Abstract
To evaluate whether pregnancy has any effect on insulin antibody levels and to test the concordance between a conventional radioimmunoassay and a new microassay for the detection of insulin antibodies, insulin antibodies were analysed in 104 mothers in early pregnancy and at delivery and in their newborn infants. Thirty-eight of the mothers had type 1 diabetes. The concordance between the assays was high in the samples taken in early pregnancy (95%), but substantially lower in the samples taken at delivery (40%) and in the cord blood samples (68%). A considerable proportion of the mothers at delivery, especially the unaffected mothers (71%), and the newborn infants of the unaffected mothers (32%) were positive for insulin antibodies in the conventional assay but not in the microassay. Insulin antibody levels increased in the mothers, significantly so in the unaffected mothers (P < 0·001), during pregnancy in the conventional assay, whereas in the microassay they decreased significantly (P < 0·01) in affected mothers and remained negative in the unaffected mothers. Since immune complexes are precipitated with protein A specific for IgG in the microassay and with polyethylene glycol lacking specificity for immunoglobulins in the conventional assay, our data indicate that insulin antibody levels decrease on average during pregnancy and that the increasing non-IgG anti-insulin activity observed in the conventional assay is induced by pregnancy and is present in both the maternal and the foetal circulation.
Keywords: insulin antibodies, insulin autoantibodies, newborn infant, pregnancy, radiobinding assay, type 1 diabetes mellitus
Introduction
Antibodies to insulin frequently occur in patients exposed to exogenous insulin treatment [1]. A humoral immune response to endogenous insulin was found to be a marker of the preclinical phase of type 1 diabetes, when patients with newly diagnosed type 1 diabetes were reported to have detectable insulin antibodies (IA) before treatment with exogenous insulin [2]. Since then insulin autoantibodies (IAA) have been successfully used as predictive markers of future type 1 diabetes together with other islet autoantibodies [3,4]. The expression of insulin mainly in β cells, the frequent appearance of IAA as the first autoantibody specificity detected in the preclinical period and the presence of IAA at high levels particularly in young children at the diagnosis of type 1 diabetes are all features that emphasize the role of insulin as a crucial antigen in the development of β-cell destruction [5,6].
There are only a few reports of follow-up data on IA during diabetic pregnancy. Controversially, some studies have reported stable IA titres during pregnancy [7,8], while a slight decrease in IA levels has been documented in others [9,10]. No detailed data are available on IAA levels in nondiabetic women during pregnancy. IA detected in the cord blood of offspring of mothers with type 1 diabetes are transplacentally transferred from the maternal circulation to the foetal circulation during pregnancy [7,8,11–13].
All sensitive assays for the detection of IA are based on RIAs [14]. They require relatively large serum volumes to achieve high sensitivity, and mainly use polyethylene glycol (PEG) precipitation to separate antibody-bound insulin from free insulin. Williams et al. [15] have recently described a new radiobinding microassay which works with a smaller serum volume and uses protein A specific for IgG instead of PEG to precipitate the insulin-insulin antibody complexes. Increased anti-insulin activity has been reported in cord blood serum [12,16], and it has been suggested that this may be non-IgG-mediated, since it was not bound by protein A [16]. So far there are no data on whether this non-Ig anti-insulin activity is present only in cord blood or whether it is also found in the maternal circulation at delivery.
We analysed IA in a population of 104 mother-infant pairs by the conventional assay as originally described by Palmer et al. [2] and by the new microassay in order to compare the concordance between the two and to investigate whether pregnancy affects the levels of IA.
