Abstract
Recent evidence has indicated that the salivary gland dysfunction associated with Sjögren's syndrome (SjS) is not necessarily due to immune-mediated destruction of acinar tissue. SjS sufferers may possess substantial reserves of acinar tissue but nevertheless be incapable of maintaining salivary flow rates in the normal range. We have investigated the ability of isolated labial gland acinar cells from SjS patients to fluid secrete by measuring agonist-evoked changes in intracellular Ca2+ ([Ca2+]i) using fura-2 microfluorimetry and activation of K+ and Cl− channels using the patch-clamp whole cell technique. We can confirm that stimulation with a super-maximal dose of acetylcholine (ACh) increased [Ca2+]i equally in both control acinar cells and those derived from SjS patients. However, at submaximal concentrations, the dose–response curve for ACh was shifted to the right by approximately one order of magnitude in acinar cells from SjS patients compared to control acinar cells. Patch-clamp measurements consistent with the presence of Ca2+-activated K+ and Cl− conductances were obtained from both control acinar cells and those obtained from SjS patients. Dose-dependent activation of the ion channels by acetylcholine was also right-shifted in acinar cells from SjS patients compared to control cells. Our data show that labial gland acinar cells from SjS patients were capable of responding to agonist stimulation by mobilizing [Ca2+]i and activating K+ and Cl− channels consistent with the requirements of fluid secretion. However, the persistent loss of sensitivity to ACh observed in from SjS patients may account for the lack of saliva production observed in these patients in vivo.
Keywords: acinar, Ca2+, human, ion-channels, xerostomia
INTRODUCTION
Sjögren's syndrome (SjS) is an autoimmune exocrinopathy that can occur independently or in association with a connective tissue disorder such a rheumatoid arthritis [1]. It is characterized by lymphocytic infiltration of the salivary and lacrimal glands [1] and severe glandular dysfunction, xerostomia and xerophthalmia. The clinical sequelae of SjS range from difficulty in speaking and eating, oral candidosis and rampant caries to chronic sialadenitis, blindness and B-cell lymphoma. In common with the majority of autoimmune diseases there exists a sexual dimorphism in the prevalence of the illness, with a male:female ratio of 9 : 1 [1]. The overall incidence of SjS is estimated to be 3% of the population [2,3], similar to that of rheumatoid arthritis. SjS is therefore one of the most common autoimmune diseases. Nevertheless, at the present time there is no specific treatment for the condition.
One reason for the dearth of clinical management options is that the symptoms of SjS were thought to result from destruction of salivary and lacrimal gland tissue. More recently, it has become clear that this is not the case and that many SjS sufferers have substantial reserves of histologically normal acinar tissue that simply does not function properly [4,5]. Re-evaluation of SjS in this light gives a much more optimistic outlook for treatment development. Whereas there can be no possibility of recovery if the acinar cells have been destroyed, reversal of acinar cell hypofunction may be attainable if the cause of the hypofunction can be determined.
Stimulus-secretion coupling in salivary gland acinar cells is initiated by release of acetylcholine (ACh) by parasympathetic nerves and mediated by a reversible, dose-dependent increase in intracellular Ca2+ (Ca2+]i) [6,7] that culminates in activation of Ca2+-dependent K+ and Cl− channels [8–10]. Disturbance of one or more of these three stages of the signal transduction process would inhibit fluid secretion. Therefore, possible causes of salivary gland hypofunction include actual [11] or functional denervation [12,13], disruption of Ca2+ mobilization and loss of Ca2+-dependent ion channels.
It is possible to assess the capacity of acinar cells to secrete fluid, independently of any changes to their nerve supply, by isolating cells from the gland. Changes in [Ca2+]i may be monitored in single isolated acinar cells in vitro using fura-2 microfluorimetry. The relationship between Ca2+ mobilization and activation of Ca2+-dependent K+ and Cl− channels may be measured using the patch-clamp technique. We have applied these techniques to assess the secretory ability of human labial gland acinar cells isolated from both healthy subjects and SjS patients to determine whether the salivary gland dysfunction associated with SjS is manifest in isolated acinar cells in vitro.
