Abstract
Otitis media with effusion (OME) is a chronic inflammation persisting in the middle ear cavity of at least 8 weeks duration. Middle ear effusion (MEE; n = 38), samples from children suffering from OME were investigated for their direct cytolytic activity or an ability to enhance complement lysis of unsensitized bystander cells. Thirteen of the 38 MEEs had direct endogenous haemolytic activity and 27 samples had an ability to enhance serum-initiated lysis. Using an enzyme immunoassay, high levels of terminal complement complexes (TCC) were detected in the MEE samples (mean 34·1 µg/ml, range 5–89 µg/ml). This indicated strong local complement activation that had progressed to the terminal stage. As one potential factor promoting complement activation we identified both monomeric and trimeric properdin in MEE by Western blotting. By stabilizing C3 and C5 convertases properdin accelerates the alternative and terminal pathways of complement. On the other hand, the membrane attack complex (MAC) inhibitor CD59, which was found to be extensively shed into the MEE in a functionally active form, may control excessive cytotoxicity of the MEE. In conclusion, intense complement activation, up to the terminal level, maintains ongoing inflammation in the middle ear cavity and can pose a threat to the local epithelium.
Keywords: CD59, properdin, OME, terminal complement complexes (TCC).
INTRODUCTION
According to the international definitions [1,2] otitis media with effusion (OME) is a chronic non-suppurative inflammation in the middle ear cleft, lasting for more than 8 weeks. It is an extremely common condition among children [3,4]. OME causes conductive hearing impairment and, for example, in England it is the most common cause for elective surgery in children [5]. While inflammatory changes are characteristic, the precise aetiopathogenetic mechanisms of OME still remain unknown. Among other mediators of inflammation complement (C) activation fragments have been shown to be present in middle ear effusion (MEE) [6–8]. The C system is an important part of both the innate and acquired immune systems in eliminating microbes. It can be activated by antibodies binding to C1q in the classical pathway or directly by C3b binding to foreign surfaces in the alternative pathway. Both activation cascades leads to terminal pathway, and finally to formation of the cytolytic membrane attack complex (MAC), formed by a heteropolymeric assembly of the terminal C components C5b, C6, C7, C8 and multiple C9 molecules [9]. Following their formation activated C components (C4b, C3b and MAC) can also become deposited on bystander host cells.
To avoid inappropriate activation of the potentially cytotoxic C system, it is strictly regulated on various steps of the activation cascade. The regulators include fluid phase regulators such as C1-inhibitor, C4-binding protein of the classical pathway, factors I and H of the alternative pathway and vitronectin of the terminal pathway. Membrane regulators which are bound to host cells include decay accelerating factor (DAF; CD55), membrane co-factor protein (MCP; CD46) and CD59 (protectin). Under physiological conditions properdin is the only known positive regulator of complement activation. It binds to and stabilizes the inherently labile alternative pathway C3 and C5 convertase complexes. It is present in human plasma at low concentrations (5–20 µg/ml) but not, e.g. in pleural, ascitic or spinal fluid [10]. It is synthesized locally by PMNs [11], monocytes and T lymphocytes [12]. Due to the strong C activation in the MEE we hypothesized that properdin could be an important positive feedback regulatory component for the ongoing complement activation in MEE during OME.
Human cells, including middle ear mucosal cells [13–15], are protected against C lysis by CD59 (membrane inhibitor of reactive lysis MIRL; protectin), a glycophosphoinositol (GPI)-anchored inhibitor of the MAC [16–18]. CD59 binds to the terminal complexes C5b-8 and C5b-9 and inhibits the cytolytic activity of complement by blocking the incorporation of C9 into membrane C5b-8 [16,19]. It can be found also in soluble form without the anchor phospholipid, but the cytolysis inhibiting activity of soluble CD59 is relatively weak [20]. Both membrane and soluble forms, in various ratios, can be found in many body fluids, e.g. in urine, seminal plasma and amniotic fluid [17,21–23].
While acute otitis media is usually caused by bacteria invading into the middle ear cleft, bacteria can be cultured only from one-third of the MEEs of patients with chronic OME. This suggests that the MEE could have bactericidal activity. With regard to the potential cytotoxicity of MEE we wanted to study whether middle ear effusions of OME patients have an ability to cause lysis of guinea pig or chicken erythrocytes or to enhance complement lysis when diffusing against components of normal human serum. To investigate the degree of terminal pathway C activation in MEE, the levels of soluble terminal complement complexes (TCC; SC5b-9) were measured. In addition, we wanted to investigate whether properdin can be found in MEE, as it could have a role in accelerating the ongoing alternative pathway C activation in the middle ear cavity. Since the expression of CD59 was recently found to be decreased on the outer surface of cells lining the middle ear cleft [15], we also examined the possibility that CD59 had become detached from the epithelial cell surfaces into the MEE.
