chemotherapy is caused by infections [1,3]. Compared to adults, the immune system of a child is even more vulnerable as it is much more ‘native’ in relation to specific cellular and humoral immune responses. This is partly due to immaturity of the immune system, partly to inexperience of the immune system (lack of memory) [4–7].
Most chemotherapeutic agents are known to suppress haematopoiesis in bone marrow causing myelosuppression and lymphocytopenia, primarily resulting in reduction or suppression of lymphocytic responses [2]. Surprisingly little is known about the short- and long-term effects of chemotherapy on lymphoid tissues and cell subpopulations and the consequences for cellular and humoral immune responses.
We focus on three different chemotherapeutic agents that are used widely in the treatment of malignancies: cisplatin (CP), methotrexate (MTX) and cyclophosphamide (CyPh). These three cytostatic agents are known to affect humoral immune responses and/or cellular constituents of B cell compartments. Some short-term effects in rats after a single dose of CP, MTX and CyPh have been reported. CP reduces specifically splenic follicles and the marginal zone in rats [8]; MTX can suppress both primary and secondary antibody responses [2,9]; CyPh causes lymphopenia of both T and B cells [2] and selective loss of lymphocytes from the marginal zone of the spleen [10].
In particular encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae are potential candidates for severe postchemotherapeutic infections [1]. The capsule of encapsulated bacteria is composed of polysaccharides generally belonging to the class of T-cell independent type 2 (TI-2) antigens. TI-2 antigens stimulate antibody production in the absence of MHC class II-restricted T cell help but do need T cell-derived factors [11]. Initiation of antibody responses to TI-2 antigens is dependent on a functional intact marginal zone [12–14]. The marginal zone is a unique compartment found only in the spleen. In humans it contains mainly marginal zone B cells with high expression of IgM and complement receptor 2 (CD21) [12,15,16].
In this study we evaluated effects of a single dose of one of the three cytostatic agents on recirculating and resident lymphoid cell populations in rats. We sacrificed rats at different time points after treatment to look at the short- and long-term effects. Bone marrow, blood and spleen were analysed by three-colour flow cytometry analysis to obtain quantitative and qualitative data of the different B cell subpopulations. Because of complement (fragment C3d) dependency of the TI-2 immune response [11], we also determined the effects on complement concentration in serum of treated rats. To obtain information about the effects on lymphoid tissue compartments in mesenteric lymph nodes and spleens, frozen sections were analysed by immunohistochemistry using a broad panel of monoclonal antibodies (Table 1) directed to B cells, T cells, monocytes, macrophages and follicular dendritic cells (FDC).
Table 1.
Reactivity of monoclonal antibodies (MoAb) used
| MoAb | Clone | Reactive with | Reference |
|---|---|---|---|
| IgM | HIS40 | Immunoglobulin M, B cells | [21] |
| IgD | MaRD | Immunoglobulin D, B cells | [21] |
| CD68 | ED1 | Macrophages | [30] |
| ED2 | ED2 | Monocytes, dendritic cells, granulocytes, macrophages | [30] |
| ED3 | ED3 | Marginal metallophilic macrophages | [30] |
| ED5 | ED5 | Follicular dendritic cells | [31] |
| TCR | R73 | αβ T cell receptor | [32] |
| HIS57 | HIS57 | Marginal zone B cells | [21] |
| CD24 | HIS50 | B cells, granulocytes | [24] |
| CD45R | HIS24 | B cells, leucocyte common antigen | [22,23] |
| CD90 | HIS51 | Thy-1, progenitor cell subset | [21] |
We focused especially on the marginal zone, since reduction of this zone could imply a higher vulnerability for encapsulated bacteria during chemotherapy. The results of this study will increase the knowledge of immunosuppressive effects of chemotherapeutic agents leading to a better understanding of infectious problems in patients receiving chemotherapy.
Materials and methods
Animals
Male Wistar rats, subgroup HsdCpb:WU (Harlan, The Netherlands) were used, aged 10–14 weeks (± 300 g). Animals were maintained under specific pathogen-free conditions and fed with standard laboratory rat food (Hope Farms, Inc., Woerden, The Netherlands). All animal experiments were approved by the Dutch Animal Experimental Committee.
Chemotherapy
Rats were injected i.v. with CP (6 mg/kg), MTX (52 mg/kg) or CyPh (40 mg/kg) under light inhalation anaesthesia (O2, N2O and halothane). A formula described by Freireich et al. [17] was used to calculate a concentration for rats based on the concentration used in humans. This calculated concentration was compared with concentrations described in the literature. We chose the concentration which was described to be proven effective [8,18–20] and closest to the calculated concentration.
