Abstract
To determine and compare the T cell response to M protein and other group A streptococcal (GAS) antigens, T cell lines (TCL) were cultured from the lesional skin of 33 psoriatic patients and 17 disease controls. GAS-reactive skin TCL were tested in proliferation assays with recombinant M6 protein, and extracts of cell wall and membrane from type M6 GAS and its corresponding M gene deletion mutant. Initially, GAS-reactive skin TCL were obtained from 16 of 25 (64%) psoriasis, and from seven of 17 (41%) control patients. Eleven psoriatic and four control GAS-reactive TCL proliferated to M6 cell wall extract, whereas all the TCL from both groups responded to the extract of M6 membrane proteins. This difference in response to the two extracts was significant for both groups of patients (psoriasis, P = 0·0335, controls, P = 0·0156). GAS-reactive TCL from a further eight psoriasis patients showed no difference in response to cell wall extract from M6 GAS (containing the M protein minus its C-terminus) compared to that of its corresponding M gene deletion mutant. Furthermore, GAS-reactive TCL did not proliferate to recombinant M6 protein. However, a small, but significant reduction in proliferation by the eight psoriatic GAS-reactive TCL to the M-negative (lacking the M protein C-terminus) compared to M6-positive membrane extract was observed (P = 0·01). These findings suggest that GAS-reactive T cells in skin lesions of chronic plaque psoriasis proliferate to streptococcal membrane and, to a lesser extent, cell wall proteins. However, psoriatic skin T cells do not recognize cell wall M protein.
Keywords: chronic plaque psoriasis, proliferation, streptococci, T cells
INTRODUCTION
Group A streptococci (GAS) are a well-recognized trigger for acute guttate psoriasis, which is associated with positive streptococcal throat cultures and raised anti-streptococcal titres [1]. Exacerbation of chronic plaque psoriasis has also been reported following streptococcal infections, suggesting that antigens from these organisms may also play a role in the chronic form of the disease [1–3].
An increased proliferative response to GAS antigens has been demonstrated in both guttate [4] and chronic plaque [5] psoriatic PBMC. In addition, T cell lines (TCL) reactive with GAS antigens have been cultured more frequently from lesional skin of both guttate and CP psoriasis patients compared with that of patients with other inflammatory skin disorders such as eczema, lichen planus or pityriasis rosea [6,7]. TCL isolated from guttate skin lesions showed strong proliferative responses to GAS of different M serotypes; T cell clones isolated from one of the GAS-reactive TCL responded in a HLA-DR-restricted manner and produced high levels of IFN-γ, but no IL-4 or TNF-α [6].
The identity of the streptococcal antigens recognized by GAS-reactive skin TCL in psoriasis has yet to be determined. One potential candidate antigen is M protein (the main antigenic determinant and virulence factor of GAS) a fibrillar molecule with an extended, α-helical coiled coil secondary structure which extends from the surface of the bacteria. M protein consists of a variable N-terminal half which defines more than 100 different serotypes and a conserved C-terminus [8]. When the M protein is translocated to the surface of the streptococcal cell wall, the C-terminal hydrophobic domain and charged tail are removed (remaining behind in the membrane) so that the protein can be properly anchored [9]. Unlike other post-streptococcal sequelae such as rheumatic fever and glomerulonephritis, the triggering of guttate psoriasis does not appear to be associated with any particular serotype [10]. Furthermore, acute psoriasis associated with group C and group G cutaneous streptococcal infections has also been reported [11]. Interestingly, these groups of streptococci also express M or M-like proteins [12,13].
Increased M protein recognition has been reported in the peripheral blood of both guttate and chronic plaque psoriasis patients, as suggested by significantly decreased T cell reactivity to trypsinized vs. untrypsinized type M22 GAS [14]. An increased response by chronic plaque patients to M protein was shown more specifically using four peptides from the C-terminal part of the M6 protein which shared sequences with keratin [15]. Significantly increased IFN-γ production by psoriatic PBMC was observed in response to 3/4 of the peptides compared to that of healthy controls and patients with atopic dermatitis [15].
Several other cell wall proteins have also recently been characterized [16–18], which could potentially be recognized by T cells in psoriatic lesions. In addition, GAS membrane consists of a minimum of 60 polypeptides which have yet to be identified [19].