MATERIALS AND METHODS
Patients and samples
Forty-nine children undergoing tympanostomy tube insertion at the Helsinki University Central Hospital or the Jorvi District Hospital, Espoo, were included in the study. All children had suffered from chronic otitis media with effusion for at least 8 weeks. Children with chronic illnesses, e.g. asthma, cleft palate or immunodeficiencies, were excluded. The samples were taken with parental consent and the study was approved of by the Ethical Committees of the Departments of Otorhinolaryngology at the Helsinki University Central Hospital and at the Jorvi Hospital. Middle ear effusion (MEE) samples were collected into Juhn Tym-Tap tubes (Xomed Surgical Products, Jacksonville, FL, USA) in connection with normal treatment. If the disease was bilateral, the samples were taken from both ears. Altogether 62 MEE samples were obtained. Forty-four of the samples collected were mucoid, 16 were mucopurulent or seromucous and two were serous. Portions of 53 MEE samples were taken for bacterial culture with standard techniques. The remaining samples were frozen within 60 min and stored at − 70°C until used. For the lysis assays the middle ear effusion samples (n = 22) were used as undiluted or after dilution into 1 ml phosphate buffered saline (PBS) (n = 16). The MEEs for immunoblotting and ELISA analyses (n = 24) were diluted in 500 µl of lithium chloride-containing phosphate buffered saline (LiCl-PBS) (2·85 mm LiCl in PBS, pH 7·4) and mechanically mixed (Vortex) until homogenized.
LiCl in PBS was used to facilitate quantification of the exact amount of the middle ear effusions in the samples as described [24]. The lithium concentration was assayed by using the Vitros 250 analyser (Chemistry System, Johnson & Johnson Clinical Diagnostics, Rochester, NY, USA). The amount of the MEE sample (µl) was calculated from the following formula: volume of the sample = ([Li]in LiCl-PBS/[Li] in sample) × 500 µl−500 µl. The percentage of MEE in the whole sample (MEE + 500 µl Li-PBS) was then defined as the correction coefficient (CC) and calculated from the formula CC = 1-([Li] in sample/[Li] in LiCl-PBS). The measured values of TCC were divided by CC to calculate the original amount of TCC in each middle ear effusion sample.
Haemolysis assays
The ability of MEE to induce complement lysis directly through the alternative pathway, or by enhancing the lysis caused by NHS, was determined in plate tests using guinea pig erythrocytes (GPE) in agarose gel or a commercially available alternative pathway haemolytic complement kit with chicken erythrocytes (AH 100; The Binding Site, Birmingham, UK). Samples for lysis assays were collected as a separate set from 33 patients (MEE samples from 38 ears) with OME. Blood from guinea pigs was collected into Alsever's solution. Two per cent GPE were mixed with 0·6% agarose (Indubiose, Biosepra, France) in MgEGTA-NaCl (5 mm MgCl2 and 10 mm ethyleneglycol tetraacetic acid) pH 7·4, which had been melted and cooled to + 54°C. After pouring the solution on glass slides, the plates were allowed to stabilize overnight at + 4°C. For lysis assays small wells with diameters of 4 mm and an interwell distance of 10–12 mm were punched in the haemolysis gels; 10 µl portions of MEE samples and normal human sera (NHS) from healthy laboratory personnel were applied on the wells. This set-up allowed diffusion of MEE samples against NHS samples and analysis of the potentially enhancing or inhibiting activity of MEE on the serum C activity. To investigate the role of different complement components polyclonal antibodies against C3c, C4 (Dako, Glostrup, Denmark), C7, C9, B (Quidel, San Diego, CA, USA) and properdin (ATAB, Scarborough, ME, USA) were applied on different wells as inhibitory antibodies. As a control a non-specific antibody against human IgG (Dade Behring, Marbung, Germany) was used. The haemolysis gels were incubated for 18 h at + 4°C, 120 min at + 37°C and thereafter 12–24 h at + 22°C and scanned. To test the ability of the C4 antibody to inhibit lysis via the classical pathway, a classical pathway haemolysis kit (THC 100; The Binding Site, Birmingham, UK) was used.
Enzyme immunoassay for quantification of SC5b-9
The levels of terminal C complexes (SC5b-9) in MEEs were measured by an enzyme immunoassay. Briefly, microtitre plate strips (Combiplate EB, Labsystems, Finland) were coated with a neoantigen-specific mouse anti-SC5b-9 antibody (Quidel) at 5 µg/ml in 100 µl of 0·1 m NaHCO3 buffer, pH 8·0, per well overnight at + 4°C. The wells were washed five times with PBS, pH 7·4, containing 0·05% Tween. The MEEs were diluted 1/20 and 1/50 in PBS containing 0·05% Tween 20. Two dilutions, both in duplicate, were added in 100 µl portions to the wells and incubated for 45 min at + 22°C. After washing (5×) a mixture of goat anti-C5 and anti-C6 antibodies (Quidel, San Diego, CA, USA) at 1 µg/ml in 0·05% Tween 20/PBS, was added. After 1 h the strips were washed again and 100 µl of horseradish peroxidase-conjugated antigoat IgG (Dako), diluted 1/5000, was added and incubated in the wells for 1 h. After five washes phenylene-diamide dihydrochloride substrate (70 µg/100 µl ureoperoxidase; Dako) was added and the reaction was stopped after 15 min with 50 µl of 2 m H2SO4. The absorbances were read at 492 nm with an ELISA plate reader (model 340, Labsystems Multiskan MCC, Finland). The TCC values in samples were calculated using a standard curve prepared with specimens of purified SC5b-9 containing 70 ng/ml, 117 ng/ml and 175 ng/ml of SC5b-9 [20,21].