Subjects and methods
Subjects
The population comprised 104 mothers and their newborn infants from families with at least one family member (mother, father and/or sibling) affected by type 1 diabetes. The mother-infant pairs were initially recruited for the second pilot phase of an intervention trial which aims at evaluating the possible effect of elimination of cow's milk proteins in early infancy on the subsequent risk of manifesting type 1 diabetes (Trial to Reduce IDDM in the Genetically at Risk, TRIGR) [17]. Thirty-eight of the 104 mothers (36·5%) had type 1 diabetes and the remaining 66 were unaffected. The mean age of the women at delivery was 31·5 ± 5·6 (SD) years (range 19·0–46·7 years) and the mean duration of diabetes in those with type 1 diabetes was 14·2 ± 7·3 years (range 2·0–28·0 years). The severity of maternal diabetes, classified according to White [18] was: 13 in class B, seven in class C, 12 in class D and six in class F. Mean individual HbA1c levels throughout pregnancy ranged from 4·6% to 7·9% (mean 6·4 ± 0·8%), as compared with a reference range of 4–6% in nondiabetic subjects [19]. More than half of the newborn infants were girls (56/104; 53·8%). Mean gestational age was 39·1 ± 1·7 weeks (range 35·1–42·6 weeks), mean birth weight 3786 ± 604 g (range 1695–5600) and mean length at birth 50·4 ± 2·4 cm (range 41·0–56·0 cm). Blood samples were obtained from the mothers at the end of the first trimester and at delivery and from the cord blood of the newborn infants. Samples from two nondiabetic mothers were not available at the end of the first trimester. Serum samples were stored at −20°C until the IA assays were performed. All three samples from each mother-infant pair were analysed in the same assay run. Written informed consent was obtained from the women. This research was approved by the Joint Ethics Committees of the participating hospitals.
IA measurements
IA were analysed with two fluid phase competitive radiobinding assays. The conventional assay was modified from that described by Palmer et al. [2], in which we used 200 μl serum. Briefly, after the removal of endogenous insulin by acid charcoal extraction, the samples were incubated with mono 125I-(TyrA14) -labelled human insulin (Amersham, Little Chalfont, Bucks, UK) for 20 h in the presence or absence of an excess of unlabelled insulin. After the incubation, antibody-bound insulin was precipitated with PEG and the precipitated radioactivity counted. The IA level was expressed in nU/ml, where 1 nU/ml corresponds to a specific binding of 0·01% of the total counts added. The intra-assay coefficient of variation (CV) was less than 5% and the interassay variation less than 8%. The cut-off limit for antibody positivity was defined as 68 nU/ml, representing the 99th percentile of 102 nondiabetic Finnish subjects. The disease sensitivity of the assay was 26% and the disease specificity 97% based on 140 samples included in the 1995 Multiple Autoantibody Workshop [20].
The microassay method was modified from that described by Williams et al. [15]. Immune complexes were precipitated with Protein A Sepharose (Pharmacia Biotech, Uppsala, Sweden) after incubation of the serum sample (5 μl/well) for 72 h with mono 125I-(TyrA14)-labelled human insulin in the presence or absence of an excess of unlabelled insulin. The volume of the incubation reaction was doubled by adding the reaction buffer (TBT; 50 mm Tris, pH 8·0, 1% (v/v) Tween 20). After thorough washing with reaction buffer, the samples were transferred from the deep well plates to microtitration plates, scintillation liquid was added and the bound activity was measured with a liquid scintillation counter (1450 MicroBeta Trilux; PerkinElmer Life Sciences Wallac, Turku, Finland). Specific binding was expressed in relative units (RU) based on a standard curve run on each plate using the MultiCalc ™ software program (PerkinElmer Life Sciences Wallac). The standard curve was constructed from nine serial dilutions of a serum from a patient with a high IA titre and a serum from an IA-negative subject. The negative human serum was taken as the lowest working point on the standard curve. Due to competition, a proportion of the samples with no detectable IA gave a cpm below the lowest standard (< 1 RU). All such samples were assigned a value of 1 RU in the statistical evaluation. The cut-off limit for antibody positivity was set at the 99th percentile in 371 nondiabetic Finnish subjects (1·56 RU). The disease sensitivity of this assay was 35% and the specificity 100% based on 140 samples included in the Multiple Autoantibody Workshop [20]. The assay was blindly compared with the microassay run in Bristol [15] by analysing 100 samples in both laboratories. A close correlation was observed between the two assays (r = 0·96, P < 0·001), and a high concordance rate (94%). The intra-assay CV was 7% and the interassay CV less than 9%. The microassay protocol does not include the removal of endogenous insulin from sera before the assay. To control for the possible effect of the serum insulin on the antibody results we removed insulin from 32 maternal sample-pairs in which one or both samples were above 1 RU in the microassay by acid charcoal extraction performed similarly as in the conventional assay. The treated sera were then re-assayed in the microassay.