METHODS
Subjects
Three female and one male patient (aged 33–50) attending the Oral Medicine Xerostomia Clinic at Liverpool University Dental School for the investigation of possible SjS were studied. The average unstimulated salivary flow rate for this group was 0·07 ± 0·06 ml/min, flow rates were increased to 0·26 ± 0·18 ml/min by chewing paraffin wax. All patients were subsequently diagnosed as having primary SjS, according to the European criteria [14]. This diagnosis was confirmed by histopathological examination of labial gland biopsies showing a focal score > 1 focus/4 mm2 [15]. All of these patients also fulfilled the San Diego criteria [16] for a positive diagnosis of primary SjS. The control group comprised four healthy individuals who attended for removal of salivary mucoceles from the lower lip.
None of the patients were taking xerogenic medication.
Minor glands were taken for experimental purposes with the informed consent of the patients and following approval by local ethical committees.
Gland collection
Labial glands were harvested from the lower lip under local anaesthesia through normal mucosa as described previously [17]. Two minor glands from these patients and 1–2 minor glands removed in association with the mucocele from the control patients were placed into culture medium and immediately transferred to the laboratory.
Acinar cell preparation
Acinar cells were isolated from the labial glands by microdissection and collagenase (Worthington Diagnostic, USA) digestion in a physiological saline containing 1 mm Ca2+ as described previously [8]. Following dispersal, cells were re-suspended in culture medium.
Microfluorimetry
Acinar cells were loaded with fura-2 by incubation for 20 min in the presence of 2 µm of cell permeable fura-2 acetoxymethylester (fura-2 AM, Molecular Probes, Eugene, OR, USA). Cells were allowed to settle onto a glass coverslip that formed the base of a perfusion chamber placed on to the stage of an inverted microscope (TMD 100, Nikon, Kingston, Surrey, UK). All experiments were carried out at 24 ± 2°C. Measurements were made using 1000× magnification on single cells, either completely isolated or part of a small [2–8] cell clump. Cells were superfused continuously at 0·5 ml/min from one of several parallel superfusion pipettes.
The ratio of UV light emitted at 510 nm following excitation at 340 nm to that emitted following excitation at 380 nm was measured using a Cairn (Cairn Research Ltd, Faversham, Kent, UK) spectrophotometer (excitation was at 96Hz, data were averaged online and collected at 4Hz). Intracellular Ca2+ activity was calculated from this ratio using the Grynkiewiez equation and custom written software.
Patch-clamp
Cells were allowed to settle on to a poly l-lysine (Sigma, Poole, UK) coated coverslip that formed the base of perfusion chamber and placed on the stage of an inverted microscope (CM, Olympus Optical Company UK, Southall, Middlesex, UK) Cells were superfused continuously at 0·5 ml/min from one of several parallel superfusion pipettes. All experiments were carried out at 24 ± 2°C.
The patch-clamp whole cell configuration was achieved with single cells using 2–4 MΩ patch-clamp pipettes pulled from borosilicate glass capillaries (Clark Electromedical Instruments, Reading, UK). Access resistance through the patch pipette was approximately three times that of the pipette itself. Cells were voltage clamped to − 40 mV using an axopatch 200a patch-clamp amplifier (Axon Instruments, Foster City, CA, USA). K+ and Cl− currents were measured separately by pulsing to 0 mV and − 80 mV, respectively, for 100 ms twice a second. Currents were digitized using the CED 1401 interface (Cambridge Electronics Design, Cambridge, UK) and stored and analysed using a PC with custom-written software [18].
Solutions
The extracellular bathing solution contained in mm: 140 NaCl, 4·7 KCl, 1·13 MgCl2, 1 CaCl2, 10 glucose buffered to pH 7·4 with 10 mm hepes. The patch-clamp pipette contained (in mm) 140 KCl, 1·13 MgCl2, 10 glucose 0·5 EGTA, 1 ATP, buffered to pH 7·4 with 10 mm hepes. The culture medium was serum free 50 : 50 Dulbecco's MEM:F12 medium plus antibiotics and antimycotics (Life Technologies, UK)
Statistical analysis
The level of statistical significance between sample means was determined using Student's t-test. Non-linear regression to calculate the EC50 for the ACh dependent increase in [Ca2+]i was performed using GraphPad Prism version 3·00 for Windows, GraphPad Software, San Diego, CA, USA.