Detection of properdin and CD59 in MEE by Western blotting
The MEE samples and the positive controls prepared from lysed red blood cells for CD59 analyses and from NHS and purified properdin (Calbiochem, La Jolla, CA, USA), for properdin analyses, were diluted in non-reducing SDS-PAGE buffer (containing 1% SDS). SDS-PAGE was performed according to the method of Laemmli [25] using 15% or 8% gels and a mini-gel system (BioRad Laboratories, Richmond, CA, USA). After SDS-PAGE the proteins were electrotransferred to a nitrocellulose filter. To prevent non-specific binding the membranes were preincubated in 5% fat-free milk in PBS at 22°C for 1·5 h. The membranes were incubated with a murine monoclonal antibody (MoAb) against CD59 (Bric 229; Bio-Products Laboratory, Elstree, UK) at 0·1 µg/ml or with a goat antibody against properdin (ATAB) at 8 µg/ml both in 5% fat-free milk in PBS (17 h) at 4°C. After three washes with PBS, peroxidase-conjugated rabbit antimouse or donkey antigoat IgG was added. To control for non-specific binding of the secondary antibody the primary antibody was omitted. The bound anti-CD59 and antiproperdin antibodies were detected by the ECL chemiluminescence method of Amersham (Amersham, UK).
Relative amounts of soluble and membrane forms of CD59 in MEE
A Triton X-114 (TX-114; Sigma Chemical Co, St Louis, MO, USA) phase separation method [26] was used for the separation of soluble and membrane forms of CD59 in MEE [20]. Fifteen MEE samples 40 µl each were pooled into three pools and TX-114 (200 µl) was added. Samples containing 2% TX-114 in PBS were first incubated for 1 h at + 4°C and thereafter centrifuged at 13 000 g for 5 min at + 4°C to remove insoluble debris. The supernatants were incubated at 37°C for 10 min until cloudy. After centrifugation at + 23°C for 10 min the aqueous and detergent phases were separated. The detergent phase was subjected to acetone precipitation prior to SDS-PAGE with 10 volumes of acetone at −20°C for 1 h, followed by pelleting of the precipitate at 13 000 g for 10 min. Thereafter three samples, from both the aqueous and detergent phases and a positive control prepared from a red blood cell lysate, were subjected to SDS-PAGE electrophoresis and immunoblotting with anti-CD59 antibody (BRIC 229) under non-reducing conditions.
Statistical analysis
A Fisher's exact test was used to compare the ‘infected’ and ‘non-infected’ MEEs by their cytolytic activity.
RESULTS
Characteristics of patients and samples
The study material consisted of 62 MEE samples from 49 children. The ages of the children varied from 7 months to 9 years 3 months (mean 2·6 years). The mean duration of OME was 3·2 months (range 2–10 months). The average amount of MEE was 161 µl (range 27–378 µl). Bacteria were recovered by culture from 18 of the 53 MEE specimens (34%) investigated. Moraxella catarrhalis was isolated in seven cases (13·2%), Haemophilus influenzae in four (7·5%), coagulase-negative staphylococci in three (5·7%), Staphylococcus aureus in two (3·8%) and Streptococcus pneumoniae in two cases (3·8%). Of all effusions, 66% showed no growth of bacteria. Seven patients had received antibiotics during a 3-week period prior to the MEE collection. In one of these cases bacteria were recovered from the MEE sample (H. influenzae).
Procytolytic and cytolytic activity in the MEE samples
The high proportion (two-thirds) of MEE samples negative for bacterial growth despite a continuing disease led us to examine the direct cytolytic activity of the MEE samples. The effect of the MEE samples on serum-induced lysis was analysed on the same plates using a set-up where it was possible to simultaneously observe direct complement lysis, enhancement of lysis and possible inhibition of lysis (Fig. 1, Fig. 2 and Table 1). In this set-up MEE samples were allowed to diffuse against fresh NHS in agar-gels containing guinea pig or chicken erythrocytes. Direct lytic activity was observed in seven cases of the 22 mucoid MEEs (Figs 1b–d, 2b,d). From the 13 mucopurulent, seromucous or serous MEEs six induced lysis of erythrocytes (Figs 1a, 2a,c).
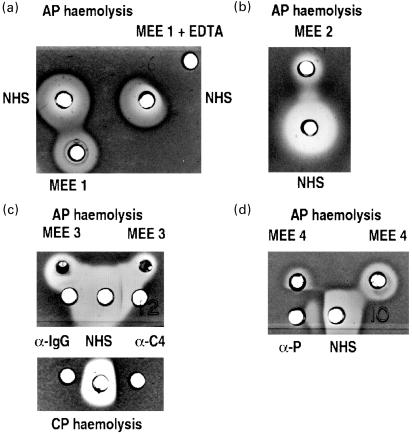
Fig. 1.
Middle ear effusions (MEE) and haemolytic activity. MEE samples from three OME patients and normal human serum (NHS) were applied in wells in AP haemolysis gel. The effect of MEE to induce haemolysis of chicken erythrocytes (ChE) or to enhance the lysis induced by NHS was observed. In panel A MEE has been directly lytic against ChE but it also enhanced the lysis induced by NHS. Both the direct lysis and the enhancement of lysis were inhibited when EDTA was added to MEE (MEE + EDTA). The NHS induced lysis of ChE was also inhibited in the area where EDTA had diffused (a). MEE number 2 had weak own cytolytic and an ability to enhance lysis caused by NHS (b). An antibody against C4 (α-C4) or a non-specific control antibody (antihuman IgG; α-IgG) did not inhibit lysis induced by MEE on an alternative pathway (AP) haemolysis gel (c). In a control both antibodies were found to inhibit the classical pathway (CP) haemolysis of NHS. An antibody against the alternative pathway accelerator properdin partially inhibited the direct MEE or NHS-induced lysis and the lysis enhanced by MEE (d).