Each treatment group consisted of 12 rats and the untreated control group of 13 rats. We sacrificed three rats of each group at 2, 7, 15 and 24 days after injection. These time points were based on a study of Dammers et al. [21]. At the same time points, untreated rats were sacrificed which served as controls. From each rat bone marrow, blood, mesenteric lymph nodes and spleen were obtained at autopsy. Blood was drawn from the heart and bone marrow cells were obtained from both femoral shafts.
Monoclonal antibodies
For three-colour flow cytometry analysis, we used the following mouse monoclonal antibodies conjugated to either fluorescein isothiocyanate (FITC), phycoerythrin (PE) or biotin: CD45R (Pharmingen, San Diego, CA, USA) and CD90, IgM, IgD and HIS57 [21] (Table 1). Streptavidin conjugated to allophycocyanin (SA-APC) (Pharmingen, San Diego, CA, USA) was used to reveal biotin. For immunohistochemistry the following primary antibodies were used: ED1, ED2, ED3, ED5 (Serotec Ltd, Oxford, UK). T cell receptor (TCR) (Pharmingen, San Diego, CA, USA) and CD45R [22,23], CD90, IgM, IgD, HIS57 [21] and CD24 [24] (Table 1). Secondary antibodies rabbit-antimouse immunoglobulins conjugated to peroxidase and goat-antirabbit immunoglobulins conjugated to peroxidase were obtained from Dako (Dako, Glostrup, Denmark).
Preparation of cell suspensions for flow cytometry
Single-cell suspensions from the spleen were prepared by mincing tissue fragments in staining medium (PBS supplemented with Dulbecco B (Oxoid, Basingstoke, UK), 5% FCS (Gibco Life Technologies, Breda, The Netherlands) and 0,03% sodium azide (Merck KgaA, Darmstadt, Germany)). Large residual fragments were removed by passing the cell suspension through a nylon gauze. Erythrocytes were lysed in ice-cold ammonium chloride solution. Blood was drawn from the heart; EDTA was added for anticoagulation. After centrifugation, the buffy coat was taken and erythrocytes were lysed with ammoniumchloride solution. Bone marrow cells were obtained by flushing both femoral shafts with ice-cold medium. Large residual fragments were removed by density gradient centrifugation through FCS. As final step, all cell suspensions were washed twice with ice-cold staining medium. After washing, cells were passed through a nylon gauze. All cell suspensions were counted on a Sysmex F-800 cell counter (Toa medical electronics Co., Ltd, Kobe, Japan). Total (absolute) numbers of nucleated cells per spleen were calculated before lysing of erythrocytes.
Flow cytometry
Three-colour flow cytometry using spleen, blood and bone marrow cells was performed by incubating about 1·4 × 106 cells with a combination of primary antibodies (IgM/CD90/IgD, IgM/CD90/HIS57 and CD45R/IgM/HIS57) in staining medium for 1 h at 4°C. Cells were washed twice in ice-cold staining medium and subsequently incubated with SA-APC for 30 min at 4°C to reveal biotin-conjugated antibodies. Cells were finally washed twice with ice-cold staining medium and resuspended in staining medium for analysis. Using a dual laser Coulter Epics-Elite flow cytometer with ESP upgrade (Coulter Corporation, Hialeah, FL, USA) 40 000 events were analysed from each sample. Data were analysed using WinList 4·0 software (Verity Software House Inc. Topsham, ME, USA). Percentages of cell populations were calculated from the total number of lymphocytes gated by forward/side scatter profile. The absolute number of cell subpopulations per spleen was established by multiplying the frequency of B cell subpopulation per spleen with total cell count.
Immunohistochemistry
Spleen and mesenteric lymph node tissues were snap frozen in liquid isopentane and stored at −80°C. Cryostat sections of 4 µm were cut and stored at −20°C until use. For staining, sections were air dried and fixed in acetone (100%) for 10 min at room temperature. Sections were incubated for 1 h with a primary monoclonal antibody in the proper dilution as determined previously. Endogenous peroxidase activity was blocked by incubating sections in 0·075% H2O2 in PBS. Subsequently, sections were incubated with rabbit-antimouse immunoglobulins conjugated to peroxidase for 30 min, followed by incubation with goat-antirabbit immunoglobulins conjugated to peroxidase for 30 min. Sections were rinsed in PBS for 5 min after each incubation step. Peroxidase activity was demonstrated by immersing the slides in sodium acetate buffer containing 0·2 mg/ml 3-amino-9-ethyl-carbazole (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) and 0·03% H2O2 for 15 min. Sections were counterstained with Mayer's haematoxylin. Results of immunohistochemistry were semiquantitatively determined comparing treated animals with controls.
