Abstract
The aim of this study was to evaluate the effects of the immunomodulating drug mycophenolic acid (MPA) on splenocytes in an animal model of systemic lupus erythematosus (SLE), using MRLlpr/lpr mice. MPA reversibly inhibits inosine 5′-monophosphate dehydrogenase, an enzyme involved in the de novo guanosine synthesis. Splenocytes were treated with MPA (at 1 or 10 µm), and stimulated with either lipopolysaccharide (LPS; 10 µg/ml) or concanavalin A (ConA; 1·25 µg/ml). In blocking experiments, guanosine (100 µm) was added to the cultures to inhibit the effects of MPA. Lymphocyte proliferation, enumeration of immunoglobulin producing cells (using ELISPOT) and quantification of anti-double-stranded (ds) DNA antibodies, IFN-γ and IL-10 (by ELISA) in supernatants were performed. In addition, cell viability was evaluated using propidium iodide and flow cytometry. We found that MPA-treated splenocytes had dramatically decreased mitogen-induced proliferation and number of immunoglobulin producing cells, down-regulated production of IFN-γ, IL-10 and IgM anti-dsDNA antibodies. The viability of MPA-treated cells was also decreased. All of the effect modulated by MPA could be neutralized by the addition of guanosine. We conclude that MPA has potent immunomodulating effects on both B and T lymphocytes, modulating not only proliferation, but also the production of cytokines, immunoglobulins and autoantibodies.
Keywords: cytokines, immunoglobulins, MPA, lymphocytes, MRLlpr/lpr
INTRODUCTION
Mycophenolic acid (MPA) is an immunomodulating drug that inhibits inosine 5′-monophosphate dehydrogenase, an enzyme that catalyses the conversion of inosine monophosphate (IMP) to xanthosine monophosphate (XMP) in the de novo guanosine synthesis [1,2]. MPA treatment of lymphocytes depletes intracellular pools of guanosine triphosphate (GTP) and deoxyguanosine triphosphate (dGTP) and inhibits proliferation. Guanosine (guo) addition neutralizes the antiproliferative effects of MPA [3]. Mycophenolate mofetil (MMF), a prodrug of MPA with improved bioavailability [4,5], is used widely in transplantation medicine to prevent graft rejection. Recently, MMF has been proposed as a candidate drug for treating autoimmune and inflammatory diseases, such as nephritis [6,7], skin disorders [8,9] and HIV infection [10].
Previous studies have demonstrated the efficacy of MMF treatment in vivo of SLE-prone NZB/W [11,12] and MRLlpr/lpr mice [13–15]. MRLlpr/lpr mice develop (i) massive enlargements of the lymph nodes and spleens, (ii) autoantibodies, (iii) hypergammaglobulinaemia (iv) severe immune complex-mediated glomerulonephritis, (v) non-erosive arthritis and (vi) vasculitis [16]. Furthermore, MRLlpr/lpr mice accumulate double negative (CD3+, B220+, CD4− and CD8−) T cells and display profound defects in T cell responsiveness, i.e. an inability to respond to ConA-induced proliferation and a deficiency in IL-2 production [17,18]. Our previous studies showed that MMF-treated MRLlpr/lpr mice lived longer, developed less severe glomerulonephritis, displayed reduced accumulations of double negative T cells, had decreased immunoglobulin and autoantibody production and increased ex vivo proliferative responses as well as increased IL-10 and IFN-γ levels in splenocyte culture supernatants following ConA stimulation [14,15]. However, van Bruggen and colleagues demonstrated only slight MMF-directed immunomodulating effects in MRLlpr/lpr mice [13]. Given these apparently contradictory findings regarding the immunomodulating properties of MMF in SLE mice, we examined the direct effects of MPA both on lymphocyte function and on the production of immunoglobulins and autoantibodies. Therefore, the aim of this study was to evaluate in vitro MPA immunomodulation of splenocytes taken from either SLE-prone MRLlpr/lpr mice or healthy C57BL/6 mice.