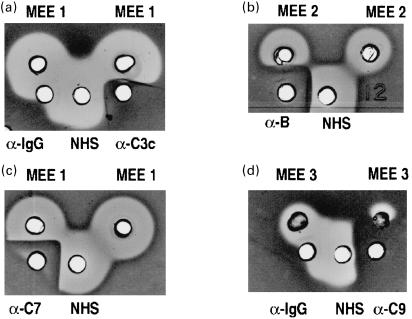
Fig. 2.
The effects of antibodies against different complement components on the haemolytic activity of different MEE samples. In addition to their own lysis the MEE samples enhanced lysis induced by NHS. An antibody against C3c (α-C3c) inhibited both the direct and enhanced lysis. A control antibody (antihuman IgG; α-IgG) had no inhibitory effect on C lysis (a). An antibody against factor B (α-B) inhibits both the direct and enhanced lysis (b). Antibodies against C7 and C9 inhibited direct lysis of MEE (c,d).
Table 1.
Cytolytic activity and enhancement of lysis by 38 MEEs*
| Type of effusion | ||
|---|---|---|
| Type of lysis | Mucoid (n = 25) | Other (n = 13) |
| Direct | 7 (28%) | 6 (46%) |
| Enhancement | 17 (68%) | 10 (76%) |
| Both | 7 (28%) | 4 (31%) |
| Inhibition | 3 (13%) | 0 |
Mucoid and other types of MEE (mucopurulent, seromucous and serous) were tested for their direct cytolytic activity or an ability to enhance or inhibit lysis of unsensitized guinea pig or chicken erythrocytes induced by NHS (see Fig. 1).
An enhancement of lysis of the unsensitized cells was observed with 27 of the 38 MEE samples (Fig. 1a–d, Fig. 2b and Table 1). Seventeen of these MEEs were mucoid and 10 mucopurulent, seromucous or serous. In 16 samples the enhancement could be detected in the absence of direct haemolytic activity (not shown). An additional observation in the qualitative assay was that three of the MEEs had a weak complement lysis inhibiting activity (not shown). Antibodies against complement components C7 and C9 inhibited both the direct and enhanced lysis (Fig. 2c,d). Ten MEEs causing an enhancement of lysis were further investigated to determine the possible origin of this phenomenon. In addition to antibodies against terminal C components also antibodies against C3c and factor B were found to inhibit the enhanced lysis (Fig. 2a,b). Antibodies against the classical pathway component C4, examined as a control, had no effect on this type of lysis, although it inhibited the classical pathway (Fig. 1c). Thus, the enhancement of lysis was interpreted to require the alternative pathway amplification cascade and not only the terminal pathway as in reactive lysis, a type of lysis that originates from the level of C56 complexes. As a control we used an antihuman IgG antibody. This antibody did not inhibit the C-mediated lysis (Figs 1c, 2a,d). With an antibody against the alternative pathway regulator properdin, variable results were obtained. In most cases the polyclonal antibody against properdin inhibited the lysis whereas in others the inhibition was only partial (Fig. 1d). Both the direct and enhanced lysis were inhibited by EDTA on the alternative pathway haemolysis gel (Fig. 1a), indicating the need for divalent cation (Mg++ in the alternative pathway)-dependent C3/C5-convertase enzymes. No difference in the ability to induce complement lysis between ‘non-infected’ and ‘infected’ MEEs was seen in Fisher's exact test (P = 0·106)
Properdin in MEE
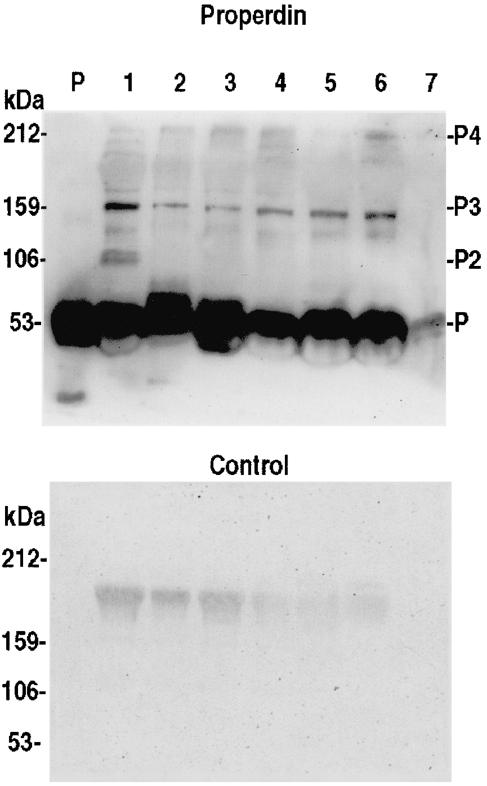
Properdin is a positive regulator of the alternative C pathway. Immunoblotting analysis of MEE samples showed that properdin was present in the middle ear fluids of OME patients (Fig. 3). The intensity of properdin bands of MEE samples (at dilutions of 1/5–1/10) corresponded to that of properdin in plasma (at a dilution of 1/10). Properdin could be detected in all the 19 MEE samples analysed. The band at 53 kDa represents the monomer form, which was abundantly seen. The dimer form at 106 kDa could not be detected while bands at 159 kDa representing the trimer were readily detectable. In some samples the 212 kDa tetramer form of properdin was also seen.