Complement assay
Concentration of complement in serum was determined by a classical pathway haemolytic assay procedure (CH50) using sensitized sheep erythrocytes. Blood was drawn from the heart and coagulated in Microtainer serum separator tubes (Becton Dickinson, New Jersey, USA) on ice for 2 h. Tubes were centrifuged at 7200 r.p.m. and serum was stored at −80°C. For the CH50 test, serum was diluted (1:10) in Veronal saline buffer (5 mm 5,5-diethylbarbituric acid sodium salt, pH 7·35, 0·15 mm CaCl2, 0·5 mm MgCl2). Sheep erythrocytes were washed three times with Veronal saline buffer and sensitized by incubation with antisheep-erythrocytes antibodies (Guinea Pig Serum, Calbiochem, San Diego, USA) for 20 min at 4°C. Each sample was incubated with 1% antibody sensitized sheep erythrocytes for 30 min at 37°C. After incubation, samples were centrifuged at 1800 r.p.m. for 10 min to pellet all unlysed cells. Absorbency of supernatants was read at 405 nm. For every sample a heat-inactivated sample was also analysed to correct for possible haemolytic serum. Complement concentrations were determined as a percentage from a reference sample (100% lysis by distilled water).
Statistical analysis
Data are represented as the mean ± standard deviation. Values of the control group are mean values of 13 animals sacrificed at different time point during the experiment. Values of the treated groups are the mean of three animals. Differences between groups were analysed by the Mann–Whitney U-test. A two-tailed P-value <0·05 was considered significant.
Results
Chemotherapy
After treatment with CP, MTX or CyPh, none of the rats showed severe signs of illness such as diarrhoea, ruffled fur or pale ears and paws. All rats survived treatment. Total cell counts per spleen were severely reduced after treatment. Normal untreated rat spleens contain around 700 million nucleated cells. Two days after CP and CyPh treatment numbers were significant reduced to approximately 300–400 million cells per spleen. Twenty-four days after MTX and CyPh treatment the numbers were still significantly reduced (Table 3).
Table 3.
Effects of chemotherapy on absolute cell numbers of splenic B cell subpopulations
| Treatment | |||||
|---|---|---|---|---|---|
| Day | Control | CP | MTX | CyPh | |
| Absolute cell numbers | 686 ± 188·9 | ||||
| per spleen | 2 | 438·3 ± 34·3* | 496·4 ± 46·3 | 300·7 ± 64·4* | |
| 7 | 580·8 ± 143·1 | 611·6 ± 25·3 | 383·8 ± 90·5* | ||
| 15 | 517·7 ± 54·3 | 998·1 ± 55·9* | 818·7 ± 209·7 | ||
| 24 | 721·9 ± 22·5 | 379·4 ± 41·0* | 395·4 ± 94·4* | ||
| NF-B | 21·7 ± 10·3 | ||||
| 2 | 8·0 ± 3·9* | 8·4 ± 0·8* | 5·8 ± 1·7* | ||
| 7 | 3·0 ± 0·5* | 7·0 ± 1·9* | 0·9 ± 0·5* | ||
| 15 | 18·2 ± 2·1 | 15·7 ± 5·8 | 30·5 ± 11·9 | ||
| 24 | 23·0 ± 2·6 | 7·8 ± 2·5* | 11·7 ± 3·8 | ||
| EFR-B | 32·8 ± 11·0 | ||||
| 2 | 15·0 ± 6·2* | 18·9 ± 0·2* | 13·2 ± 3·3* | ||
| 7 | 8·9 ± 1·6* | 14·8 ± 0·9* | 4·7 ± 2·8* | ||
| 15 | 28·2 ± 2·7 | 35·1 ± 8·3 | 56·5 ± 14·7* | ||
| 24 | 37·7 ± 1·2 | 13·8 ± 3·2* | 23·1 ± 7·3 | ||
| RF-B | 76·1 ± 18·2 | ||||
| 2 | 69·5 ± 30·7 | 66·3 ± 8·7 | 19·4 ± 6·5* | ||
| 7 | 106·1 ± 31·4 | 93·7 ± 8·9 | 30·6 ± 5·0* | ||
| 15 | 87·5 ± 10·6 | 129·1 ± 18·2* | 34·4 ± 6·8* | ||
| 24 | 92·7 ± 5·0 | 50·7 ± 14·4 | 36·4 ± 8·2* | ||
| MZ-B | 26·3 ± 8·9 | ||||
| 2 | 25·7 ± 10·7 | 16·9 ± 4·4 | 5·1 ± 1·4* | ||
| 7 | 24·8 ± 4·4 | 23·1 ± 6·0 | 7·8 ± 2·3* | ||
| 15 | 20·2 ± 1·6 | 21·9 ± 4·4 | 9·7 ± 3·7* | ||
| 24 | 17·9 ± 0·5 | 9·9 ± 1·3* | 8·8 ± 3·0* | ||
Effects on B cell populations were analysed by three-colour flow cytometry using IgM, CD90 and HIS57. Absolute cell numbers (×106 ± standard deviation) were determined by multiplying the frequency of the B cell subpopulation by the absolute number of cells per spleen.