MATERIALS AND METHODS
Mice
MRLlpr/lpr and C57BL/6 mice were originally purchased from Bomholtgård (Ry, Denmark). MRLlpr/lpr mice were bred in the animal facility of the Department of Rheumatology, University of Göteborg. The mice were housed 3–10 animals in each cage under standard conditions of temperature and light and were fed standard laboratory chow ad libitum. Female mice aged between 3 and 4 months (MRLlpr/lpr) and between 10 and 15 months (C57BL/6) were used in the experiments.
Single cell preparation
Spleens were freshly isolated, mashed and passed through a nylon wool sieve to give a single cell suspension. Cells from two or three spleens were pooled and considered as one observation. Cells were centrifuged at 515 g for 5 min and the pelleted cells were resuspended in Tris-buffered 0·83% ammonium chloride in order to lyse erythrocytes. The total number of cells was calculated after two washes in PBS before being used.
In vitro treatment with MPA
Spleen cells (1 × 106/ml) were incubated in 96-, 24- or 6-well flat-bottomed microtitre plates (Nunc, Roskilde, Denmark) in volumes of 0·2, 2 or 10 ml, respectively. A complete medium consisting of Iscove's medium (GIBCO, Paisley, UK) supplemented with 10% fetal calf serum (FCS) (Biological Ind., Beit Haemek, Israel), 2 mm glutamine, 5 × 10−5 m 2-mercaptoethanol and 50 µg/ml gentamycin was used. MPA (Roche Pharmaceutical, Basel, Switzerland) was dissolved in dimethylsulphoxide (DMSO; Merck, Darmstadt, Germany) and further diluted in medium to give final concentrations of 0·1, 1 and 10 µm MPA. Cells incubated in complete medium alone were used as controls, and in some experiments DMSO (without MPA) was included to exclude any potential effects of DMSO alone. Guanosine hydrate (guo; product number G12000, Sigma-Aldrich, St Louis, MO, USA) was dissolved in 0·4 m NaOH and diluted further in complete medium to a final concentration of 100 µm in order to neutralize the effects of MPA. Ten µm guo had no effect on MPA treatment while 75 µm was as effective as 100 µm (data not shown). In order to stimulate the splenocytes to produce cytokines, immunoglobulins and to proliferate, the B cell mitogen lipopolysaccaride (LPS; Sigma) or the T cell mitogen concanavalin A (ConA; Miles Yeda, Rehovot, Israel) was included [19] at the final concentrations of 10 or 1·25 µg/ml, respectively. Cell cultures were incubated at 37°C in 5% CO2 and 95% humidity for 3–4 days.
Lymphocyte proliferation
Spleen cells (1 × 106/ml) were incubated for 72 h in 96-well flat-bottomed microtitre plates with LPS or ConA or in the absence of either mitogen and subsequently treated with MPA and/or guo as described above. For the final 18 h of culture 1 µCi[3H]-thymidine (Amersham, Buckinghamshire, UK) was added to each well. The cultures were harvested into glass-fibre filters, processed and counted in a β-counter. The cells were set up in triplicate, and the results are expressed as the mean of the counts per minute (cpm).
Enumeration of immunoglobulin-producing cells
The ELISPOT technique [20] was used, with modifications, as described previously [14] for enumeration of IgM and IgG producing cells in splenocyte cultures. The numbers of immunoglobulin-secreting cells were expressed as the frequency of spot-forming cells per 106 mononuclear cells (SFC/106 MNC).
Cytokine and anti-double-stranded (ds) DNA antibody analyses
After 72 or 96 h of incubation of splenocytes as described above, the supernatants were collected and stored in −20°C awaiting cytokine (96 h ConA stimulated MRLlpr/lpr supernatants) and anti-dsDNA antibody analyses (72 h C57BL/6 and MRLlpr/lpr supernatants stimulated with LPS) with ELISA. IFN-γ ELISA was performed as described previously [15]. The Opt-EIA™ Set for murine IL-10 (Pharmingen, San Diego, CA, USA) was used according to the manufacturer's instructions. Anti-dsDNA antibodies of IgM isotype were measured by ELISA as described previously [14].