Statistics
The correlation analyses were performed using Spearman's nonparametric rank correlation test (rs). Differences in antibody levels were evaluated with Wilcoxon's rank-sum test, and differences in antibody frequencies with the two independent proportions test. A two-tailed P-value of 0·05 or less was considered to indicate statistical significance. Statistical analyses were performed using the SPSS statistical software package (SPSS Inc., Chicago, IL., USA) or Arcus QuickStat Biomedical statistical software (Addison Wesley Longman Ltd, Research Solutions, Cambridge, UK).
Results
Frequencies and levels of IA in pregnant women during pregnancy and in their newborn infants in cord blood
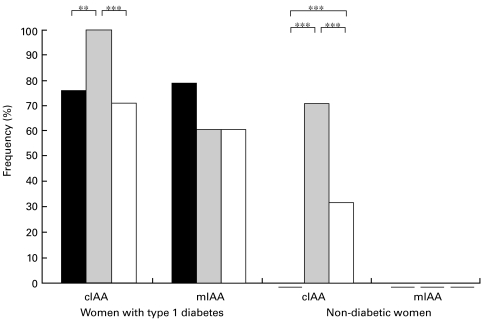
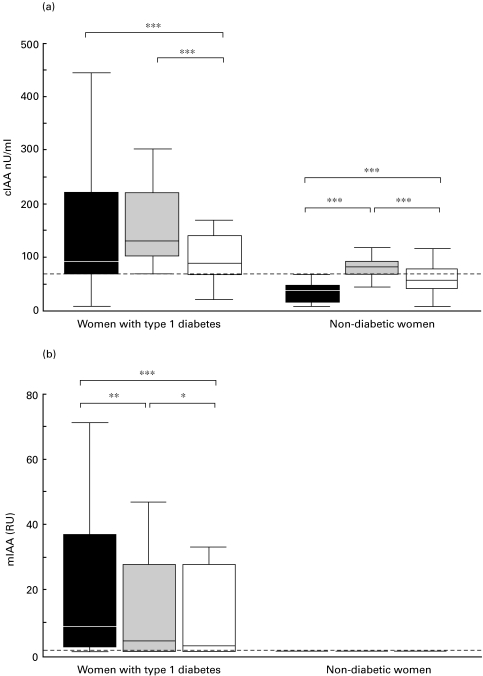
None of the 64 unaffected women tested positive for IAA at the end of the first trimester according to the conventional assay. Surprisingly, 47 (71%) out of them were found to be positive for IAA at delivery (Fig. 1). The increase in IAA levels during pregnancy was highly significant (Fig. 2a). No correlation was observed between the two maternal samples. Twenty-one (32%) out of the newborn infants of unaffected mothers tested positive for IAA in cord blood (Fig. 1). The cord blood IAA levels were significantly higher than maternal levels in early pregnancy but significantly lower than maternal levels at delivery (Fig. 2a). A weak correlation was observed between the cord blood IA levels and maternal IA levels in early pregnancy (rs = 0·27; P = 0·026) and at delivery (rs = 0·28; P = 0·021). Among 38 mothers with type 1 diabetes, 29 (76%) were positive for IA in early pregnancy and all at delivery (Fig. 1). A nonsignificant increase was observed in the IA levels during pregnancy (Fig. 2a) and a close correlation between the two maternal samples (Table 1) was seen. As expected, IA were frequently detected in the offspring of affected mothers (27; 71%, Fig. 1). The cord blood IA levels were significantly lower than maternal levels in early and late pregnancy (Fig. 2a) and correlated slightly more closely with the maternal IA levels at delivery than with the maternal levels in early pregnancy (Table 1).
Fig. 1.
Frequency (%) of IA at the end of the first trimester (▪), at delivery ( ) and in cord blood (□) in women with type 1 diabetes and unaffected women according to the conventional assay (cIAA) and the microassay (mIAA). None of the nondiabetic women tested positive for IAA in early pregnancy according to any assay or at delivery according to the microassay, and none of the newborn infants of the unaffected mothers had IAA according to the microassay. ** P < 0·01, *** P < 0·001.
) and in cord blood (□) in women with type 1 diabetes and unaffected women according to the conventional assay (cIAA) and the microassay (mIAA). None of the nondiabetic women tested positive for IAA in early pregnancy according to any assay or at delivery according to the microassay, and none of the newborn infants of the unaffected mothers had IAA according to the microassay. ** P < 0·01, *** P < 0·001.