Indicates a significant difference with control values (P < 0·05)
B cell subsets in normal rat bone marrow, blood and spleen as determined by flow cytometry analysis
Lymphocytes were gated by forward/side scatter (FSC/SSC) profile and further analysed. Different B cell subpopulations could be distinguished in the rat by three-colour flow cytometry, using combinations of CD90, CD45R, IgD, IgM and HIS57 monoclonal antibodies [21]. In bone marrow large numbers of pro/pre B cells were present which were CD90+, CD45Rlow, IgD− and IgM−. In addition, newly formed B (NF-B) cells were found which were CD90+, CD45Rhi, IgDlow and IgMhi (Fig. 1b, c) [21]. In blood, besides NF-B cells two additional B cell subpopulations could be found, the immature early recirculating follicular B (ERF-B) cells which were CD90+, CD45Rhi, IgDhi and IgMlow and the mature, naive recirculating follicular B (RF-B) cells which were CD90−, CD45Rhi, IgDhi and IgMlow [21]. In the spleen, besides NF-B cells, ERF-B cells and RF-B cells (Fig. 2a, b), marginal zone B (MZ-B) cells could be distinguished which were CD90−, CD45Rlow, IgDlow/negative and IgMhi (Fig. 2a, b). In addition, MZ-B cells were the only cells that strongly stained with HIS57 (Fig. 2c) [21].
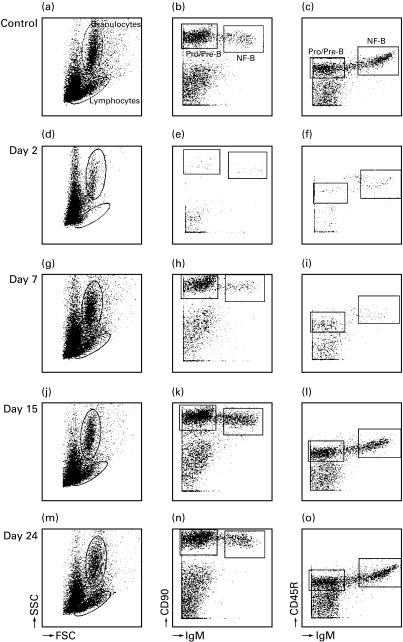
Fig. 1.
Effects of CP on cell subpopulations in the bone marrow at 2, 7, 15 and 24 days after a single i.v. injection determined by three-colour flow cytometry. Lymphocytes and granulocytes were distinguished by FSC/SSC profile. Lymphocytes were gated for further analysis by the expression of IgM and CD90 (b, e, h, k, n) and by IgM and CD45R (c, f, i, l, o). Two B cell populations could be distinguished: pro/pre B cells (IgM− and CD90+) and NF-B cells (IgM+ and CD90+). Lymphocytes were almost completely depleted at 2 days after treatment (d, e, f). The figures were established using all events collected by flow cytometry (40 000) and after gating for lymphocytes based on FSC/SSC profile.
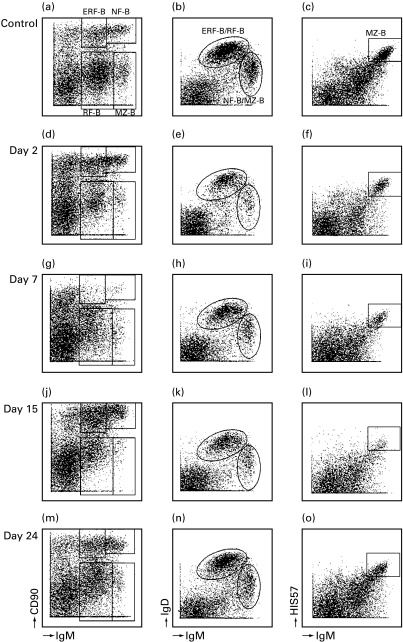
Fig. 2.
Effects of CyPh on B cell populations in the spleen at 2, 7, 15 and 24 days after treatment as determined by three-colour flow cytometry. Lymphocytes were analysed by IgM and CD90 (a, d, g, j, m), IgM and IgD (b, e, h, k, n) and by IgM and HIS57 (c, f, i, l, o). Four different B cell populations could be distinguished: NF-B cells, ERF-B cells, RF-B cells and MZ-B cells (a, b, c). At day 7 NF-B cells, ERF-B cells and MZ-B cells were severely reduced (g, i). The figures were established using all events collected by flow cytometry (40 000) and after gating for lymphocytes based on FSC/SSC profile.