Cell viability
The viability of freshly isolated spleen cells was determined by the trypan blue exclusion method. MRLlpr/lpr splenocyte viability in 72-h cultures in the presence or absence of MPA and/or guo and further stimulated with LPS, was investigated using flow cytometry. Briefly, cells were harvested from the plates, counted and washed in PBS. The cells were resuspended in 200–300 µl of PBS and 10 µl of 50 µg/ml propidium iodide (Sigma) was added 30–60 s before analysis in a FACSTAR (Beckton-Dickinson).
Statistical analysis
The unpaired Student's t-test was used as the statistical method. Results are presented as the mean ± the standard deviation (s.d.). In Figs 1, 2 and 3 all individual observations are shown. For statistical analysis the results from MPA-treated cells were compared with the results from cells not treated with MPA; P < 0·05 was considered statistically significant.
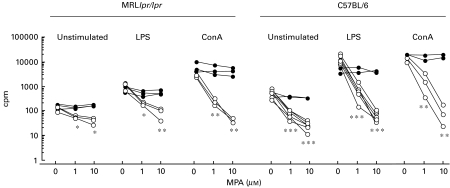
Fig. 1.
Proliferative responses (cpm) of spleen cell cultures from C57BL/6 (n = 6) and MRLlpr/lpr mice (n = 3) 72 h after in vitro treatment with different doses of MPA in the presence of (•) or in the absence of (○) 100 µm guanosine. The cells (1 × 106/ml) were either untreated or stimulated with LPS (10 µg/ml) or ConA (1·25 µg/ml). Individual mouse spleen cell cultures are shown. Regarding C57BL/6 mice, not all splenocyte cultures were stimulated with ConA or given guo, explaining the discrepancy of numbers of observations in the figure. Irrespective of the of mouse strain or whether or not mitogen was present, both 1 and 10 µm MPA significantly decreased proliferative responses compared to cultures where no MPA was added. Similar results were obtained in three pilot experiments for each mouse strain, data not shown. *P < 0·05, **P < 0·01 and ***P < 0·001.
All data were also analysed with the non-parametric Mann–Whitney test with the same results regarding statistical significance (not shown).
RESULTS
MPA has potent anti-proliferative effects on splenocyte cultures in vitro
Spleen cells from 4-month-old MRLlpr/lpr and 10–14-month-old C57BL/6 mice were treated with different concentrations of MPA and stimulated with either LPS or ConA or left non-stimulated for 72 h in vitro. The results presented in Fig. 1 show that MPA had a dose-dependent antiproliferative effect on both MRLlpr/lpr and C57BL/6 spleen cells, and that the highest dose of MPA (10 µm) completely inhibited proliferative responses to either LPS or ConA. As expected, lymphocytes from MRLlpr/lpr mice displayed weaker mitogen-stimulated proliferative responses compared to lymphocytes from C57BL/6 mice. The addition of guo (100 µm) completely abrogated the antiproliferative effects of MPA on both non-stimulated and mitogen-exposed cells. The addition of DMSO to the cell cultures did not affect proliferation (data not shown).
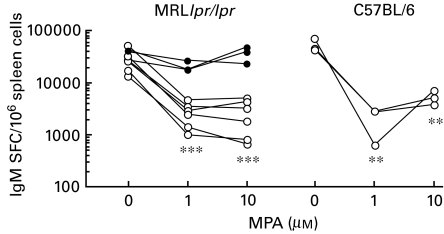
MPA inhibits LPS induced Ig production
Isolated spleen cells, pooled from either MRLlpr/lpr mice (two experiments n = 3 in each) or C57BL/6 mice (three individuals from one experiment) were stimulated with LPS and treated with MPA in the presence or absence of guo for 3 days. The frequencies of IgM- and IgG-producing cells were examined.
The frequency of IgM-producing cells was significantly decreased after MPA treatment at doses of 1 and 10 µm in both mouse strains (Fig. 2). The neutralizing effect of guo on MPA-treated MRLlpr/lpr splenocytes is shown in Fig. 2. Similar levels of guo neutralization were found in a pilot study with C57BL/6 splenocytes (data not shown). The number of IgM SFC/106 spleen cells in freshly isolated spleens (day 0) was 1470 ± 240 for C57BL/6 mice (n = 3) and 2580 ± 1240 for MRLlpr/lpr mice (n = 6).