RESULTS
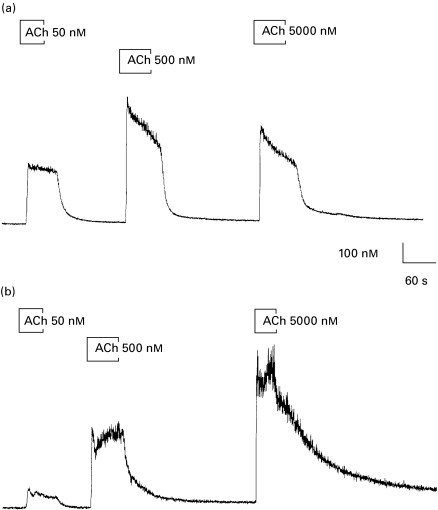
The data in Fig. 1 are representative of the time-course of the changes in [Ca2+]i stimulated by ACh over three orders of magnitude observed in labial gland acinar cells derived from control (Fig. 1a) and SjS (Fig. 1b) patients. In all cases there was a characteristic rapid increase in [Ca2+]i within 5 s of stimulation that persisted for the duration of the stimulus. Intracellular Ca2+ activity rapidly returned to prestimulus levels following removal of the ACh. The [Ca2+]i prior to stimulation averaged 61 ± 7 (n = 9) in control cells and 69 ± 6 (n = 7) in cells from SjS patients, low resting [Ca2+]i was used as an indicator of health, cells or cell preparations with elevated resting [Ca2+]i were discarded. The maximum response to ACh was similar in both control acinar cells and those from SjS patients; however, there was a clear difference between the two groups in the dose dependency of the response. This may be seen most clearly in the much smaller increase in [Ca2+]i stimulated by 50 nm ACh in cells from SjS patients compared to that observed following the same stimulus in control cells.
Fig. 1.
Time course of ACh-evoked changes in [Ca2+]i. Change in [Ca2+]i in response to stimulation by successive application of ACh at 50, 500 and 5000 nm in control labial gland acinar cells (a) and cells obtained from a SjS patient (b).
The difference in ACh dose-dependency between control and SjS cells, illustrated in Fig. 1 was a consistent observation. The average ACh-evoked changes (plateau-baseline) in [Ca2+]i from a total of eight patients (four control and four SjS) are shown in Fig. 2 (each point is the average of measurements made from four to nine cells). The EC50 for the ACh-dependent increase in Ca2+ rose significantly from 28 ± 10 to 176·8 ± 20 nm (P < 0·05). Thus, acinar cells from Sjögren's syndrome patients showed little or no response to ACh concentrations below 50 nm and responded maximally to an ACh concentration between 500 and 5000 nm. Control acinar cells responded consistently to 5 nm ACh and returned a maximum response to an ACh concentration between 50 and 500 nm. The response to 50 nm ACh was significantly greater (P < 0·05) in control cells (217 ± 40, n = 9 observations from n = 4 patients) compared to that in cells from SjS patients (88 ± 18, n = 7 observations from n = 4 patients). There was no significant difference in the magnitude of the maximum change in [Ca2+]i between control cells (in response to 500 or 5000 nm ACh) and those obtained from SjS patients (in response to 5000 nm ACh). Although not significantly greater, the response to 5000 nm ACh seen in cells from SjS patients, shown in Fig. 2, was larger than that elicited in control cells by 5000 nm ACh. This observation is consistent with those made in the only previous study of agonist-evoked changes in [Ca2+]i in labial gland acinar cells from SjS patients [19]; however, the effect shown here may be due to use-dependent effect of ACh i.e having once responded maximally to a lower concentration of ACh, the cells were not capable of a second maximal response to the subsequent challenge with 5000 nm ACh.
Fig. 2.
Average ACh-evoked changes in [Ca2+]I. Mean change in [Ca2+]i following stimulation by ACh in labial acinar cells derived from control and SjS patients plotted against log10 ACh concentration. The curves are best fit lines to a simple Michaelis–Menten function. □, Control; ○, SjS.
