Abstract
The diagnosis of the antiphospholipid syndrome (APS) requires both a typical clinical event plus a persistently positive test in an assay for either anticardiolipin (aCL) antibodies or a lupus anticoagulant (LA). Enzyme linked immunosorbent assays (ELISA) specific for autoantibodies against β2-glycoprotein I (β2GPI) or prothrombin are also used, but none of the tests are adequately sensitive or specific. A chromogenic assay was developed that measures the effect of test antibody or plasma samples on in vitro thrombin formation. It is able to detect both LA and β2GPI-dependent aCL antibodies and may have greater specificity for APS than currently available tests. Using this method various monoclonal antibodies (MoAbs) were examined, from mice immunized with β2GPI, mice with a spontaneous animal model of APS, and from three humans with APS. Plasma and affinity purified antibodies from patients with APS and control groups were also examined. Thrombin inhibition was more sensitive to perturbation by MoAbs than a combination of tests for LA (P < 0·05) and at lower antibody concentrations (12·5 µg/ml versus 100 µg/ml). There was a significant correlation between inhibition of thrombin generation and the level of MoAb reactivity to β2GPI (r = 0·90; P < 0·001) but not to CL (r = 0·06; P = 0·76). Plasma and affinity purified antibodies from patients with APS also inhibited thrombin generation, and significantly more so than patients with aPL from causes other than APS. APS patient samples showed thrombin inhibition in the presence of anti-β2GPI or antiprothrombin antibodies. All MoAbs binding β2GPI showed inhibition of thrombin generation, while MoAbs binding domain I of β2GPI had more LA effect.
Keywords: antiphospholipid syndrome, autoimmunity, β2-glycoprotein I, lupus anticoagulant, thrombin generation
INTRODUCTION
The antiphospholipid syndrome (APS) is characterized by venous and arterial thrombosis, recurrent fetal loss and thrombocytopenia and occurs in association with autoimmune antiphospholipid (aPL) antibodies [1,2]. These antibodies have traditionally been detected by solid phase immunoassays using cardiolipin (CL) as the target antigen or by in vitro phospholipid dependent functional coagulation assays (‘lupus anticoagulant’) (LA) [3]. However, the real target antigen for these autoantibodies is not phospholipid but rather phospholipid binding plasma proteins, in particular β2-glycoprotein I (β2GPI) and prothrombin [4–6].
Another group of aPL antibodies occurs in patients with a variety of infections including syphilis, malaria and AIDS [7,8] and following exposure to certain medications [9]. These aPL antibodies are not associated with clinical manifestations of the APS, in particular thrombosis. Many are true aPL antibodies, binding directly to phospholipid in a charge-dependent manner and are inhibited by β2GPI which competes for the same binding site [7]. They are detected by current CL solid phase assays and may induce prolongation of in vitro coagulation assays for the determination of LA [8]. Thus the current conventional laboratory assays for aPL antibodies do not always distinguish between these two groups.
A chromogenic amidolytic assay of thrombin activity offers certain potential advantages over the use of clot formation as an end-point, given that thrombin formation is the focal point in both coagulant and anticoagulant pathways [10]. This type of assay often detects the effect of substances known to affect coagulation clinically, yet which do not show an effect on standard clotting tests [10]. Similar amidolytic types of assays have been used previously to assess the procoagulant activity of cell preparations [11] and, using a different methodology, to assess purified antiphospholipid antibodies [12].
Although most aPL are detected by the aCL and the various LA tests, none of the available methodologies detect all aPL and they are prone to positive results in cases of infection, specific coagulation factor inhibitors or therapeutic anticoagulants.
In the present study we describe a chromogenic assay of thrombin generation which is more sensitive to perturbation by antibodies than conventional coagulation assays for LA, and distinguishes between antibodies associated with APS and those found in other conditions. The assay is marginally sensitive to the presence of anticoagulation, but significantly more sensitive to the presence of aPL in test samples.