Fig. 2.
IgM SFC per 1 × 106 spleen cells from 3–4-month-old MRLlpr/lpr mice (from two experiments, n = 3 in each) and 10–11-month-old C57BL/6 mice (n = 3) after 72 h in vitro treatment with MPA (0, 1 or 10 µm) ± 100 µm guanosine. LPS (10 µg/ml) was used as the mitogen in all cultures. Results of MPA treatment of separate spleen cell pools are shown. Similar results were obtained in two pilot experiments for MRLlpr/lpr mice and in one for C57BL/6 mice, data not shown. **P < 0·01 and ***P < 0·001. ○, No guanosine; •, 100 μm guanosine.
The suppressive effect of MPA on IgG SFC was less pronounced than on IgM (Table 1). In C57BL/6 mice MPA significantly decreased the frequency of SFC. Furthermore, MPA decreased IgG SFC in MRLlpr/lpr in one of two experiments using the high dose of MPA. The addition of guo to the MRLlpr/lpr splenocyte cultures neutralized the effects of MPA. DMSO alone did not affect the spot producing capacity of the cells (data not shown). As expected, the levels of IgG SFC in freshly isolated spleen cells were several-fold higher in MRLlpr/lpr than in C57BL/6 mice.
Table 1.
IgG spot-forming cells (SFC) per million spleen cells from MRLlpr/lpr and C57BL/6 mice. Splenocytes were isolated and treated with different concentrations of MPA in the presence or absence of 100 µm guanosine in vitro for 72 h. LPS (10 µg/ml) was used as mitogen in all cultures. The spontaneous production of IgG by freshly isolated splenocytes is also presented. For statistical analysis the MPA treated cells were compared with cells not treated with MPA. Similar results were obtained in two pilot experiments with MRLlpr/lpr mice and in one with C57BL/6 mice
| IgG SFC per million spleen cells (103) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MPA (µm) | MPA (µm) + Guanosine (100 µm) | ||||||||||
| Mice | n | Age (months) | Before treatment | 0 | 0·1 | 1 | 10 | 0 | 0·1 | 1 | 10 |
| C57BL/6 | 3 | 11 | 1·6 ± 0·7 | 7·3 ± 0·7 | 4·8 ± 1·1* | 2·2 ± 1·3** | 2·2 ± 0·9** | n.d. | n.d. | n.d. | n.d. |
| MRLlpr/lpr | 3 | 3 | 10·2 ± 4·5 | 10·4 ± 3·3 | 9·6 ± 4·8 | 4·5 ± 2·7 | 3·7 ± 0·4* | n.d. | n.d. | n.d. | n.d. |
| MRLlpr/lpr | 3 | 4 | 6·1 ± 1·1 | 5·4 ± 2·3 | n.d. | 3·6 ± 0·9 | 3·1 ± 1·7 | 5·4 ± 0·9 | n.d. | 5·0 ± 1·4 | 4·7 ± 2·0 |
P < 0·05
P < 0·01. Data are presented as mean ± s.d., n.d. = not done.
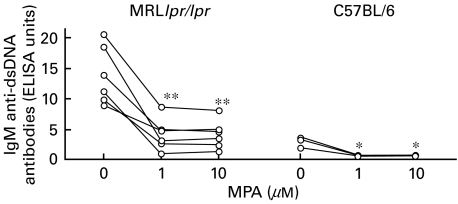
MPA reduces IgM anti-dsDNA antibody levels
Splenocyte culture supernatants from MRLlpr/lpr and C57BL/6 mice, treated in vitro with MPA in the absence or presence of guo and stimulated with LPS, were taken after 72 h and analysed for IgM anti-dsDNA antibodies. MPA significantly decreased the levels of IgM anti-dsDNA antibodies in spleen cell cultures from both MRLlpr/lpr and C57BL/6 mice (Fig. 3). As expected, the levels of dsDNA antibodies in cultures without MPA were higher in MRLlpr/lpr mice than in C57BL/6 mice.
Fig. 3.