Flow cytometry analysis of bone marrow cells after treatment
All three cytostatic agents resulted in severe depletion of cells in the bone marrow 2 days after treatment as revealed by FSC/SSC analysis. Dotplots of CP treatment (Fig. 1) are representative for all three agents. Two days after CP treatment, granulocytes were mildly depressed (Fig. 1d). Pro/pre-B cells and NF-B cells were completely absent (Fig. 1e, f). Seven days after treatment pro/pre B cells and granulocytes started to reappear (Fig. 1g). Bone marrow composition appeared normal 15 days after treatment (Fig. 1j, k, l and Table 2). MTX also severely effected the bone marrow composition. In contrast to CP, 24 days after MTX treatment NF-B cells were still significantly lower compared to controls (Table 2). CyPh showed severe reduction of pro/pre B cells and NF-B cells with mild granulocyte depression. Fifteen days after treatment NF-B cells were starting to recover. Twenty-four days after treatment B cell subsets in bone morrow appeared normal (Table 2).
Table 2.
Effects of chemotherapy on percentage of B cell populations in BM
| Treatment | |||||
|---|---|---|---|---|---|
| Day | Control | CP | MTX | CyPh | |
| Pro/Pre | 12·9 ± 4·3 | ||||
| 2 | 0·4 ± 0·3* | 2·5 ± 1·2* | 0·2 ± 0·1* | ||
| 7 | 4·4 ± 1·8* | 11·3 ± 3·5 | 6·6 ± 1·0* | ||
| 15 | 13·2 ± 1·1 | 6 ± 3·3* | 6·9 ± 2·1* | ||
| 24 | 12·7 ± 1·3 | 6·9 ± 1·6 | 12·8 ± 3·8 | ||
| NF-B | 5·2 ± 2·1 | ||||
| 2 | 0·6 ± 0·4* | 1·5 ± 0·5* | 0·3 ± 0·2* | ||
| 7 | 0·7 ± 0·2* | 1 ± 0·2* | 0·1 ± 0·0* | ||
| 15 | 3·9 ± 0·5 | 1·8 ± 0·5* | 2·9 ± 0·6 | ||
| 24 | 4·2 ± 0·4 | 2·7 ± 0·8* | 3·9 ± 0·8 | ||
Effects were determined by three-colour flow-cytometry using IgM, CD90 and CD45R. Relative numbers (% ± standard deviation) of B cell populations were established by multiplying the frequency of the B cell subpopulation by the percentage of lymphocytes gated by FSC/SSC.
Indicates a significant difference with control values (P < 0·05).
Flow cytometry analysis of peripheral blood B lymphocytes after treatment
NF-B cells, ERF-B cells and RF-B cells were reduced 2 days after CP treatment. Blood composition appeared normal 15 days after treatment. MTX showed a slight reduction in B cell populations, which recovered to normal values 15–24 days after treatment. CyPh caused a severe reduction of all B cell populations in the blood. Fifteen days after treatment NF-B cells and ERF-B cells were starting to recover. RF-B cells were still not fully recovered at that time. Twenty-four days after treatment blood cells appeared normal (data not shown).
Flow cytometry analysis of B cells in spleen after treatment
Two days after CP treatment NF-B cells and ERF-B cells were significantly reduced. At day 15 NF-B cells and ERF-B cells started to recover. No severe effects on RF-B cells were seen. MZ-B cells were slightly lower at 24 days after treatment (Table 3). MTX reduced NF-B cells and ERF-B cells at 2 days after treatment. At 15 days after treatment RF-B cells were higher compared to control values. At 24 days after treatment MZ-B cells were significantly lower (Table 3). CyPh treatment also had a severe impact on NF-B cells and ERF-B cells (Fig. 2d, g). At day 15 these two populations recovered (Fig. 2j). RF-B cells and MZ-B cells were also severely affected by CyPh (Fig. 2d, f). At day 24 these populations had still not recovered to control values (Table 3 and Fig. 2 m, o).
Immunohistochemical analysis of spleen tissue
Spleen sections of treated animals were stained with a broad panel of monoclonal antibodies directed to B cells, T cells, macrophages and monocytes (Table 1). No difference in cellular staining intensity was observed between spleen sections of treated and control animals, only a difference in the number of positive cells. All three agents resulted in a reduced number of CD90 positive cells in the lymphocyte corona (Table 4). The spleen sections of CP treated rats and the spleen sections of control rats showed no clear difference. MTX also showed hardly any difference in positive cells, except for a slight decrease of CD24 positive B cells in the lymphocyte corona and the marginal zone 7–15 days after treatment (Table 4).
Table 4.