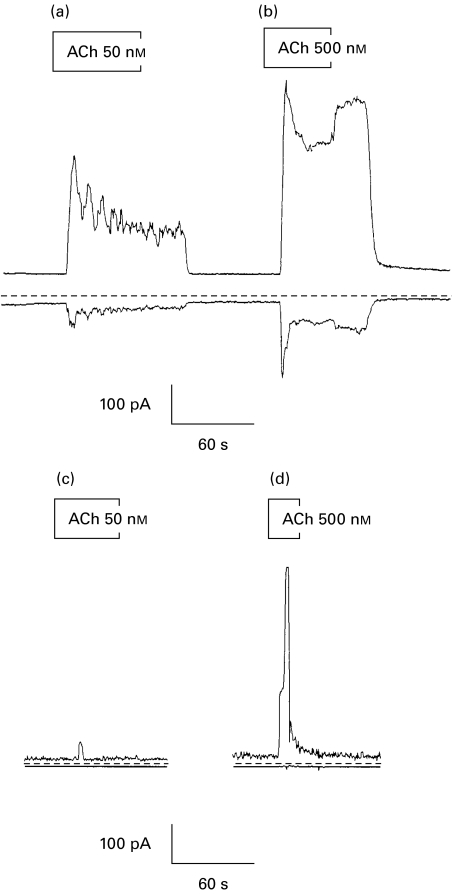
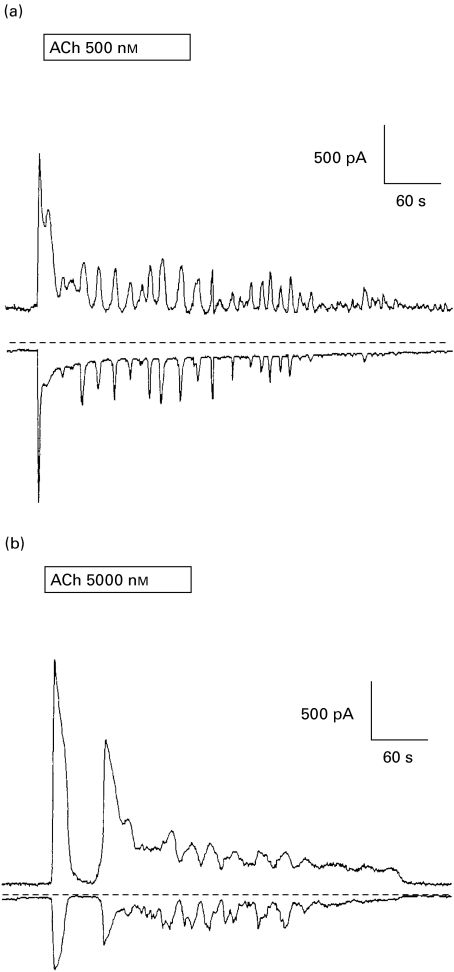
The agonist-evoked changes in [Ca2+]i in acinar cells serve to activate the Ca2+-dependent ion channels that drive fluid secretion. We have demonstrated that acinar cells from SjS patients are capable of mobilizing [Ca2+]i, albeit with a lower sensitivity to ACh than control cells. It is therefore of critical importance to determine whether acinar cells from SjS patients, who have considerably reduced salivary flow rates, possess K+ and Cl− channels and whether these channels are sensitive to the increased [Ca2+]i stimulated by ACh. Agonist-evoked changes in Cl− conductance may be determined from changes in total conductance measured at the K+ reversal potential. Similarly, changes in K+ conductance may be measured at the Cl− reversal potential. This technique has been used for many years to separate similar conductances in rodent exocrine acinar cells [18]. The data in Fig. 3 show ACh stimulated changes in both K+ and Cl− current stimulated by ACh in control (Fig. 3a–b) and in cells from SjS patients (Fig. 3c–d). The resting K+ and Cl− conductances appear smaller in cells from SjS patients compared to control cells. This could indicate that cells from SjS patients were smaller than control cells, however, we did not observe any consistent differences in the cell size. Furthermore, the difference in resting conductance was not statistically significant and the range of resting currents from both control and SjS cells fall within that seen in mouse [8,20] and human (P.M Smith, unpublished observations) major salivary gland cells. The data shown in Fig. 3 indicate a much lower responsiveness to ACh in acinar cells from SjS patients than those from control subjects. Nevertheless, both the K+ and the Cl− conductances were activated in SjS cells by 5000 nm ACh, as shown in Fig. 4. This trace also shows a striking oscillatory component to the response to ACh that most probably reflects oscillatory changes in Ca2+ [21,22]. Both control (Fig. 4a) cells and cells from SjS patients (Fig. 4b) were capable of an oscillatory response to ACh; however, this response was observed in response to a 10-fold lower ACh concentration in control cells compared to SjS cells. Together, the data in Figs 3 and 4 indicate that labial glands have both K+ and Cl− conductances and that these conductances could be increased in both control and SjS cells, consistent with the requirements of fluid secretion, by ACh.