Fig. 3.
Detection of properdin in six different MEE samples by Western blotting (lanes 2–7). The MEE samples (10 µl, diluted 1/5–1/10) were run on a 15% SDS-PAGE gel under nonreducing conditions and immunoblotted with a polyclonal antiproperdin antibody. Purified properdin (P; 0·1 µg/lane) and normal human serum (lane 1, diluted 1/10) were used as positive controls. A monomeric form of properdin (m.w. 53 kDa) can be detected in all samples. The bands at 106, 159 and 212 kDa represent the dimeric, trimeric and tetrameric forms of properdin, respectively (P2, P3, P4). In the control immunoblot the antiproperdin antibody was omitted. Lanes 2–7 represent samples 3, 6, 12, 13, 18 and 22 in Table 2, respectively.
Quantification SC5b-9 in MEE
In addition to MAC formation on cell membranes the terminal C components can assemble in the fluid phase into the SC5b-9 soluble complexes. To determine the extent of terminal complement pathway activation in MEEs the levels of C5b-9 complexes were measured by a C5b-9 neoepitope-specific ELISA. In comparison to levels usually observed in plasma after C activation the levels of TCC were highly elevated in all MEEs (Table 2). The mean value was 34·1 µg/ml (range 5–89 µg/ml).
Table 2.
The nature of middle ear effusion, duration of OME, TCC levels and the presence of CD59 in MEE
| Sample | Effusion* | Duration (mo)† | TCC (µg/ml)‡ | CD59§ |
|---|---|---|---|---|
| 1 | M | 2 | 21·5 | + + |
| 2 | M | 10 | 45 | + |
| 3 | M | 10 | 23·4 | n.d. |
| 4 | M | 4 | 24·4 | + + |
| 5 | SM | 2 | 61·6 | + |
| 6 | M | 2 | 19·1 | + + |
| 7 | M | 3 | 37·4 | n.d. |
| 8 | M | 3 | 16·8 | n.d. |
| 9 | M | 2·5 | 23·5 | + + |
| 10 | SM | 4 | 45·2 | + + |
| 11 | SM | 4 | 89·3 | + + |
| 12 | M | 3·5 | 25·9 | + + |
| 13 | M | 2 | 31·9 | + + |
| 14 | M | 2 | 40 | n.d. |
| 15 | M | 5 | 55·4 | n.d. |
| 16 | M | 5 | 65·2 | – |
| 17 | M | 3 | 4·8 | + + |
| 18 | M | 6 | 32·1 | + + + |
| 19 | M | 6 | 16·2 | + + + |
| 20 | M | 5·5 | 31·3 | + |
| 21 | M | 2 | 7 | + + |
| 22 | M | 2 | n.d. | + + |
| 23 | MP | 3 | n.d. | + + + |
| 24 | SM | 5 | n.d. | + + |
The nature of the effusion was mucoid (M) in 19 MEE samples, seromucous (SM) in four and mucopurulent (MP) in one MEE sample.
Duration of the effusion (months).
TCC (SC5b-9) levels were measured by a neoepitope-specific EIA.
Semiquantitative estimation (band intensity) of CD59 in the MEE samples by immunoblotting: + + + = strong, + + = moderate, + = weak, - = no CD59 detected and n.d. = not done. Due to the limited amount of MEE not all determinations could be carried out from all samples.
CD59 in MEE
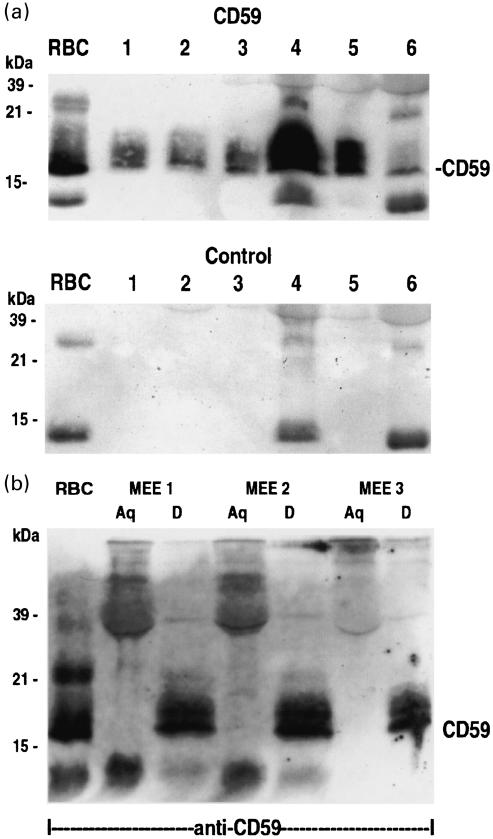
As we observed earlier, that CD59 was strongly expressed on the middle ear epithelial cells but reduced on the outer surface of the cells in OME [15], we now wanted establish whether it became detached from the cells to the MEE. Western blotting analysis of MEE samples with the Bric 229 monoclonal anti-CD59 antibody showed a typical 19–21 kDa CD59 smear after SDS-PAGE under non-reducing conditions (Fig. 4a). The size of the CD59 band of MEEs corresponded to that of CD59 in a red blood cell lysate (Fig. 4). CD59 could be detected in 17 of the 21 MEE samples analysed. In controls where the anti-CD59 antibody was omitted, non-specifically stained bands were seen, but could be discriminated easily from the CD59-specific bands. In normal plasma samples CD59 was hardly detectable by this technique (not shown). The data from Western blotting analysis in relation to the other parameters is presented in Table 2. No apparent correlation between the presence of CD59 and TCC levels was observed.