IgM anti-dsDNA antibodies in supernatants from spleen cells, isolated from MRLlpr/lpr mice (from two experiments, n = 3 in each) and C57BL/6 mice (n = 3), cultured with MPA and stimulated with LPS (10 µg/ml) in vitro for 72 h. MPA treatment of separate spleen cell pools are shown. *P < 0·05 and **P < 0·01.
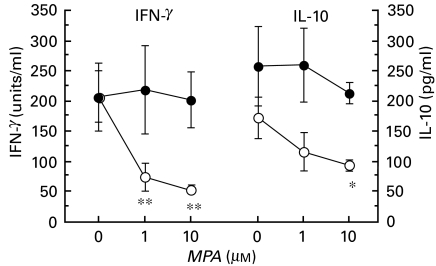
MPA reduces ConA-induced cytokine production
Cultured splenocyte supernatants from MRLlpr/lpr mice, treated in vitro with MPA in the presence or absence of guo and stimulated with ConA, were taken after 96 h and assayed for IFN-γ and IL-10. Both IFN-γ and IL-10 levels were reduced in a dose-dependent manner after MPA treatment (Fig. 4). Adding guo to cultures treated with MPA restored the IFN-γ and IL-10 levels to those of untreated splenocyte supernatants. The addition of DMSO alone did not affect the cytokine levels in splenocyte culture supernatants.
Fig. 4.
Cytokine levels in culture supernatants of spleen cells, isolated from 4 month-old MRLlpr/lpr mice (n = 3). The cells were cultured with MPA in the presence or absence of guanosine, and stimulated with ConA in vitro for 96 h. The IFN-γ (units, where 1 unit = 87·7 pg/ml) and IL-10 (pg/ml) levels were measured with ELISA. Data are presented as mean ± s.d. N = 3 for each mean value. Similar results for IFN-γ were obtained in two pilot experiments, data not shown. *P < 0·05 and **P < 0·01. ○, No guanosine; •, 100 μm guanosine.
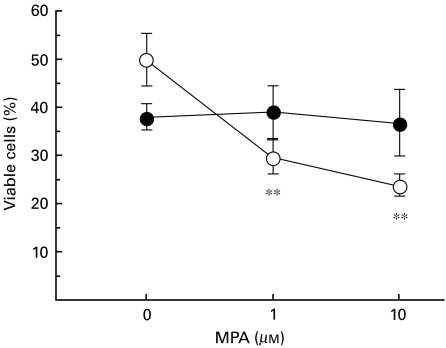
Guanosine abrogates MPA-induced cell death in LPS-stimulated spleen cells
The viability of freshly isolated spleen cells always exceeded 95% as determined by trypan blue exclusion. We considered the possibility that MPA might be cytotoxic which could affect our results. To investigate this, we stained cultured MRLlpr/lpr spleen cells with propidium iodide. Figure 5 shows that MPA decreased the viability of spleen cells in LPS-stimulated cultures. The addition of guo to the cultures blocked the MPA-induced decrease in viability, suggesting that MPA-induced cell death was due to IMPDH depletion. Thus, MPA was not cytotoxic per se in the concentrations used, but deprived cells of guo. However, guo alone reduced the viability of LPS-stimulated MRLlpr/lpr spleen cells in culture. The addition of DMSO did not affect viability (data not shown).
Fig. 5.
MRLlpr/lpr (n = 3) spleen cell viability after 3 days in culture in the presence or absence of MPA and guo. All cultures were stimulated with LPS (10 µg/ml). Cells were harvested, washed and transferred to FACS tubes. Propidium iodide was added to the cells 1 min prior to analysis. Similar results were obtained in two pilot experiments, data not shown. Mean ± s.d. is presented. **P < 0·01. ○, No guanosine; •, 100 μm guanosine.
DISCUSSION
This study shows that IMPDH depletion with MPA has significant modulatory effects on murine T and B lymphocyte functions in vitro, both in healthy C57BL/6 mice and in SLE-prone MRLlpr/lpr mice. It is important to note that the concentrations of MPA used in these experiments were within the range found in human plasma 2 h after a single oral dose of 1 g MMF [21].