The aim of this study was to determine whether GAS-reactive skin TCL from chronic plaque psoriasis patients proliferated to M protein and/or other streptococcal cell wall and membrane proteins. This was determined using purified recombinant M6 (rM6) protein, and extracts of cell wall and membrane from M6 GAS and its corresponding isogenic M gene deletion mutant. Psoriatic T cell responses were compared to those of a group of disease controls consisting of patients with other inflammatory skin diseases.
MATERIALS AND METHODS
Patients and biopsies
Initially 25, and then later a further eight untreated patients with chronic plaque psoriasis (total 33) and 17 untreated disease controls (nine lichen planus, three pityriasis rosea, two dermatitis herpetiformis, three non-specific dermatitis) were studied.
The psoriasis group consisted of 22 males and 11 females, age range 18–80 years with < 1–75% skin involvement and varying degrees of disease activity. Of 17 patients tested for serological evidence of streptococcal infection only one, whose skin flare had followed a sore throat, had raised antistreptolysin-O, anti-DNase B and antihyaluronidase titres, and another had a raised anti-DNase B titre only.
The disease control group consisted of 10 males and 7 females, age range 27–83 years.
Two 3-mm punch biopsies (25 psoriasis and all control patients) or a shave biopsy (eight psoriasis patients) of lesional skin and 40 ml heparinized blood were taken.
This study was approved by St Mary's Local Research Ethics Committee (Kensington, Chelsea and Westminster Health Authority). Patient consent was obtained prior to collection of samples.
Streptococcal extracts
Strep-A is a mixture of four heat-killed and sonicated streptococcal throat isolates from patients with psoriasis [6]. One of the isolates was serotyped as M4, T4 and two as M12, T12; it was not possible to type the fourth. Using the lysomotrophic agent NH4Cl, this streptococcal preparation has been shown to require antigen processing for presentation to T cells (unpublished data).
The streptococcal cell wall and membrane extracts were prepared from the D471 strain of type M6 GAS and a corresponding M gene deletion mutant, strain JRS 75 [20]. Briefly, 50 ml of GAS-containing Todd-Hewitt broth was centrifuged and the pelleted bacteria washed and resuspended in 30% raffinose in 50 mm phosphate buffer, pH 6·1 containing 5 mm EDTA and 5 mm dithiothreitol. Phage-associated lysin was added and incubated for 90 min at 37°C to remove the streptococcal cell wall, and the resulting protoplasts were sedimented at 15 000 g for 30 min. The supernatant (cell wall extract) which contained M protein and other surface proteins together with digested cell wall components was collected, dialysed and stored at − 20°C.
The pelleted protoplasts, which retain the COOH-terminal 19 hydrophobic amino acids and charged tail of the M molecule after cell wall removal with lysin [21], were resuspended in 1 ml of PBS, subjected to three freeze/thaw cycles, and centrifuged at 100 000 g for 45 min. The membrane pellet was resuspended in 1 ml of PBS and stored at − 20°C.
Recombinant M6 (rM6) protein was purified from Escherichia coli as described previously [22].
Optimal concentrations of each streptococcal preparation were determined using a range of dilutions in skin TCL proliferation assays (data not shown).
Isolation of PBMC
PBMC were isolated from heparinized blood by Ficoll-Hypaque gradient centrifugation (Lymphoprep, Robins Scientific Europe, Knowle, UK) and resuspended in RPMI 1640 medium (Gibco BRL, Paisley, UK) supplemented with antibiotics, 2 mm glutamine (Gibco BRL) and 10% human A+ serum (supplemented medium).
Isolation of skin TCL
The skin punch biopsies were washed in saline to remove any extraneous blood, trimmed free of fat, chopped into small fragments and digested briefly with 1 mg/ml collagenase (Sigma-Aldrich Co. Ltd, Poole, UK) for 30 min at 37°C. Previous studies have shown that skin TCL obtained from whole skin punch biopsies consist predominately of dermal T cells as indicated by the prevalence of CD4+ over CD8+ T cells [6]. The shave biopsies (which were taken at sufficient depth to reveal bleeding points) were washed in saline and digested in dispase (Roche Molecular Biochemicals, Lewes, UK) for 1 h at 37°C. The epidermis was then removed for use in other studies, and the remaining dermis washed twice and cut into small fragments.
After two washes in medium, TCL were established by culturing the skin fragments in supplemented medium containing 1 µg/ml Strep-A for 3 days, adding 20 U/ml IL-2 (Lymphocult-T, Biotest UK Ltd, Solihull, UK), and then culturing for a further 8–11 days, adding IL-2 every 3–4 days. Antigen-presenting cells were not added at this stage since dermal dendritic cells migrate out of the skin together with the T cells forming clusters. The TCL were further expanded by culture with a mixture of 1 × 106 30 Gy-irradiated, autologous PBMC, 1 µg/ml Strep-A and 20 U/ml IL-2.