Fig. 4.
(a) Western blotting analysis of CD59 in six different MEE samples. The MEE samples were diluted in a non-reducing sample buffer and run on a 15% SDS-PAGE gel, transferred to a nitrocellulose membrane and immunostained with the BRIC 229 (anti-CD59) MoAb. As a positive control CD59 prepared from a lysate of human red blood cells (RBC) was used. CD59 can be detected on all samples shown. In the control the anti-CD59 antibody was omitted. Lanes 1–6 represent samples 6, 12, 17, 18, 19 and 20 in Table 2, respectively. (b) Western blotting analysis of three pools of MEE samples after Triton X-114 phase separation. Aqueous (Aq) and detergent (D) phases from the TX-114 phase separation were immunoblotted using the BRIC 229 anti-CD mAb. A positive control was prepared from a red blood cell lysate (RBC). CD59 (mw 18 kDa) can be detected in the RBC, and only in the detergent phases (D) of the pooled MEE samples at 19–21 kDa.
To examine whether CD59 had retained its phospholipid-moiety in the MEE a Triton X-114 phase separation and Western blotting analysis three sets of pools from 15 MEEs was performed. In all three pools of MEE samples CD59 could be seen in the detergent phase at 19–21 kDa, but not in the aqueous phase. This indicates that CD59 retains it phospholipid-tail while shedding form the epithelium into the MEE.
DISCUSSION
We have hypothesized that OME is a self-perpetuating inflammatory condition where the endogenous mediators, particularly components of the complement system, can maintain the disease. In this study we found that MEEs induced lysis of target cells (guinea pig or chicken erythrocytes) either directly or by enhancing the cytolytic activity of serum. Properdin and other components of the alternative pathway were identified as factors promoting also the latter type of cytolytic activity. High levels of SC5b-9 complexes in the MEEs indicated that C activation had progressed to the terminal stage in vivo.
The fact that MEE-induced lysis occurred in the presence of MgEGTA and was inhibited by anti-C7 and C9 antibodies, but not with anti-C4 antibodies, indicated that the lysis occurred through the alternative and/or the terminal C pathway. Inhibition of lysis by anti-C3c, anti-B and antiproperdin antibodies indicated that lysis was initiated through the alternative pathway. The properdin-dependence of the lysis and the presence of functionally active trimeric and tetrameric forms of properdin [27,28] indicated that properdin is important in promoting complement activation in OME. In cases where properdin antibodies did not inhibit the lysis it may have been initiated by properdin-free C3/C5 convertases or by preformed C5b6 complexes that lead to cytolysis when encountering the MAC components C7, C8 and C9. C8 and C9 are produced in the liver and enter inflammatory sites from the circulating plasma whereas C7 is primarily produced by polymorphonuclear leucocytes (PMN), monocytes and macrophages [29,30]. These cells have been demonstrated in MEE [31]. However, the whole alternative pathway activation cascade is needed, as we could not observe C lysis in the presence of C3c antibody. If the activation would undergo reactive lysis, which refers to the lysis of unsensitized bystander cells by the terminal C components (C5b6, C7, C8 and C9) acting independently of the early C components [32–34], MEE could also lyse cells in the presence of inhibitory antibodies against the early C components. However, no such type of lysis was detected, indicating that the lysis occurred through the alternative pathway. C activation leads to release of anaphylatoxins (C3a, C5a) in the MEE [8] attracting PMNs into the middle ear cavity. Collectively, the local conditions thus favour formation of terminal C complexes. Our model system simulated conditions in the middle ear cavity. Alternative pathway and early late pathway (C5, C6) components were presumed to originate from the MEE, C7 from the neighbouring serum and C8 and C9 from either source. When merging together in vivo, the components could form potentially cytotoxic MAC complexes, which may affect the integrity of the middle ear epithelium. On the other hand, the opsonizing or cytolytic complement activity can keep the middle ear free from bacteria. The MEE fluids have been shown to be bactericidal at least against non-typable H. influenzae. This property was demonstrated to be complement-dependent [35]. The cytolytic C activity of the MEE fluids may thus explain why so few bacteria can be cultured from MEE. However, other factors must also contribute to the survival of bacteria in the middle ear because we found cytotoxic complement activity also in samples with bacterial growth. It is possible that bacteria persisting within the middle ear have mechanisms to evade complement attack.