Fig. 2.
IA levels in samples taken from the women at the end of the first trimester (▪) and at delivery ( ) and in the cord blood of newborn infants (□) according to the conventional assay (cIAA; a) and the microassay (mIAA; b). The broken line indicates the cut-off limit for IA positivity (1·56 RU in the mIAA and 68 nU/ml in the cIAA). Each box plot represents the median, and the 25th and 75th percentiles. The error bars represent the lowest and the highest values that are not outliers. Statistical significance evaluated with Wilcoxon's rank-sum test. * P < 0·05, ** P < 0·01, *** P < 0·001.
) and in the cord blood of newborn infants (□) according to the conventional assay (cIAA; a) and the microassay (mIAA; b). The broken line indicates the cut-off limit for IA positivity (1·56 RU in the mIAA and 68 nU/ml in the cIAA). Each box plot represents the median, and the 25th and 75th percentiles. The error bars represent the lowest and the highest values that are not outliers. Statistical significance evaluated with Wilcoxon's rank-sum test. * P < 0·05, ** P < 0·01, *** P < 0·001.
Table 1.
Spearman's nonparametric rank correlation analyses for samples taken from women with type 1 diabetes at the end of the first trimester (n = 38) and at delivery (n = 38), and from the cord blood of the newborn infants (n = 38)
| Conventional assay | Micro-assay | |||
|---|---|---|---|---|
| Early pregnancy | Delivery | Early pregnancy | Delivery | |
| Early pregnancy | 0·87* | 0·83* | ||
| Cord blood | 0·73* | 0·80* | 0·79* | 0·97* |
P < 0·001
No IAA were detected in the unaffected mothers and in their newborn infants according to the microassay. Since all the unaffected women were negative (1 RU) on both occasions, changes in IA levels during pregnancy could not be assessed. Of the 38 affected women, a higher proportion tested positive in early pregnancy (30; 79%) than at delivery (23; 61%, Fig. 1). A significant decrease in the IA levels was observed during pregnancy (Fig. 2b), and the two maternal samples correlated closely. Similarly, a significant decrease (P < 0·01) was also observed during pregnancy in the samples from which serum insulin was removed before the assay (data not shown). Among the newborn infants of affected mothers, 23 (61%) were positive for IA in cord blood (Fig. 1). IA levels in both maternal samples were significantly higher than the levels in the newborn infants (Fig. 2b). The cord blood IA levels correlated closely with the maternal IA levels (Table 1).
Comparison between the conventional assay and the new microassay
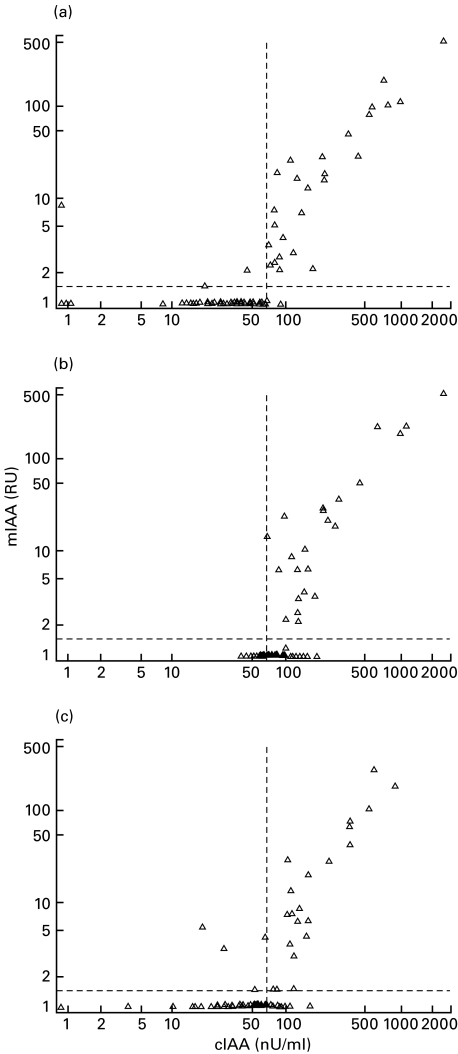
Altogether 310 samples were measured by both assays. Sixty-nine samples (22%) were positive in both assays, 93 (31%) only in the conventional assay (range 68–195 nU/ml; median 87 nU/ml) and seven (2%) only in the microassay (range 1·56–11·83 RU; median 3·79 RU). One hundred 41 samples (45%) had IA levels below the cut-off limit for positivity in both assays. Overall, 68% of the samples were concordant. The concordance rate was conspicuously higher in the samples taken in early pregnancy (97 out of 102; 95%, Fig. 3a) than in those taken at delivery (42 out of 104; 40%, Fig. 3b) or in cord blood (71 out of 104; 68%, Fig. 3c). The IA levels of the affected women and their newborn infants correlated between the two assays. The closest correlation between the assays was observed in the maternal samples from early pregnancy (rs = 0·72, P < 0·001), followed by the samples taken at delivery (rs = 0·64, P < 0·001), with the weakest in the cord blood samples (rs = 0·55, P < 0·001). No correlation analyses could be performed between the assays for IAA levels in the unaffected mothers and their newborn infants, since all the samples from these subjects were assigned an IAA level of 1 RU in the microassay.