MPA dramatically decrease the proliferative response of spleen cells to T and B cell mitogens, an effect neutralized by addition of guo, suggesting that MPA acted via depletion of guanine nucleotides. Previously, it was demonstrated in vitro that MMF treated MRLlpr/lpr spleen cells displayed suppressed proliferative responses to ConA [13]. These findings corroborate earlier in vitro studies where human peripheral blood cells stimulated with phytohaemagglutinin (PHA) and treated with 1 µm MPA showed a suppressed proliferation, an effect reversible by addition of guo [3]. Recently, we have shown that spleen cells from MRLlpr/lpr mice treated with MMF in vivo displayed increased proliferative responses to ConA compared to controls [15]. In addition, we demonstrated that spleen cells from MMF treated MRLlpr/lpr mice were more normal (i.e. had less double negative T cells) than untreated mice and therefore responded better to ConA.
MPA also decreased the ability of B lymphocytes, from both MRLlpr/lpr and C57BL/6 mice, to produce immunoglobulins actively after stimulation with LPS in vitro, an effect reversed by adding guo to the cultures. As expected, the effect of MPA was most pronounced in IgM-producing cells, the dominant Ig isotype following LPS stimulation [22,23]. Furthermore, the frequency of IgG-secreting cells was decreased by addition of MPA. Notably, MPA treatment of MRLlpr/lpr spleen cell cultures stimulated with LPS suppressed levels of IgG-producing cells below those of freshly isolated cells. These results corroborate our previous in vivo experiments demonstrating decreased immunoglobulin production in MRLlpr/lpr mice after MMF treatment [14]. MRLlpr/lpr mice display increased IgG SFC and have greatly increased levels of IgG in serum. When these mice were treated with MMF, the number of IgG-producing cells was decreased and serum levels of IgG and IgG autoantibodies were lowered [14]. Thus, in vitro, MPA-induced or in vivo, MMF-induced guanosine depletion caused a decrease in antibody production in MRLlpr/lpr mice.
MPA significantly decreased the levels of IgM anti-dsDNA antibodies in supernatants of LPS-stimulated splenocytes from both MRLlpr/lpr and C57BL/6 mice. IgG anti-dsDNA antibodies are considered the most pathogenic autoantibodies in SLE disease. In MRLlpr/lpr mice, serum IgG anti-dsDNA antibodies dominate over IgM, and it has been shown that after in vivo treatment with MMF only IgG anti-dsDNA antibody levels decreased [14]. However, in our present experiments, IgM was favoured over IgG, and therefore we analysed the effects of MPA on IgM anti-dsDNA antibody levels. The in vitro results with MPA reported here support our previous results that MMF has the ability to down-regulate anti-dsDNA antibody levels in vivo.
IFN-γ and IL-10 production was down-regulated by MPA treatment and the effect was reversed by adding guo to the cultures. In our previous study we found no differences in the production of these cytokines in the serum of mice treated with MMF. When performing pure in vitro experiments, interfering factors present in vivo are minimized. In the complex in vivo situation there might be compensatory mechanisms affecting the cytokine balance and thereby influencing the results. On the contrary, IFN-γ and IL-10 in supernatants from ConA stimulated spleen cells treated in vivo with MMF were significantly increased, a phenomenon coupled to the increased ex vivo proliferative response discussed above [15].
An additional effect of MPA is the potential to induce apoptosis. Cohn et al. have shown that MPA increases apoptosis in human lymphoid and monocytic cell lines [24], and Li et al. showed that prolonged depletion of GTP induced by MPA led to the apoptosis of insulin-secreting β-cells in vitro [25]. However, Hauser et al. could not detect apoptosis in a study where they showed antiproliferative effects of MMF on mesangial cells [26]. Recently it was shown that MPA induced the apoptosis and cell death of activated CD4+ T lymphocytes from HIV infected humans in vitro. Treatment of HIV patients with MMF decreased the number of dividing CD4+ and CD8+ T cells without affecting the total CD4+ and CD8+ populations in peripheral blood cells [10]. Thus, MMF can selectively induce cell death in certain cell populations, and in our previous study [15] showed that the frequency of double-negative T cells in spleen was decreased, whereas no difference was found regarding the frequency of CD4+ and CD8+ T cells. In our present study, the viability of MRLlpr/lpr cells was only slightly decreased after MPA treatment. By adding guo to the cultures, MPA-induced cell death was decreased and in the presence of both guo and MPA the drug was not cytotoxic.