Fig. 3.
Time course of ACh-evoked changes in K+ and Cl− currents. Representative traces showing the time course of changes in K+ (upper trace) and Cl− current (lower trace) measured in acinar cells from control (a–b) and SjS patients (c–d) in response to ACh stimulation (50–500 nm). The dotted line indicates 0 current.
Fig. 4.
Time course of oscillatory ACh-evoked changes in K+ and Cl− currents. Representative traces showing the time course of changes in K+ (upper trace) and Cl− current (lower trace) measured in a control acinar cell (a) and a cell from a SjS patients (b) in response to ACh stimulation of 500 and 5000 nm, respectively. The dotted line indicates 0 current.
DISCUSSION
The resting and stimulated salivary flow rates of the SjS patients in this study were 10–20% of those expected of a healthy subject [23]. In these patients, neither the major nor the minor glands were capable of normal basal function and were barely able to respond to the increased level of stimulation following mastication that would, in a healthy individual produce a copious flow of saliva. Nevertheless, our data demonstrate clearly that labial gland acinar cells isolated from patients suffering from SjS are capable of mobilizing [Ca2+]i in response to stimulation by ACh. These findings confirm those of a recent study by Pedersen et al. [19] of Ca2+ mobilization, stimulated by a variety of agonists, in labial gland acinar cells from SjS patients. The authors of this study also make the point that labial gland function can provide a useful mirror of major gland activity in SjS.
Our findings go beyond those previously published in two key areas.
First, we have demonstrated that labial glands from SjS patients have functioning Ca2+-dependent K+ and Cl− channels. The fluid secretion that underlies saliva production is driven by Cl− efflux from the cells through the Ca2+-activated Cl− channel. Concomitant activation of the K+ channel maintains the cell negative membrane potential that drives Cl− from the cell [10]. Loss of either K+ or Cl− channels from the membrane or even loss of the Ca2+ sensitivity of these channels could account for acinar cell hypofunction even if Ca2+ mobilization were unaffected. Our data show that this is not the case.
Secondly, we have shown that the dose–response curve for ACh was shifted to the right in acinar cells from SjS patients compared to control. At submaximal concentrations acinar cells from SjS patients required a 10-fold greater ACh concentration than control cells to elicit the same increase in Ca2+. This will have a significant impact on the ability of these cells to fluid secrete in response to anything less than maximum stimulation. Although it is difficult to assess the agonist concentration to which acinar cells are exposed in vivo, it is likely to be variable in order to accommodate different salivary flow rates, and to include the lower end of the range. Furthermore, much recent work, particularly that with rodent acinar cells, showing local and oscillatory patterns of Ca2+ mobilization emphasize the role of nanomolar rather than micromolar agonist concentration in these responses [8,22,24].
The reduction in ACh sensitivity that we have observed in acinar cells from SjS patients could be significant factor underlying the salivary dysfunction and reduced salivary flow rates in this condition. At present, we do not know the mechanism responsible for the reduction in sensitivity to ACh; however, we have work in progress to elucidate possible roles for muscarinic M3 antibodies [25–27] and also the putative second messenger cyclic adenosine diphosphate ribose (cADPr) [28–30], which is itself regulated by cGMP [29,31] and nitric oxide (NO) levels [32–35].
Our data indicate that salivary stimulation with methods such as chewing gum, or muscarinic agonists such as pilocarpine, are likely to be effective in patients with SjS because the salivary gland acinar cells of these patients are capable of secretion if stimulated sufficiently strongly. Furthermore, as salivary gland atrophy is a well-known consequence of dysfunction [36], it is possible that the eventual loss of the salivary gland cells seen in SjS is a consequence of acinar cell hypofunction rather than a cause of it. Therefore, stimulation of salivary glands during the early stages of the disease could retard loss of glandular tissue.
Acknowledgments
We would like to thank Dr C. Balmer, Dr K. Downes and Dr J. Rostron for providing tissue samples, Dr J. Stanbury for technical support and the University of Liverpool SjS Group for help and advice during this study. A.R.H. is supported by a Wellcome Trust Prize Studentship. This project was supported by a grant from the Royal Liverpool and Broadgreen University Hospital NHS Trust.