Fig. 3.
Comparison of IA results obtained by the conventional assay (cIAA) and the microassay (mIAA) for samples taken (a) at the end of the first trimester (n = 102) and (b) at delivery (n = 104) and (c) cord blood samples (n = 104). The broken lines indicate the cut-off limit for IA positivity (1·56 RU in the mIAA and 68 nU/ml in the cIAA). Spearman's nonparametric rank correlation analyses gave an rs of 0·72 for samples taken in early pregnancy, 0·64 for samples taken at delivery and 0·55 for cord blood samples (P < 0·001 for all).
The observed discrepancy between the assays in the samples taken at delivery and in cord blood was mainly due to an increase in the IA level in the unaffected mothers and their infants in the conventional assay. None of the women turned negative for IA during pregnancy, whereas 56 initially negative mothers had low positive levels at delivery (range 68–162 nU/ml, median 86 nU/ml) according to the conventional assay. Among these 56 mothers, 47 were unaffected, while nine had type 1 diabetes. According to the microassay, one mother with type 1 diabetes seroconverted to positivity for IA, while eight became negative during pregnancy. The initially IA negative mother had a relatively high titre at delivery (30·2 RU). Seroconversion to IA negativity occurred in women who initially had low IA levels (range 1·57–10·6 RU, median 3·4 RU). If IA were detected in cord blood, the corresponding maternal sample taken at delivery was also positive with the exception of four cases in the conventional assay and a single case in the microassay. In the case detected by the microassay, the mother had tested positive for IA in early pregnancy (3·24 RU). The IA levels in all five cord blood samples found to be positive despite an IA negative maternal sample were close to the cut-off limit for antibody positivity (1·59 RU, 74, 90, 94 and 114 nU/ml).
Discussion
In this study, we demonstrate that there is a discrepancy between the conventional assay and the microassay in the detection of IA in pregnant women and their newborn infants. The main discrepancy was that a considerable proportion of the mothers at delivery and their newborn infants tested positive for IA in the conventional assay but not in the microassay. The samples identified as positive only in the conventional assay had relative low IA levels. The inability of the microassay to detect these samples as positive ones is hardly a consequence of an insensitive assay, since the affected women found to have low IA levels in the conventional assay in early pregnancy also tested positive in the microassay. The sensitivity of our microassay format to detect patients with newly diagnosed type 1 diabetes as IA positive subjects (54%) [21] does not differ significantly from those sensitivities achieved by microassay formats in studies published previously (50–70%) [15,22]. In those studies, a high concordance between the microassay and the conventional assay was observed. Such series did not include any pregnant women or newborn infants, however. Haemolysis has been reported to induce falsely positive IAA results in the conventional assay. In the present study, haemolytic samples were equally often concordant or discordant as nonhaemolytic ones. The lack of a clear effect of haemolysis on the conventionally quantified IAA levels may be a consequence of the acid charcoal pretreatment of the samples in the present protocol, while no pretreatment was performed in the study reporting falsely positive IAA levels due to haemolysis [23].