Taken together, the results suggest strongly that depletion of guanine nucleotides attenuate lymphocytic functions, including proliferation and cytokine and immunoglobulin production. In the present study we focused on lymphocytes and their activities following exposure to MPA. We used unfractionated spleen cells and therefore other cells, such as macrophages, were present in the cultures. Direct or indirect effects of MPA on macrophages, adhesion molecules or nitric oxide production cannot be excluded. In the context of SLE these factors have significance, since they might contribute to the beneficial effects of MMF seen in SLE-prone mice. SLE is an immune complex-mediated disease, involving autoantibody production by B lymphocytes as well as T lymphocyte helper functions. MMF affects both cell populations and has the potential to suppress ongoing autoimmune processes in lupus. Indeed, case reports of SLE patients successfully treated with MMF where other therapies failed indicate that the drug could be therapeutic in this disease [27–29]. Interestingly, Chan and colleagues recently reported that for the treatment of diffuse proliferative lupus nephritis MMF was as effective as cyclophosphamide [6].
In summary, we show that MPA treatment of MRLlpr/lpr spleen cells in vitro modulated activities of the acquired immune system, including lymphocyte proliferation and the production of cytokines, immunoglobulins and autoantibodies.
Acknowledgments
We thank Dr Vincent Collins for critically reading the manuscript. This study was supported by grants from the Börje Dahlin foundation, the Göteborg Medical Society, the Swedish association against Rheumatism, the King Gustav V's 80 years foundation, the Anna-Greta Craaford foundation, Reumaforsknings fond Margareta, the Medical Faculty of the University of Göteborg (LUA) and the Swedish Medical Research Council.
REFERENCES
- 1.Sintchak MD, Fleming MA, Futer O, et al. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell. 1996;85:921–30. doi: 10.1016/s0092-8674(00)81275-1. [DOI] [PubMed] [Google Scholar]
- 2.Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–4. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Allison AC, Almquist SJ, Muller CD, et al. In vitro immunosuppressive effects of mycophenolic acid and an ester pro-drug, RS-61443. Transplant Proc. 1991;23:10–4. [PubMed] [Google Scholar]
- 4.Morris RE, Wang J, Blum JR, et al. Immunosuppressive effects of the morpholinoethyl ester of mycophenolic acid (RS-61443) in rat and nonhuman primate recipients of heart allografts. Transplant Proc. 1991;23:19–25. [PubMed] [Google Scholar]
- 5.Lee WA, Gu L, Miksztal AR, et al. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res. 1990;7:161–6. doi: 10.1023/a:1015828802490. [DOI] [PubMed] [Google Scholar]
- 6.Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong-Guangzhou Nephrology Study Group. N Engl J Med. 2000;343:1156–62. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 7.Briggs WA, Choi MJ, Scheel PJ., Jr Successful mycophenolate mofetil treatment of glomerular disease (see comments) Am J Kidney Dis. 1998;31:213–7. doi: 10.1053/ajkd.1998.v31.pm9469489. [DOI] [PubMed] [Google Scholar]
- 8.Nousari HC, Sragovich A, Kimyai-Asadi A, et al. Mycophenolate mofetil in autoimmune and inflammatory skin disorders. J Am Acad Dermatol. 1999;40:265–8. doi: 10.1016/s0190-9622(99)70203-3. [DOI] [PubMed] [Google Scholar]
- 9.Jayne D. Non-transplant uses of mycophenolate mofetil. Curr Opin Nephrol Hypertens. 1999;8:563–7. doi: 10.1097/00041552-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chapuis AG, Paolo Rizzardi G, D'Agostino C, et al. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat Med. 2000;6:762–8. doi: 10.1038/77489. [DOI] [PubMed] [Google Scholar]