Effects of chemotherapy on B cells in the spleen determined by immunohistochemistry
| IgM | CD90 | CD24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgD | HIS57 | CD45R | ||||||||
| Treatment | Day | Cor. | MZ | Cor. | Cor. | PALS | MZ | Cor. | Cor. | MZ |
| CP | 2 | – | ↓ | – | ↓↓ | – | – | – | – | – |
| 7 | – | – | – | ↓↓ | – | – | – | ↓ | – | |
| 15 | – | – | – | ↓↓ | ↓ | – | – | – | – | |
| 24 | – | – | – | ↓↓ | – | – | – | – | – | |
| MTX | 2 | ↓ | – | – | ↓↓ | – | – | – | – | – |
| 7 | – | – | – | ↓↓ | – | – | – | ↓ | ↓ | |
| 15 | – | – | – | ↓↓ | – | – | ↓ | ↓ | ↓ | |
| 24 | – | – | – | ↓↓ | – | – | – | – | – | |
| CyPh | 2 | – | ↓ | – | ↓↓ | – | ↓↓ | ↓ | ↓ | ↓↓ |
| 7 | – | ↓ | – | ↓↓ | ↓↓ | ↓↓ | ↓↓ | – | ↓↓ | |
| 15 | ↓ | ↓ | – | ↓ | – | ↓ | ↓ | ↓ | ↓↓ | |
| 24 | – | – | – | ↓ | – | ↓ | – | – | – | |
Spleen sections were stained for IgM, IgD, CD90, His57, CD45R and CD24. The number of positive cells were semiquantitative scored. – Indicates no difference compared to controls
indicates a reduction 25% compared to controls
indicates a reduction of 50% compared to control. Cor., lymphocyte corona. MZ, marginal zone. PALS, periarteriolar lymphatic sheath.
The number of IgM positive cells in the lymphocyte corona were slightly decreased after CyPh treatment. The decrease of NF-B cells and ERF-B cells as determined by flow cytometry was confirmed by finding a decrease of CD90 positive cells in spleen sections of CP, MTX or CyPh treated animals. The decrease of MZ-B cells by CyPh was also seen in spleen sections stained with HIS57 (Table 4). No influence on monocytes/macrophage, FDC and T cells was observed as demonstrated by staining with different monoclonal antibodies (CD68, ED2, ED3, ED5 and TCR).
Immunohistochemical analysis of mesenteric lymph nodes
Mesenteric lymph nodes were stained with antibodies against IgM, IgD, CD45R, CD90 and TCR (Table 1). No clear difference was observed after CP or MTX treatment, only a slight decrease in immature (CD90 positive) B cells was observed compared to controls. CyPh showed a decrease in the size of follicles as determined by a reduced number of CD45R, IgM and IgD positive cells. All three cytostatic agents hardly affected the T cell population in the lymph nodes as determined by TCR expression (data not shown).
Complement assay
Relative total complement concentrations were determined by a haemolytic assay. A reference value was obtained by lysing sheep erythrocytes with de-mineralized water (100% lysis). Complement concentrations of samples were calculated as a percentage of the reference value. MTX caused a significant decrease (P < 0·05) in complement concentrations 2 days after treatment. Concentrations reached normal values again at day 7. CP and CyPh did not affect the complement concentration. In all groups a high individual variation was noted.
Discussion
Chemotherapy has, besides the beneficial therapeutic effect, severe impact on the immune system [1]. Increasing the knowledge of immunosuppressive effects of cytostatic agents may lead to better supportive care, because of better attention to problems of infection. We investigated the effects of three different cytostatic agents in rats by treating rats with a single dose of each cytostatic agent. We used a dose that was described to be proven effective [18] to obtain information regarding the short- and long-term effects of cytostatic agents on B cell populations [8,19,20].
All three agents are primarily active during the S-phase of the cell cycle [25], affecting most severely rapidly dividing cells in the bone marrow. Pro/pre B cells in the bone marrow recovered seven to 15 days after CP or CyPh treatment followed by the recovery of NF-B cells. Twenty-four days after MTX treatment the NF-B cell population in the bone marrow was still significantly reduced.
Bone marrow depression caused an inhibition in B cell differentiation. Pro/pre B cells in bone marrow differentiate to NF-B cells which migrate to the blood [21]. In spleen, NF-B cells further differentiate to ERF-B cells followed by differentiation to RF-B cells [21]. A small fraction of RF-B cells differentiates to MZ-B cell [21]. As a result of the reduced number of pro/pre B cells in the bone marrow, immature (CD90+) B cell subpopulations with a short life span in blood and spleen (NF-B cells and ERF-B cells) decreased 2–7 days after treatment. Mature (CD90) B cell populations with a relatively long life span, such as RF-B cells and MZ-B cells, declined in numbers by 24 days after CP and MTX treatment. The reduction of the mature B cell populations is explained by a decreased number of lymphoid precursor cells differentiating into these more mature phenotypes. CP and MTX did not affect the mature B cell populations directly. CyPh, however, actively affected the RF-B cell and MZ-B cell populations, confirming data of Kumararatne et al. [19]. Although CyPh influences the S-phase, it can affect cells at all phases of the cell cycle [26]. Two days after treatment, as well as the pro/pre B cells, the NF-B cells and the ERF-B cells, the RF-B cells and the MZ-B cells were also essentially reduced compared to control animals. Twenty-four days after treatment, the RF-B cells and the MZ-B cells were still significantly reduced.