In some cases, skin fragments were divided into two, one half grown with Strep-A and the other with rM6 (10 µg/ml).
Proliferation assay
After 10–14 days culture, 2·5 × 104 skin TCL plus equal numbers of irradiated, autologous PBMC were cultured for 3 days in the presence or absence of optimal concentrations of each of the streptococcal preparations in 96-well plates. Tritiated thymidine (4 µCi/ml, specific activity 5 Ci/mmole; Amersham International, Amersham, UK) was added for the last 6 h of culture; proliferation was assessed by thymidine incorporation measured in a β scintillation counter and expressed as the mean cpm of three replicates.
The stimulation index (SI) was calculated as the mean cpm in the presence of antigen divided by the mean cpm in the absence of antigen.
Phenotyping of skin TCL
Skin TCL were phenotyped by incubating on ice with Leu 4-FITC (anti-CD3), or Leu 2a-PE/Leu 3a-FITC mixture (Simultest, anti-CD8 and anti-CD4, respectively) (Becton Dickinson Ltd, Oxford, UK). Staining with control mouse IgG (Coulter Electronics Ltd), followed by FITC-conjugated sheep antimouse Ig (Sigma-Aldrich Co. Ltd) was used to measure non-specific staining. Stained cells were fixed in 2% paraformaldehyde in PBS and kept at 4°C until analysis on an EPICS Profile flow cytometer (Coulter Electronics).
Statistics
Data were analysed with GraphPad Instat software using paired and unpaired t tests or the Wilcoxon signed rank test, depending upon whether or not the data conformed to a Gaussian distribution.
RESULTS
GAS-reactive skin TCL, as assessed by proliferation to Strep-A, were obtained from 16 of the initial group of 25 (64%) psoriatic and seven of 17 (41%) control patients (data not shown).
Phenotype of psoriatic skin TCL
Ten psoriatic skin TCL were phenotyped; all were 95·8–100% CD3+ and eight were 73·4–100% CD4+ and 0–16·7% CD8+. One TCL was predominately CD8+ (82%) and a second was comprised mainly of CD4−, CD8− T cells with only 6·4% and 27·4% of T cells staining for CD4 and CD8 antigens, respectively. One TCL, which was predominately CD4+, also contained 20·7% CD4+, CD8+ T cells.
Increased response to membrane vs. cell wall GAS proteins
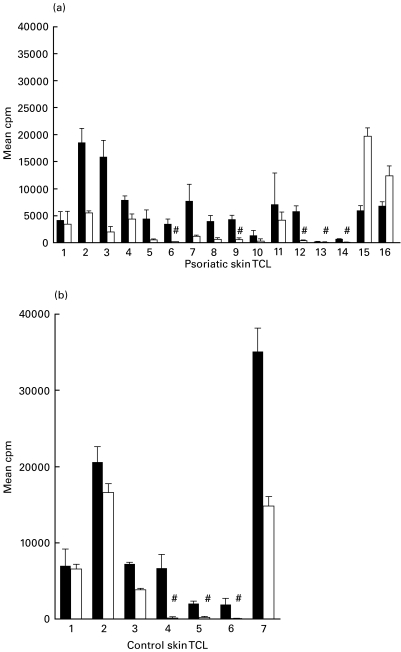
All of the psoriatic and control TCL were significantly stimulated (SI > 3) by the GAS membrane extracts and showed a wide range of responses (Fig. 1). In contrast, significant responses to the M6 GAS cell wall extract were observed in only 11 (68·7%) psoriatic and four (57%) control TCL compared to medium alone (Fig. 1). When the response to the two streptococcal extracts was compared, both groups of TCL showed significantly greater proliferation to the membrane protein mixture (psoriasis, P = 0·0335; controls, P = 0·0156).
Fig. 1.
Increased response to membrane versus cell wall GAS proteins by skin TCL. Proliferative response in mean cpm ± s.d of skin TCL from (a) psoriasis and (b) control patients to cell wall and membrane extracts from M6 GAS.Background mean cpm in medium alone ranged from 70 to 571 (psoriasis) and 231–2390 (controls). # indicates skin TCL that had a SI of < 3. M6 membrane versus M6 cell wall: psoriasis, P = 0·0335; controls P = 0·0156. ▪, M6 membrane; □, M6 cell wall.