The concentrations of C activation products in MEE were among the highest found under any pathological condition. Compared to TCC levels for example in the urine of membranous glomerulonephritis patients (5·6 + 0·2 µg/ml; mean + s.d.) [20] or those in plasmas of infants undergoing cardiopulmonary bypass (0·12–1·34 µg/ml) [36] the values in MEE were more than 10-fold. In our earlier study we have shown that C activation does not usually proceed to the MAC stage on the surface of the middle ear epithelial cells by demonstrating low or absent expression of the C5b-9 neoepitope [15]. It is likely that the membrane regulators MCP and CD59 divert C activation away from membranes to the fluid phase. Nevertheless, high amounts of TCC in middle ear effusions indicate that the epithelial cells and migrating leucocytes are exposed to complement attacks. Non-lethal amounts of MAC on nucleated cells initiate Ca2+ influx and induce release of further mediators of inflammation. These include phospholipase A2 that can catalyse the release of arachidonic acid from cell membranes [37–39] as well as that of prostaglandins, leucotrienes, cytokines and reactive oxygen metabolites, all demonstrated to be present in MEE and suggested to be important in the pathogenesis of otitis media [8,40,41].
As judged from the immunoblotting results the MEE fluids contained relatively high amounts of CD59. In light of the low amounts of CD59 in plasma and a strong CD59 expression in the middle ear epithelium it appears likely that CD59 in MEE has become shed from the middle ear epithelial cells. This was supported by the decreased surface expression and a granular or vesicular staining pattern of the epithelium in the immunofluorescence studies [15]. Similar observations have been made with patients suffering from membranous glomerulonephritis where CD59 becomes shed from the glomerular and tubular cells into urine [20]. While the CD59 protein was shown to retain its phospholipid moiety it can still be able to act as an inhibitor of MAC. This, together with CD59 remaining in the middle ear epithelial cells would confer resistance to MAC. In addition, the cells are also able to remove potentially lytic MAC complexes from cell membranes and recover [42]. Thus, the damage to the epithelial cell lining may remain limited and lead primarily only to sublethal inflammatory changes.
As delineated in the present study conditions promoting complement activation and a continuing inflammation can maintain chronic otitis media with effusion. Thus, instead of — or in addition to — antibiotics other approaches to suppress persistent inflammation in OME should be considered. The engineering of complement regulators may provide new substances to be used as anti-inflammatory agents and in the prevention of complement-mediated local tissue damage [43].
Acknowledgments
We thank Marjatta Ahonen for skilful technical assistance and Tessa Lehtinen for lithium analyses. This study was supported by The Academy of Finland, The Sigrid Jusélius Foundation, Finska Läkaresällskapet Foundation, The University of Helsinki and The Helsinki University Central Hospital Funds.
REFERENCES
- 1.Klein JO, Tos M, Burchard H, et al. Panel reports, definition and classification. Ann Otol Rhinol Laryngol. 1989;98(Suppl. 139):10. [PubMed] [Google Scholar]
- 2.Harkness P, Topham J. Classification of otitis media. Laryngoscope. 1998;108:1539–43. doi: 10.1097/00005537-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Casselbrant ML, Brostoff LM, Flaherty MR, et al. Otitis media with effusion in preschool children. Laryngoscope. 1985;95:428–36. doi: 10.1288/00005537-198504000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Tos M, Holm-Jensen S, Sorensen CH, et al. Spontaneous course and frequency of secretory otitis in 4-year-old children. Arch Otolaryngol. 1982;108:4–10. doi: 10.1001/archotol.1982.00790490006002. [DOI] [PubMed] [Google Scholar]
- 5.Freemantle N. The treatment of persistent glue ear in childrenEffective health care. Leeds: University of Leeds; 1992. [Google Scholar]
- 6.Meri S, Lehtinen T, Palva T. Complement in chronic secretory otitis media. Arch Otolaryngol. 1984;110:774–8. doi: 10.1001/archotol.1984.00800380004002. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein JM, Schenkein HA, Genco RJ, et al. Complement activity in middle ear effusions. Clin Exp Immunol. 1978;33:340–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Närkiö-Mäkelä M, Teppo A-M, Meri S. Complement C3 cleavage and cytokines interleukin-1β and tumour necrosis-factor-α in otitis media with effusion. Laryngoscope. 2000;110:1745–9. doi: 10.1097/00005537-200010000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Volanakis JE. Overview of the complement system. In: Volanakis JE, Frank MM, editors. The human complement system in health and disease. New York: Marcel Dekker Inc; 1998. pp. 9–32. [Google Scholar]
- 10.Pillemer L, Blum L, Lepow IH, et al. The properdin system and immunity: demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954;120:279–85. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- 11.Wirthmueller U, Dewald B, Thelen M, et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol. 1997;158:4444–51. [PubMed] [Google Scholar]
- 12.Schwaeble W, Dippold WG, Schäfer MK-H, et al. Properdin, a positive regulator of complement activation, is expressed in human T cell lines and peripheral blood T cells. J Immunol. 1993;151:2521–8. [PubMed] [Google Scholar]
- 13.Meri S, Waldmann H, Lachmann PJ. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991;65:532–7. [PubMed] [Google Scholar]