We propose that the discrepancy between the assays is due to non-Ig-associated anti-insulin activity detected by the conventional assay. Initially, the normal range of IAA in the cord blood of infants of nondiabetic mothers was documented to be higher than that in older children and adults [12,24]. Later the anti-insulin activity detected as elevated IAA concentrations in cord blood was shown to be likely due to a cross-reacting molecule that binds insulin but is not part of the IgG fraction, based on the fact that this activity was not bound by protein A or G [16]. Our results confirm this hypothesis, since we did not observe any elevated IAA levels in cord blood with the microassay, in which the separation of antibody-bound insulin from free labelled insulin is based on protein A binding rather than PEG as in the conventional assay. The anti-insulin activity does not result from the weak capacity of protein A to bind the IgG3 subclass [25], since we also measured IA using protein G binding all IgG subclasses, and the results obtained were highly concordant.
Our results show as a primary finding that normal pregnancy induces the same kind of non-Ig-associated anti-insulin activity in the maternal circulation as is observed in cord blood, since elevated IAA levels in mothers at delivery were seen in the conventional assay but not in the microassay. This activity was clearly observed in women without type 1 diabetes and in their newborn infants. It is probably also present in mothers with type 1 diabetes at delivery and in their newborn infants, but it is masked by the high IA levels resulting from exogenous insulin treatment. The present observations suggest that this activity is present in higher concentrations in the maternal than in the foetal circulation, since 71% of the mothers testing negative for IAA in early pregnancy were positive at delivery, while only 32% of their newborn infants were positive according to the conventional assay, and since the increase in IAA levels seen during pregnancy was higher than the difference in IAA levels between the cord blood and the maternal sample from early pregnancy. Bilbao et al. [16] reported that the anti-insulin activity is detected in 96% of the newborn infants born to nondiabetic mothers. Additional studies are needed to sort out this discrepancy in frequency.
Insulin-like growth factor binding proteins (IGFBPs) which are capable of binding insulin as well as insulin-like growth factors, although with a much lower affinity for insulin, could be potential candidates for the increasing insulin-binding activity. It is known that maternal IGFBP-1 and foetal IGFBP-2 and IGFBP-3 concentrations increase during pregnancy and that the level of IGFBP-1 is higher in foetal and cord blood than in adults [26,27]. In a preliminary assessment, however, we could not find any correlation between the conventionally assayed IAA levels and IGFBP-1 or IGFBP-3 concentrations in the cord blood of normal newborn infants (Ronkainen et al. unpublished observation).
Our results obtained by the microassay indicate that IAA are rarely present in nondiabetic women in early or late pregnancy. A decrease in IA levels was observed during pregnancy in the women with type 1 diabetes. The decrease can not result from the potentially higher concentrations of circulating insulin in late pregnancy that could inhibit the binding of labelled insulin to antibodies, since a significant decrease in IA levels during pregnancy was also seen in the samples pretreated by acid charcoal to remove insulin present in the sample. Previously both stable and decreasing IA levels have been reported during pregancy [7–10]. The decrease in antibody concentrations observed during pregnancy may be a consequence of haemodilution [28]. Although normal pregnancy is considered to represent a Th2 –biased state with a suppressed cell-mediated immune response [29,30], it may not result in an enhanced humoral immune response to insulin.
Based on our results of the microassay, we confirm earlier reports that more than half of the infants of mothers with type 1 diabetes have IA at birth [12], and the close correlation between the IA levels in the newborn infant and those in the maternal circulation verifies the claim that the IA in cord blood represent transplacentally transferred antibodies. Our results indicate that there are no specific autoantibodies to insulin in the cord blood of infants with their father or a sibling affected by type 1 diabetes.
Having set out to evaluate the effect of pregnancy on IA and to test the concordance between the new microassay and the conventional radiobinding assay, we conclude that the methods utilizing PEG as a precipitating agent are not reliable for the detection of IA in pregnant mothers and newborn infants. We emphasize that the haemodilution effect should be considered when analysing changes in antibody levels or in concentrations of other serum proteins during pregnancy. In addition, we observed an anti-insulin activity that is not associated with IgG but is induced by pregnancy and is present in both the maternal and foetal circulation at delivery.