- 11.McMurray RW, Elbourne KB, Lagoo A, et al. Mycophenolate mofetil suppresses autoimmunity and mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus. J Rheumatol. 1998;25:2364–70. [PubMed] [Google Scholar]
- 12.Corna D, Morigi M, Facchinetti D, et al. Mycophenolate mofetil limits renal damage and prolongs life in murine lupus autoimmune disease. Kidney Int. 1997;51:1583–9. doi: 10.1038/ki.1997.217. [DOI] [PubMed] [Google Scholar]
- 13.Van Bruggen MC, Walgreen B, Rijke TP, et al. Attenuation of murine lupus nephritis by mycophenolate mofetil. J Am Soc Nephrol. 1998;9:1407–15. doi: 10.1681/ASN.V981407. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson CA, Svensson L, Carlsten H. Beneficial effect of the inosine monophosphate dehydrogenase inhibitor mycophenolate mofetil on survival and severity of glomerulonephritis in systemic lupus erythematosus (SLE)-prone MRLlpr/lpr mice. Clin Exp Immunol. 1999;116:534–41. doi: 10.1046/j.1365-2249.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson CA, Erlandsson M, Svensson L, et al. Mycophenolate mofetil ameliorates perivascular T lymphocyte inflammation and reduces the double-negative T cell population in SLE-prone MRLlpr/lpr mice. Cell Immunol. 1999;197:136–44. doi: 10.1006/cimm.1999.1570. [DOI] [PubMed] [Google Scholar]
- 16.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 17.Altman A, Theofilopoulos AN, Weiner R, et al. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981;154:791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang HE, Hsueh YP, Wu CC, et al. Atypical signaling defects prevent IL-2 gene expression in lpr/lpr CD4- CD8- cells. J Biomed Sci. 1998;5:297–304. doi: 10.1007/BF02255862. [DOI] [PubMed] [Google Scholar]
- 19.Andersson J, Moller G, Sjoberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972;4:381–93. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- 20.Czerkinsky CC, Nilsson LA, Nygren H, et al. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 21.Na-Bangchang K, Supasyndh O, Supaporn T, et al. Simple and sensitive high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;738:169–73. doi: 10.1016/s0378-4347(99)00487-9. [DOI] [PubMed] [Google Scholar]
- 22.Tarkowski A, Kjellson B, Carlsten H, et al. Frequency and phenotypic feature of autoantibody-producing cell precursors in the preclinical stage of murine lupus. Immunology. 1990;71:335–40. [PMC free article] [PubMed] [Google Scholar]
- 23.Kearney JF, Lawton AR. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975;115:671–6. [PubMed] [Google Scholar]
- 24.Cohn RG, Mirkovich A, Dunlap B, et al. Mycophenolic acid increases apoptosis, lysosomes and lipid droplets in human lymphoid and monocytic cell lines. Transplantation. 1999;68:411–8. doi: 10.1097/00007890-199908150-00014. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Segu VB, Rabaglia ME, et al. Prolonged depletion of guanosine triphosphate induces death of insulin-secreting cells by apoptosis. Endocrinology. 1998;139:3752–62. doi: 10.1210/endo.139.9.6207. [DOI] [PubMed] [Google Scholar]
- 26.Hauser IA, Renders L, Radeke HH, et al. Mycophenolate mofetil inhibits rat and human mesangial cell proliferation by guanosine depletion. Nephrol Dial Transplant. 1999;14:58–63. doi: 10.1093/ndt/14.1.58. [DOI] [PubMed] [Google Scholar]
- 27.Glicklich D, Acharya A. Mycophenolate mofetil therapy for lupus nephritis refractory to intravenous cyclophosphamide. Am J Kidney Dis. 1998;32:318–22. doi: 10.1053/ajkd.1998.v32.pm9708620. [DOI] [PubMed] [Google Scholar]
- 28.Dooley MA, Cosio FG, Nachman PH, et al. Mycophenolate mofetil therapy in lupus nephritis: clinical observations. J Am Soc Nephrol. 1999;10:833–9. doi: 10.1681/ASN.V104833. [DOI] [PubMed] [Google Scholar]
- 29.Gaubitz M, Schorat A, Schotte H, et al. Mycophenolate mofetil for the treatment of systemic lupus erythematosus: an open pilot trial. Lupus. 1999;8:731–6. doi: 10.1191/096120399678840927. [DOI] [PubMed] [Google Scholar]