The reduction of the number of immature, CD90+ B cells by the three cytostatic agents was also demonstrated by immunochemistry. CD90+ cells were reduced in lymph nodes and spleens 2–7 days after treatment and were detectable again at 24 days after treatment. The severe reduction of MZ-B cells by CyPh was also seen in frozen sections of spleen tissue. With these three cytostatic agents no essential effect was observed on T cells, monocytes, macrophages and FDC.
RF-B cells and MZ-B cell were reduced for a relatively long period of time after treatment with MTX or CyPh. RF-B cells are mature recirculating, naive B cells capable to differentiate into plasma cells after antigenic activation. Reduction of the RF-B cell population might lead to an impaired immune response capacity. The MZ-B cells in their specific anatomical context have been shown to be important for the initiation of the TI-2 antibody response [12,14]. MZ-B cells are mainly located in the marginal zone of the spleen, and only in this typical low flow compartment are able to meet and respond to low or non-opsonized antigens. Other cells present are macrophages, dendritic cells and reticular cells [16]. These cells seem not to be affected by the tested cytostatic agents, as far as can be demonstrated immunohistologically. The late recovery of the marginal zone implies that treated patients are also at risk for development of severe infections caused by TI-2 antigenic encapsulated bacteria such as S. pneumoniae [1]. This risk of developing an infection caused by TI-2 antigens appears to be present for a considerable period (at least 24 days after a single dose) of time after treatment. The immune response against TI-2 antigens also depends on the presence of complement [12,13,27]. One of the chemotherapeutics, MTX, affected complement concentrations (at 2 days after treatment), enhancing the impairment of the immune response against TI-2 antigens.
For the first time we have demonstrated the short- and long-term effects of chemotherapy on different B cell populations in the bone marrow, blood, lymph nodes and spleen. The long-term effects on RF-B cells and MZ-B cells were particularly impressive. Dammers et al. also demonstrated that the marginal zone of rats treated with a single dose of adriamycin was not recovered at 24 days after treatment [21]. The consequences are even more pronounced because of the slow differentiation of RF-B cells to MZ-B cells [10,19,21]. The renewal rate of the marginal zone has been estimated to be about 0·5% of the total number of MZ-B cells per day [28]. This would indicate that the total recovery of the marginal zone of rats after a single dose of CTX would take considerable time. The slow recovery of the marginal zone after depletion due to chemotherapy has also been demonstrated in humans. Nakayama et al. showed the reconstitution of the white pulp after CyPh treatment and total body irradiation. The recovery of the marginal zone was poor up to 10 months [29].
Extrapolating the data obtained in this study from rats to humans, who in general are treated with multidose and combination chemotherapy, may imply that these patients are vulnerable for infections for a long period of time after treatment, extending beyond the period of bone marrow depression.
Acknowledgments
This study was supported by the Dutch Foundation for Paediatric Oncology Research Groningen, grant 96–09. The authors would like to thank Geert Mesander for helping with the flow cytometry analysis.
References
- 1.Viscoli C, Castagnola E, Rogers D. Infections in the compromised child. Baillière's Clin Haematol. 1991;4:511–43. doi: 10.1016/s0950-3536(05)80169-6. [DOI] [PubMed] [Google Scholar]
- 2.Gourley MF, Seldin MF, Steinberg AD. Inflammation: basic principles and clinical correlation. New York: Raven Press; 1992. Immunoregulatory agents; pp. 1103–26. [Google Scholar]
- 3.Pizzo PA, Robichaud KJ, Wesley R, Commers JR. Fever in the pediatric and young adult patient with cancer. A prospective study of 1001 episodes. Medicine. 1982;61:153–65. doi: 10.1097/00005792-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Timens W, Rozeboom T, Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology. 1987;60:603–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immuno-architecture of human fetal lymphoid tissue. Virchows Arch Pathol Anat. 1988;413:563–71. doi: 10.1007/BF00750398. [DOI] [PubMed] [Google Scholar]
- 6.Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset: strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989;19:2163–6. doi: 10.1002/eji.1830191129. [DOI] [PubMed] [Google Scholar]
- 7.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infants: possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–6. [PubMed] [Google Scholar]
- 8.Milicevic Z, Splepcevic V, Nikolic D, Zivanovic V, Milicevic NM. Effects of cis-diamminedichloroplatinum II (cisplatin) on the splenic tissue of rats: a histoquantitative study. Exp Mol Pathol. 1994;61:77–81. doi: 10.1006/exmp.1994.1027. [DOI] [PubMed] [Google Scholar]