No difference in the skin T cell response to the cell wall and membrane extracts was observed between the psoriasis and control groups.
The second group of eight GAS-reactive skin TCL was used for the following experiments.
Psoriatic TCL do not respond to cell wall M protein
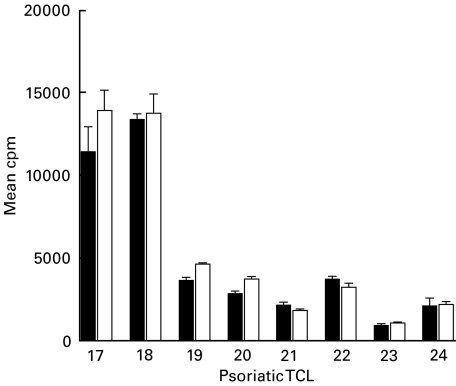
To determine if GAS-reactive skin TCL recognized M protein, a major antigenic determinant present on the cell wall of the organism, their proliferative responses to cell wall extract from type M6 GAS and its isogenic M gene deletion mutant were compared (Fig. 2). All eight TCL had significant responses (SI > 3) to M6 cell wall extract; no difference in the response to the corresponding M-negative extract was detected.
Fig. 2.
No difference in response of psoriatic skin TCL to M-negative compared to M6-positive GAS cell wall extracts. Proliferative response (mean cpm ± s.d.) of psoriatic skin TCL to cell wall extract from type M6 GAS (M6) compared to that of the corresponding M gene deletion mutant (M-). Background mean cpm in medium alone ranged from 85 to 420. ▪, M6 cell wall; □, M‐cell wall.
Furthermore, rM6 failed to induce a proliferative response in any of the other 11 psoriatic TCL tested (data not shown). When six psoriatic skin samples were cultured with rM6, only four grew out TCL. Three of these four TCL had sufficient T cell numbers to test with rM6 in a proliferation assay and all were unresponsive (data not shown).
Psoriatic TCL recognize M and non-M membrane proteins
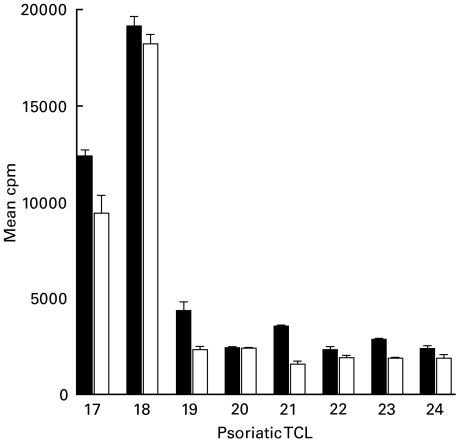
The hydrophobic amino acids and charged tail region at the M protein C-terminus remain in the streptococcal membrane after removal of the streptococcal cell wall with lysin. Thus comparison of responses to membrane extracts from type M6 GAS and its corresponding M gene deletion mutant allows detection of T cell responses to this highly conserved part of the molecule. All the psoriatic TCL showed significant responses to M6 membrane extract. Furthermore, a small, but significant decrease in the response to M-negative compared to M6-positive membrane extract was observed (P = 0·01) (Fig. 3). However, in most cases, the majority of the T cell response was to non-M membrane proteins present in both extracts.
Fig. 3.
Small decrease in response of psoriatic skin TCL to M-negative compared to M6-positive GAS membrane extracts.Proliferative response (mean cpm ± s.d.) of psoriatic skin TCL to membrane extract from type M6 GAS (M6) compared to that of the corresponding M gene deletion mutant (M-). Background mean cpm as in Fig. 2 legend. M6 versus M-membrane: P = 0·01. ▪, M6 membrane; □, M6 cell wall.
DISCUSSION
This study has demonstrated that TCL isolated from skin lesions from both psoriasis and other inflammatory skin diseases show similar responses to GAS antigens with significantly stronger proliferation to membrane compared to cell wall streptococcal proteins. The responses by psoriatic TCL were directed predominately against non-M proteins in both the cell wall and membrane extracts, except for a small, but significant response to the highly conserved hydrophobic domain and charged tail region of M protein embedded in the GAS membrane.