REFERENCES
- 1.Scully C. Sjogren’s syndrome: clinical and laboratory features, immunopathogenesis, and management. Oral Surg Oral Med Oral Pathol. 1986;62:510–23. doi: 10.1016/0030-4220(86)90313-0. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsson LT, Axell TE, Hansen BU, Henricsson VJ, Larsson A, Lieberkind K, et al. Dry eyes or mouth - an epidemiological study in Swedish adults, with special reference to primary Sjogren’s syndrome. J Autoimmun. 1989;2:521–7. doi: 10.1016/0896-8411(89)90185-6. [DOI] [PubMed] [Google Scholar]
- 3.Thomas E, Hay EM, Hajeer A, Silman AJ. Sjogren’s syndrome: a community-based study of prevalence and impact [see comments] Br J Rheumatol. 1998;37:1069–76. doi: 10.1093/rheumatology/37.10.1069. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys-Beher MG, Brayer J, Yamachika S, Peck AB, Jonsson R. An alternative perspective to the immune response in autoimmune exocrinopathy: induction of functional quiescence rather than destructive autoaggression. Scand J Immunol. 1999;49:7–10. doi: 10.1046/j.1365-3083.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 5.Fox RI, Maruyama T. Pathogenesis and treatment of Sjogren’s syndrome. Curr Opin Rheumatol. 1997;9:393–9. doi: 10.1097/00002281-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Petersen OH. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol (Lond) 1992;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdez IH, Paulais M, Fox PC, Turner RJ. Microfluorometric studies of intracellular Ca2+ and Na+ concentrations in normal human labial gland acini. Am J Physiol. 1994;267:G601–7. doi: 10.1152/ajpgi.1994.267.4.G601. [DOI] [PubMed] [Google Scholar]
- 8.Smith PM, Gallacher DV. Acetylcholine- and caffeine-evoked repetitive transient Ca2+- activated K+ and C1- currents in mouse submandibular cells. J Physiol (Lond) 1992;449:109–20. doi: 10.1113/jphysiol.1992.sp019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner RJ, Paulais M, Valdez II, Evans RL, Fox PC. Ion transport and signalling in human labial glands. Arch Oral Biol. 1999;44(Suppl.):S15–9. doi: 10.1016/s0003-9969(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 10.Smith PM, Gallacher DV. Electrophysiological correlates of fluid secretion by salivary acini. In: Garret JR, Ekstrom J, Anderson LC, editors. Glandular mechanisms of salivary secretion. Basel: Karger; 1998. pp. 36–54. [Google Scholar]
- 11.Konttinen YT, Hukkanen M, Kemppinen P, Segerberg M, Sorsa T, Malmstrom M, et al. Peptide-containing nerves in labial salivary glands in Sjogren’s syndrome. Arthritis Rheum. 1992;35:815–20. doi: 10.1002/art.1780350717. [DOI] [PubMed] [Google Scholar]
- 12.Dawson LJ, Smith PM, Moots RJ, Field EA. Sjogren’s syndrome-time for a new approach [editorial] Rheumatology (Oxf) 2000;39:234–7. doi: 10.1093/rheumatology/39.3.234. [DOI] [PubMed] [Google Scholar]
- 13.Dawson LJ, Christmas SE, Smith PM. An investigation of interactions between the immune system and stimulus- secretion coupling in mouse submandibular acinar cells. A possible mechanism to account for reduced salivary flow rates associated with the onset of Sjogren’s syndrome. Rheumatology (Oxf) 2000;39:1226–33. doi: 10.1093/rheumatology/39.11.1226. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, et al. Preliminary criteria for the classification of Sjogren’s syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 15.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren’s disease. J Clin Pathol. 1968;21:656–60. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjogren’s syndrome. Proposed criteria for classification. Arthritis Rheum. 1986;29:577–85. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 17.Daniels TE. Labial salivary gland biopsy in Sjogren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984;27:147–56. doi: 10.1002/art.1780270205. [DOI] [PubMed] [Google Scholar]
- 18.Smith PM. Patch-clamp whole-cell pulse protocol measurements using a microcomputer. J Physiol (Lond) 1992;446:72P. [Google Scholar]