- 9.Cronstein BN. Molecular mechanism of methotrexate action in inflammation. Inflammation. 1992;16:411–23. doi: 10.1007/BF00918968. [DOI] [PubMed] [Google Scholar]
- 10.Kumararatne DS, Gagnon RF, Smart Y. Selective loss of large lymphocytes from the marginal zone of the white pulp in rat spleens following a single dose of cyclophosphamide. A study using quantitative histological methods. Immunology. 1980;40:123–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 12.Harms G, Hardonk MJ, Timens W. In vitro complement-dependent binding and in vivo kinetics of pneumococcal polysaccharide TI-2 antigens in the rat spleen marginal zone and follicle. Infect Immun. 1996;64:4220–5. doi: 10.1128/iai.64.10.4220-4225.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peset Llopis M-J, Harms G, Hardonk MJ, Timens W. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J Allergy Clin Immunol. 1996;97:1015–24. doi: 10.1016/s0091-6749(96)80078-9. [DOI] [PubMed] [Google Scholar]
- 14.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–12. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 15.Spencer J, Perry ME, Dunn WD. Human marginal-zone B cells. Immunol Today. 1998;19:421–6. doi: 10.1016/s0167-5699(98)01308-5. [DOI] [PubMed] [Google Scholar]
- 16.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytology. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 17.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–44. [PubMed] [Google Scholar]
- 18.Devineni D, Klein SA, Gallo JM. In vivo microdialysis to characterize drug transport in brain tumors: analysis of methotrexate uptake in rat glioma-2 (RG-2)-bearing rats. Cancer Chemother Pharmacol. 1996;38:499–507. doi: 10.1007/s002800050518. [DOI] [PubMed] [Google Scholar]
- 19.Kumararatne DS, MacLennan IC. Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur J Immunol. 1981;11:865–9. doi: 10.1002/eji.1830111104. [DOI] [PubMed] [Google Scholar]
- 20.Yasutomi M, Ito M, Hayashi S, et al. Establishment of a concordant xenogeneic splenocyte injection model for the dynamic study of the marginal zone in the spleen. J Heart Lung Transpl. 1998;17:452–9. [PubMed] [Google Scholar]
- 21.Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FGM. The origin of marginal zone B cells in the rat. Eur J Immunol. 1999;29:1522–31. doi: 10.1002/(SICI)1521-4141(199905)29:05<1522::AID-IMMU1522>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Kroese FGM, Butcher EC, Lalor PA, Stall AM, Herzenberg LA. The rat B cell system: the anatomical localization of flow cytometry-defined B cell subpopulations. Eur J Immunol. 1990;20:1527–34. doi: 10.1002/eji.1830200718. [DOI] [PubMed] [Google Scholar]
- 23.Kroese FGM, Wubbena AS, Opstelten D, et al. B lymphocyte differentiation in the rat: production and characterization of monoclonal antibodies to B lineage-associated antigens. Eur J Immunol. 1987;17:921–8. doi: 10.1002/eji.1830170705. [DOI] [PubMed] [Google Scholar]
- 24.Hermans MH, Deenen GJ, De-Boer N, Bo W, Kroese FGM, Opstelten D. Expression of HIS50 Ag: a rat homologue of mouse heat-stable antigen and human CD24 on B lymphoid cells in the rat. Immunology. 1997;90:14–22. doi: 10.1046/j.1365-2567.1997.d01-2130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowal CD, Larner JM, Wingard LB. Human pharmacology, molecular to clinical. London: Wolfe Publishing Ltd; 1991. Individual antineoplastic drugs; pp. 589–605. [Google Scholar]
- 26.Molrine DC, Ambrossino DM. Immunization in immunocompromised cancer patients. Infect Med. 1996;13:259–80. [Google Scholar]
- 27.Timens W, Harms G, Peset Llopis M-J, Hardonk MJ. Leukocyte typing v white cell differentiation antigens. Oxford: Oxford University Press; 1994. Pneumococcal polysaccharides localize on splenic marginal zone B cells and follicular dendritic cells, together with complement fragment C3d, without relation to known CD21 epitopes; pp. 39–40. [Google Scholar]
- 28.Gray D. Population kinetics of rat peripheral B cells. J Exp Med. 1988;167:805–16. doi: 10.1084/jem.167.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama A, Hirabayashi N, Ito M, et al. White pulp reconstruction after human bone marrow transplantation. Am J Pathol. 1993;143:1111–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Beelen RHJ, Eestermans IL, Dijkstra CD. Monoclonal antibodies ED1, ED2 and ED3 against macrophages: expression of recognized antigens in different stages of differentiation. Transplant Proc. 1987;XIX:3166–70. [Google Scholar]
- 31.Jeurissen SH, Dijkstra CD. Characteristics and functional aspects of nonlymphoid cells in rat germinal centers, recognized by two monoclonal antibodies ED5 and ED6. Eur J Immunol. 1986;16:562–8. doi: 10.1002/eji.1830160518. [DOI] [PubMed] [Google Scholar]
- 32.Hunig T, Wallny HJ, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]