Acknowledgments
This research was supported by the Juvenile Diabetes Foundation International (grants 192612 and 195003), the Novo Nordisk Insulin Foundation and the Aage and Johannes Louis-Hansen Foundation. The TRIGR Study was supported by the European Commission (BMH4-CT96–0233), Helsinki University Central Hospital, the Sigrid Jusélius Foundation, University of Helsinki, the Liv and Hälsa Foundation, and the Dorothea Olivia, Karl Walter and Jarl Walter Perklén Memorial Fund. We thank Susanna Heikkilä and Riitta Päkkilä for their skilful technical assistance.
The Finnish Trial to Reduce IDDM in the Genetically at Risk (TRIGR) Study Group is composed of the following members: Principal investigator: H.K. Åkerblom.
Local investigators: V. Eskola, H. Haavisto, R. Jokisalo, A.-L. Järvenpää, U. Kaski, J. Komulainen, P. Korpela, M.-L. Käär, P. Lautala, A-M. Hämäläinen, K. Niemi, A. Nuuja, M. Renlund, M. Salo, T. Talvitie, T. Uotila, G. Wetterstrand.
Special investigators: J. Ilonen, J. Karjalainen, P. Klemetti, M. Knip, P. Kulmala, J. Paronen, A. Reunanen, T. Saukkonen, E. Savilahti, K. Savola, K. Teramo, O. Vaarala, S.M. Virtanen.
References
- 1.Greenbaum CJ, Palmer JP. Insulin antibodies and insulin autoantibodies. Diabetic Med. 1991;8:97–105. doi: 10.1111/j.1464-5491.1991.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 2.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin dependent diabetes before insulin treatment. Science. 1983;222:1337–9. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, MacLaren NK, Riley WJ, Winter WE, Fisk DD, Spillar RP. Are insulin autoantibodies markers for insulin-dependent diabetes mellitus? Diabetes. 1986;35:894–8. doi: 10.2337/diab.35.8.894. [DOI] [PubMed] [Google Scholar]
- 4.Kulmala P, Savola K, Petersen JS, et al. Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J Clin Invest. 1998;101:327–36. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardi P, Ziegler AG, Matthews JH, et al. Concentration of insulin autoantibodies at onset of type I diabetes: inverse log-linear correlation with age. Diabetes Care. 1988;9:736–9. doi: 10.2337/diacare.11.9.736. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Ziegler R, Vardi P, Jackson RA, Soeldner JS, Eisenbarth GS. Life-table analysis of progression to diabetes of anti-insulin autoantibody-positive relatives of individuals with type 1 diabetes. Diabetes. 1989;38:1320–5. doi: 10.2337/diab.38.10.1320. [DOI] [PubMed] [Google Scholar]
- 7.Mylvaganam R, Stowers JM. Steel JM, Wallace J, MacHendry JC, Wright AD. Insulin immunogenicity in pregnancy: Maternal and fetal studies. Diabetologia. 1983;24:19–25. doi: 10.1007/BF00275942. [DOI] [PubMed] [Google Scholar]
- 8.Di Mario U, Fallucca F, Gargiulo P, Tiberti C, Scardellato A, Arduini P, Pachi A, Andreani D. Insulin-anti-insulin complexes in diabetic women and their neonates. Diabetologia. 1984;27:83–6. doi: 10.1007/BF00275654. [DOI] [PubMed] [Google Scholar]
- 9.Exon PD, Dixon K, Malins JM. Insulin antibodies in diabetic pregnancy. Lancet. 1974;2:126–8. doi: 10.1016/s0140-6736(74)91555-4. [DOI] [PubMed] [Google Scholar]
- 10.Dozio N, Beretta A, Castiglioni M, Rosa S, Scavini M, Belloni C, Poloniato A. Insulin antibodies do not preclude optimization of metabolic control in women with IDDM during pregnancy. Diabetes Care. 1996;19:979–82. doi: 10.2337/diacare.19.9.979. [DOI] [PubMed] [Google Scholar]
- 11.Spellacy WN, Goetz FC. Insulin antibodies in pregnancy. Lancet. 1963;2:222–4. doi: 10.1016/s0140-6736(63)90118-1. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler A-G, Hillebrand B, Rabl W, et al. On the appearance of islet associated autoimmunity in offspring of diabetic mothers: a prospective study from birth. Diabetologia. 1993;36:402–8. doi: 10.1007/BF00402275. [DOI] [PubMed] [Google Scholar]