- 19.Pedersen AM, Dissing S, Fahrenkrug J, Hannibal J, Reibel J, Nauntofte B. Innervation pattern and Ca2+ signalling in labial salivary glands of healthy individuals and patients with primary Sjogren’s syndrome (pSS) J Oral Pathol Med. 2000;29:97–109. doi: 10.1034/j.1600-0714.2000.290301.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith PM, Harmer AR, Letcher AJ, Irvine RF. The effect of inositol 1,3,4,5-tetrakisphosphate on inositol trisphosphate-induced Ca2+ mobilization in freshly isolated and cultured mouse lacrimal acinar cells [In Process Citation] Biochem J. 2000;347(Part; 1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 21.Gallacher DV, Smith PM. Autonomic transmitters and Ca2+-activated cellular responses in salivary glands in vitro. In: Garret JR, Ekstrom J, Anderson LC, editors. Neural mechanisms of salivary secretion. Karger: Basel; 1999. pp. 80–93. [Google Scholar]
- 22.Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–8. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- 23.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66:648–53. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 24.Petersen OH, Burdakov D, Tepikin AV. Polarity in intracellular calcium signaling. Bioessays. 1999;21:851–60. doi: 10.1002/(SICI)1521-1878(199910)21:10<851::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Bacman S, Sterin-Borda L, Camusso JJ, Arana R, Hubscher O, Borda E. Circulating antibodies against rat parotid gland M3 muscarinic receptors in primary Sjogren’s syndrome. Clin Exp Immunol. 1996;104:454–9. doi: 10.1046/j.1365-2249.1996.42748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacman S, Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda E. Autoantibodies against lacrimal gland M3 muscarinic acetylcholine receptors in patients with primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci. 1998;39:151–6. [PubMed] [Google Scholar]
- 27.Robinson CP, Brayer J, Yamachika S, Esch TR, Peck AB, Stewart CA, et al. Transfer of human serum IgG to nonobese diabetic Igç null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren’s syndrome. Proc Natl Acad Sci USA. 1998;95:7538–43. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harmer AR, Smith PM, Gallacher DV. The role of InsP3, cADPr and NAADP in Ca2+ signalling in mouse submandibular acinar cells. Biochem J. 259 doi: 10.1042/0264-6021:3530555. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galione A. Cyclic ADP-ribose: a new way to control calcium. Science. 1993;259:325–6. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- 30.Looms D, Nauntofte B, Dissing S. ADP ribosyl cyclase activity in rat parotid acinar cells. Eur J Morphol. 1998;36(Suppl.):181–5. [PubMed] [Google Scholar]
- 31.Clementi E, Riccio M, Sciorati C, Nistico G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. Role of the nitric oxide'cGMP pathway. J Biol Chem. 1996;271:17739–45. doi: 10.1074/jbc.271.30.17739. [DOI] [PubMed] [Google Scholar]
- 32.Michikawa H, Mitsui Y, Fujita-Yoshigaki J, Hara-Yokoyama M, Furuyama S, Sugiya H. cGMP production is coupled to Ca (2+) -dependent nitric oxide generation in rabbit parotid acinar cells. Cell Calcium. 1998;23:405–12. doi: 10.1016/s0143-4160(98)90097-5. [DOI] [PubMed] [Google Scholar]
- 33.Sugiya H, Michikawa H, Mitsui Y, Fujita-Yoshigaki J, Hara-Yokoyama M, Furuyama S, et al. Ca2+-nitric oxide-cGMP signaling in rabbit parotid acinar cells. Eur J Morphol. 1998;36(Suppl.):194–7. [PubMed] [Google Scholar]
- 34.Konttinen YT, Platts LA, Tuominen S, Eklund KK, Santavirta N, Tornwall J, et al. Role of nitric oxide in Sjogren’s syndrome. Arthritis Rheum. 1997;40:875–83. doi: 10.1002/art.1780400515. [DOI] [PubMed] [Google Scholar]
- 35.Perez Leiros C, Sterin-Borda L, Hubscher O, Arana R, Borda ES. Activation of nitric oxide signaling through muscarinic receptors in submandibular glands by primary Sjogren syndrome antibodies. Clin Immunol. 1999;90:190–5. doi: 10.1006/clim.1998.4640. [DOI] [PubMed] [Google Scholar]
- 36.Scott J, Liu P, Smith PM. Morphological and functional characteristics of acinar atrophy and recovery in the duct-ligated parotid gland of the rat. J Dent Res. 1999;78:1711–19. doi: 10.1177/00220345990780110801. [DOI] [PubMed] [Google Scholar]