- 14.Nose M, Katoh M, Okada N, et al. Tissue distribution of HRF20, a novel factor preventing the membrane attack of homologous complement, and its predominant expression on endothelial cells in vivo. Immunology. 1990;70:145–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Närkiö-Mäkelä M, Jero J, Meri S. Complement activation and expression of membrane regulators in the middle ear mucosa in otitis media with effusion. Clin Exp Immunol. 1999;116:401–9. doi: 10.1046/j.1365-2249.1999.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meri S, Morgan BP, Davies A, et al. Human protectin (CD59), an 18, 000–20, 000 MW complement lysis restricting factor, inhibits C5b−8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;71:1–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Davies A, Simmons DL, Hale G, et al. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989;170:637–54. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holguin MH, Fredrick LR, Bernshaw NJ, et al. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989;84:7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rollins SA, Zhao J, Ninomiya H, et al. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991;146:2345–51. [PubMed] [Google Scholar]
- 20.Lehto L, Honkanen E, Teppo A-M, et al. Urinary excretion of protectin (CD59), complement SC5b-9 and cytokines in membranous glomerulonephritis. Kidney Int. 1995;7:1403–11. doi: 10.1038/ki.1995.197. [DOI] [PubMed] [Google Scholar]
- 21.Lehto T, Meri S. Interactions of soluble CD59 with the terminal complement complexes: CD59 and C9 compete for a nascent epitope on C8. J Immunol. 1993;151:4941–9. [PubMed] [Google Scholar]
- 22.Rooney IA, Heuser JE, Atkinson JP. GPI-anchored complement regulatory proteins in seminal plasma. J Clin Invest. 1996;97:1675–86. doi: 10.1172/JCI118594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rooney IA, Morgan BP. Characterization of the membrane attack complex inhibitory protein CD59 antigen on human amniotic cells and in amniotic fluid. Immunology. 1992;76:541–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Virolainen A, Mäkelä MJ, Esko E, et al. New method to assess dilution of secretions for immunological and microbiological assays. J Clin Microbiol. 1993;31:1382–4. doi: 10.1128/jcm.31.5.1382-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature (Lond) 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Brusca JS, Radolf JD. Isolation of integral membran proteins by phase separation with triton X-114. In: Walter H, Johansson G, editors. Aqueous two-phase systems. San Diego, CA: Academic Press; 1994. pp. 183–91. [Google Scholar]
- 27.Schwaeble WJ, Reid KBM. Does properdin crosslink the cellular and the humoral immune response? Immunol Today. 1999;20:17–21. doi: 10.1016/s0167-5699(98)01376-0. [DOI] [PubMed] [Google Scholar]
- 28.Pangburn MK. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J Immunol. 1989;142:202–7. [PubMed] [Google Scholar]
- 29.Naughton MA, Walport MJ, Würtzner R, et al. Organ-specific contribution to circulating C7 levels by the bone marrow and liver in humans. Eur J Immunol. 1996;26:2108–12. doi: 10.1002/eji.1830260922. [DOI] [PubMed] [Google Scholar]
- 30.Høgåsen AKM, Würzner R, Abrahamsen TG, et al. Human polymorphonuclear leukocytes store large amounts of terminal complement components C7 and C6, which may be released on stimulation. J Immunol. 1995;154:4734–40. [PubMed] [Google Scholar]
- 31.Nassif PS, Simpson SQ, Izzo AA, et al. Interleukin 8 concentration predicts the neutrophil count in middle ear effusion. Laryngoscope. 1997;107:1223–7. doi: 10.1097/00005537-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Lachmann PJ, Thompson RA. Reactive lysis: the complement mediated lysis of unsensitized cells. II. The characterization of the reactor factor as C56, and the role of C8 and C9. J Exp Med. 1970;131:643–7. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLeod B, Baker P, Gewurz H. Studies on the inhibition of C56 initiated lysis (reactive lysis) I. Description of the phenomenon and methods of assay. Immunology. 1974;26:1145–57. [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson RA, Rowe DS. Reactive haemolysis- A distinctive form of red cell lysis. Immunology. 1968;14:745–62. [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu T, Harada T, Majima Y, et al. Bactericidal activity of middle ear effusion on a single isolate of non-typable Haemophilus influenzae. Int J Ped Otolaryngol. 1988;16:211–17. doi: 10.1016/0165-5876(88)90032-8. [DOI] [PubMed] [Google Scholar]
- 36.Ashraf SS, Tian Y, Zacharrias S, et al. Effects of cardiopulmonary bypass on neonatal and paediatric inflammatory profiles. Eur J Cardiothoracic Surg. 1997;12:862–8. doi: 10.1016/s1010-7940(97)00261-3. [DOI] [PubMed] [Google Scholar]
- 37.Panesar M, Papillon J, McTavish AJ, et al. Activation of phospholipase A2 by complement C5b-9 in glomerular epithelial cells. J Immunol. 1997;159:3584–94. [PubMed] [Google Scholar]
- 38.Tedesco F, Pausa M, Nardon E, et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–27. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264:1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung TTK. Prostaglandins, leukotrienes, and other arachidonic acid metabolites in the pathogenesis of otitis media. Laryngoscope. 1988;98:980–93. doi: 10.1288/00005537-198809000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Brodsky L, Faden H, Bernstein J, et al. Arachidonic acid metabolites in middle ear effusions of children. Ann Otol Rhinol Laryngol. 1991;100:589–92. doi: 10.1177/000348949110000714. [DOI] [PubMed] [Google Scholar]
- 42.Campbell AK, Morgan BP. Monoclonal antibodies demonstrate protection of polymorphonuclear leucocytes against complement attack. Nature. 1985;317:164–6. doi: 10.1038/317164a0. [DOI] [PubMed] [Google Scholar]
- 43.Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:59–87. [PubMed] [Google Scholar]