- 13.Martikainen A, Saukkonen T, Kulmala PK, et al. Disease-associated antibodies in offspring of mothers with IDDM. Diabetes. 1996;45:1706–10. doi: 10.2337/diab.45.12.1706. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the fourth international workshop on the standardization of insulin autoantibody measurement. J Clin Endocrinol Metab. 1992;74:1040–4. doi: 10.1210/jcem.74.5.1569152. [DOI] [PubMed] [Google Scholar]
- 15.Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM. A novel micro-assay for insulin autoantibodies. J Autoimmun. 1997;10:473–8. doi: 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 16.Bilbao JR, Calvo B, Urrutia I, Linares A, Castano L. Anti-insulin activity in normal newborn cord-blood serum. Diabetes. 1997;46:713–6. doi: 10.2337/diab.46.4.713. [DOI] [PubMed] [Google Scholar]
- 17.Åkerblom HK, Savilahti E, Saukkonen T, et al. The case for elimination of cow's milk in early infancy in the prevention of Type I diabetes: the Finnish experience. Diabetes Metab Rev. 1993;9:269–78. doi: 10.1002/dmr.5610090407. [DOI] [PubMed] [Google Scholar]
- 18.White P. Pregnancy and diabetes, medical aspects. Med Clin N Am. 1965;49:1015–24. doi: 10.1016/s0025-7125(16)33292-8. [DOI] [PubMed] [Google Scholar]
- 19.Stenman U-H, Pesonen K, Ylinen K, Huhtala M-L, Teramo K. Rapid chromatographic quantitation of glycosylated haemoglobins. J Chromatogr. 1984;297:327–32. doi: 10.1016/s0021-9673(01)89052-x. [DOI] [PubMed] [Google Scholar]
- 20.Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in Type 1 diabetes. Combinatorial islet cell autoantibody workshop. Diabetes. 1998;47:1857–66. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 21.Sabbah E, Savola K, Kulmala P, et al. Diabetes-associated autoantibodies in relation to clinical characteristics and natural course in children with newly diagnosed type 1 diabetes. J Clin Endocrinol Metab. 1999;84:1534–9. doi: 10.1210/jcem.84.5.5669. [DOI] [PubMed] [Google Scholar]
- 22.Naserke HE, Dozio N, Ziegler AG, Bonifacio E. Comparison of a novel micro-assay for insulin autoantibodies with the conventional radiobinding assay. Diabetelogia. 1998;41:681–3. doi: 10.1007/s001250050968. [DOI] [PubMed] [Google Scholar]
- 23.Naserke HE, Bonifacio E, Ziegler AG. Immunoglobulin G insulin autoantibodies in BABYDIAB offspring appear postnatally: Sensitive early detection using a protein A/G-based radiobinding assay. J Clin Endocrinol Metab. 1999;84:1239–43. doi: 10.1210/jcem.84.4.5597. [DOI] [PubMed] [Google Scholar]
- 24.Wellik SR, de Vaciana M, Morgan MA, Berkowitz KM, Arquilla ER. Naturally occurring insulin autoantibodies in neonates of normal pregnancies and their relationships to insulinemia and birth weight. Am J Obstet Gynecol. 1995;173:1878–84. doi: 10.1016/0002-9378(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 25.Kronvall G, Williams RC. Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969;103:828–33. [PubMed] [Google Scholar]
- 26.Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–31. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 27.Langford K, Nicolaides K, Miell JP. Maternal and fetal insulin-like factors and their binding proteins in the second and third trimesters of human pregnancy. Hum Reprod. 1998;13:1389–93. doi: 10.1093/humrep/13.5.1389. [DOI] [PubMed] [Google Scholar]
- 28.Ailus KT. A follow-up study of immunoglobulin levels and autoantibodies in an unselected pregnant population. Am J Reprod Immunol. 1994;31:189–96. doi: 10.1111/j.1600-0897.1994.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 29.Reinhard G, Noll A, Schlebusch H, Mallmann P, Ruecker V. Shifts in the TH1/TH2 balance during human pregnancy correlate with apoptotic changes. Biochem Biophys Res Commun. 1998;245:933–8. doi: 10.1006/bbrc.1998.8549. [DOI] [PubMed] [Google Scholar]
- 30.Wilder R. Hormones, pregnancy, and autoimmune diseases. Ann NY Acad Sci. 1998;840:45–5. 33. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]