The presence of GAS-reactive T cells in skin lesions of both psoriasis and other inflammatory skin diseases is not surprising, since streptococcal infections are common and most individuals have probably experienced them at some time in their lives. In both groups of patients skin T cell proliferative responses were, in most cases, much higher to membrane compared to cell wall proteins. A similar differential proliferative response to these GAS proteins has been observed in PBMC from psoriatic and nonpsoriatic individuals (B. S. Baker et al., submitted). This may indicate that streptococcal membrane proteins are more antigenic and/or that the T cell repertoire specific for membrane proteins is more extensive than that for the cell wall proteins.
To determine if skin T cells from psoriatic patients respond to M protein, a major antigenic determinant expressed by GAS, cell wall and membrane extracts from M6 GAS were compared to those of an isogenic M gene deletion mutant. The cell wall extract from M6 GAS contains the majority of the M protein molecule with the exception of the hydrophobic domain and charged tail at the C-terminus which remain behind in the cell membrane [21]. No difference between the response of psoriatic T cells to cell wall extracts with or without M protein was detected. Furthermore, the psoriatic skin TCL did not proliferate to rM6 protein confirming a lack of response to cell wall M protein. In support of these findings, the IFN-γ response by psoriatic skin TCL to GAS cell wall proteins has also been shown to be directed against non-M proteins [23,24].
In contrast, a subpopulation of psoriatic skin T cells was reactive to the highly conserved C-terminus of the M protein molecule present in the membrane as shown by a small but significant decrease in response to the M gene deletion mutant compared to M6 GAS extract. The majority of the proliferative response to the membrane proteins was, however, directed to non-M proteins present in extracts of both M-positive and M-negative GAS.
The GAS-reactive TCL were cultured from lesional skin using a mixture of types M4, M12 and an unknown M type of GAS (Strep-A [6]) but were subsequently tested with cell wall proteins from type M6 GAS. This would allow only T cells specific for the C-terminal half of the M molecule, which is conserved between M serotypes, to be detected. The conserved part of the M protein molecule is considered to be of more potential relevance to the psoriatic process based upon the lack of association of particular serotypes with the triggering of psoriasis [10]. Therefore, it cannot be excluded that T cells specific for the serotype-specific N-terminus of M protein are present in these skin TCL; these would not be stimulated by the M6 cell wall extract leading to an underestimation of the GAS cell wall response.
These findings for T cells cultured from skin lesions contrast with those reported for peripheral blood T cells from psoriatic patients [15]. Similarly, a discrepancy between intestinal and peripheral blood T cell responses to gluten has been reported in patients with coeliac disease [25]. In the peripheral blood study, an increased IFN-γ response to peptides homologous for M protein and keratin 17 was demonstrated by circulating T cells from psoriasis patients, compared to controls or patients with atopic dermatitis [15]. In addition, we have shown a significant decrease in proliferation by psoriatic PBMC to cell wall extract from the M gene deletion mutant compared to that from M6 GAS (B. S. Baker et al., submitted). Thus cell wall M protein-specific T cells are present in the blood of psoriatic patients but do not appear to migrate to the skin. It should be borne in mind, however, that after two rounds of in vitro expansion with Strep-A the relative proportions of T cells specific for different GAS antigens may have altered with respect to the situation in vivo.
It is likely therefore that streptococcal protein(s) other than M protein are responsible for T cell activation in the skin in psoriasis. In addition, non-streptococcal proteins (bacterial/autoantigen/viral) are also implicated in some patients by our inability to culture GAS-reactive T cells from one-third of psoriasis patients studied [7, this study].
This study has shown that there are T cells in the lesional skin of some patients with psoriasis, or other inflammatory skin diseases, which proliferate to antigens present on the membrane and, to a lesser extent, cell wall of GAS. However, cell wall M protein does not appear to be recognized by psoriatic skin T cells. The identity of the streptococcal protein(s) involved in the induction and maintenance of the psoriatic process remains to be established.
Acknowledgments
The authors are grateful to Dr I. Jonsdottir for supplying the streptococcal isolate, Strep-A. This work was supported by a grant from the British Skin Foundation to Dr B. Baker.
REFERENCES
- 1.Norholm-Pedersen A. Infections and psoriasis. Acta Dermatol Venereol (Stockh) 1952;32:159–67. [PubMed] [Google Scholar]
- 2.Norrlind R. Significance of infections in origin of psoriasis. Acta Rheumatol Scand. 1955;1:135–44. [PubMed] [Google Scholar]
- 3.Cohen-Tervaert WC, Esseveld H. A study of the incidence of haemolytic streptococci in the throat in patients with psoriasis vulgaris, with reference to their role in the pathogenesis of this disease. Dermatologica. 1970;140:282–90. doi: 10.1159/000252565. [DOI] [PubMed] [Google Scholar]
- 4.Gross WL, Packhauser U, Hahn G, et al. Lymphocyte activation by streptococcal antigens in psoriasis. Br J Dermatol. 1977;97:529–36. doi: 10.1111/j.1365-2133.1977.tb14130.x. [DOI] [PubMed] [Google Scholar]
- 5.Baker BS, Powles AV, Malkani AK, et al. Altered cell-mediated immunity to group A haemolytic streptococcal antigens in chronic plaque psoriasis. Br J Dermatol. 1991;125:38–42. doi: 10.1111/j.1365-2133.1991.tb06036.x. [DOI] [PubMed] [Google Scholar]
- 6.Baker BS, Bokth S, Powles AV, et al. Group A streptococcal antigen-specific T lymphocytes in guttate psoriatic lesions. Br J Dermatol. 1993;128:493–9. doi: 10.1111/j.1365-2133.1993.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 7.Baker BS, Brown D, Porter W, et al. T lymphocytes reactive for group A streptococcal antigens in chronic plaque psoriatic lesions. Arch Dermatol Res. 1999;291:564–6. doi: 10.1007/s004030050455. [DOI] [PubMed] [Google Scholar]
- 8.Fischetti VA. Streptococcal M protein: molecular design and biological behaviour. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarre WW, Schneewind O. Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39–42. [PubMed] [Google Scholar]
- 11.Henderson CA, Highet AS. Acute psoriasis associated with Lancefield group C and group G cutaneous streptococcal infections. Br J Dermatol. 1988;118:559–61. doi: 10.1111/j.1365-2133.1988.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 12.Woolcock JB. Purification and antigenicity of an M-like protein of Streptococcus equi. Infect Immun. 1974;10:116–22. doi: 10.1128/iai.10.1.116-122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KF, Fischetti VA. Biological and immunochemical identity of M protein on group G streptococci with M protein on group A streptococci. Infect Immun. 1987;55:502–6. doi: 10.1128/iai.55.3.502-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker BS, Bokth S, Garioch JJ, et al. Decreased T cell reactivity to trypsinized group A, type M22 streptococci in psoriasis. Acta Dermatol Venereol (Stockh) 1994;74:276–8. doi: 10.2340/0001555574276278. [DOI] [PubMed] [Google Scholar]
- 15.Sigmundsdottir H, Sigurgeirsson B, Troye-Blomberg M, et al. Circulating T cells of patients with active psoriasis respond to streptococcal M peptides sharing sequences with human epidermal keratins. Scand J Immunol. 1997;45:688–97. doi: 10.1046/j.1365-3083.1997.d01-438.x. [DOI] [PubMed] [Google Scholar]
- 16.Pancholi V, Fischetti VA. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–26. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneewind O, Jones KF, Fischetti VA. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J Bacteriol. 1990;172:3310–7. doi: 10.1128/jb.172.6.3310-3317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansky E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van de Rijn I, Zabriskie JB, McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterisation of the streptococcal antigen. J Exp Med. 1977;146:579–99. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischetti VA, Jones KF, Scott JR. Size variation of the M protein in group A streptococci. J Exp Med. 1985;161:1384–401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pancholi V, Fischetti VA. Isolation and characterisation of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988;170:2618–24. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischetti VA, Jones KF, Manjula BN, Scott JR. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification and comparison with streptococcal-derived M protein. J Exp Med. 1984;159:1083–95. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown DW, Baker BS, Ovigne J-M, et al. Skin CD4+ T cells produce interferon-γ in vitro in response to streptococcal antigens in chronic plaque psoriasis. J Invest Dermatol. 2000;114:576–80. doi: 10.1046/j.1523-1747.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown DW, Baker BS, Ovigne J‐M, et al. Non‐M protein(s) on the cell wall and membrane of group A streptococci induce(s) IFN‐γ production by dermal CD4+ T cells in psoriasis. Arch Dermatol Res. 2001;293:165–70. doi: 10.1007/s004030100218. [DOI] [PubMed] [Google Scholar]
- 25.McAdam SN, Sollid LM. Getting to grips with gluten. Gut. 2000;47:743–5. doi: 10.1136/gut.47.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]