MATERIALS AND METHODS
In vitro assay for thrombin generation
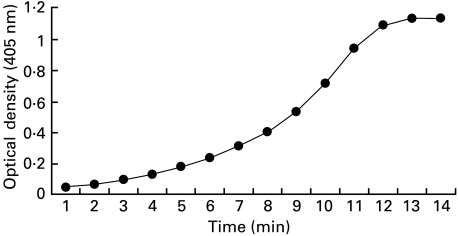
A chromogenic assay (patent pending) was used to determine the rate of thrombin generation over time (Fig. 1). This uses Spectrozyme TH (no. 238 L, American Diagnostica, Greenwich, CT, USA), a p-nitroanilide-peptide substrate cleaved with some specificity by thrombin (given human thrombin 5 nm at 23°C, 0·01 m HEPES/Tris, 0·5 m NaCl the Km = 3·24 × 10−6 m and Vmax = 0·17 µm/min NIH U). The colour generated is read by optical absorbance; as the formation of fibrin interferes with this all plasma must be defibrinated prior to testing. Fresh, not frozen, citrated plasma was spun at 3000 g for 30 min then filtered at 0·22 µm to produce essentially platelet-free plasma. Defibrination was by heating to 53°C in a shaking water bath for 20 min then centrifugation in Eppendorf tubes at 10 000 g for 15 min; the supernatant can then be stored at −70°C. Residual Factor V activity after defibrination was determined by admixture with Factor V deficient human plasma and found to be 10% (mean of 3, range 8–12%), which is unlikely to have a significant impact in isolation on thrombin generation [13].
Fig. 1.
Spontaneous in vitro thrombin generation of normal pooled plasma detected with a chromogenic substrate over 20 min.
Thromboplastin (with calcium 11·6 mm) (T-7280, Sigma) diluted 1/10 in 0·9% NaCl was added to 96-well ELISA plates (25 µl/well). Pooled, aliquoted, defibrinated plasma collected from healthy volunteers was used for all experiments. This was added to each well (50 µl/well) and the plate incubated at 37°C for 10 min. Spectrozyme TH 1 mm in 0·9% NaCl (50 µl/well) and 30 mm CaCl2 (50 µl/well) were added sequentially. The final volume was 175 µl, Spectrozyme TH concentration was 0·286 mm, and calcium concentration 10·2 mm. Background thrombin generation from any activation of the samples during handling and defibrination was determined with 0·9% saline replacing thromboplastin. Positive pooled plasma controls without the addition of test samples were performed as the reference for calculating the degree of inhibition caused by the test sample.
Test antibodies were added in 0·9% saline at a final concentration of 12·5 µg/ml. The plates were read immediately and every 2 min at 405 nm until pNA generation, representing substrate consumption by generated thrombin had reached a plateau, approximately 15–20 min in controls, with prolongation in the presence of abnormal test samples. Plotting thrombin generation over time yielded a sigmoidal curve. Alteration in the rate of thrombin generation by MoAbs was examined in duplicate wells, three time points on the linear portion of the curve were chosen (corresponding to the point of inflection of the curve), the OD at each time point of the wells with MoAb divided by the OD of the corresponding well without antibody and the result expressed as the mean of the percentages calculated for each of the three time points. The assay was highly reproducible with intraplate and interplate coefficients of variation of less than 10%.
The assay was slightly modified for the analysis of patient plasma. Twenty-five µl of undiluted patient plasma instead of purified antibody was added prior to the addition of 50 µl of pooled normal plasma diluted 1 : 1 with 0·9% saline. Following a 10-min incubation at 37°C, the addition of chromogenic substrate and CaCl2, the plates were read immediately and every 15–30 s using an automated ELISA plate reader (Molecular Devices Spectro Max 250 with Softmax Pro 1·2 software). Change in the rate of thrombin generation by patient plasma was expressed as the percentage of the optical density reached by the test sample compared with the normal control for each plate (pooled plasma only), at the time-point at which the normal control sample reached half its maximum optical density.
Measurement of lupus anticoagulant
All coagulation tests were performed on an Automated Coagulation Laboratory (ACL-3000 Plus, Coagulation System Instrumentation Laboratory, Milan, Italy). The dilute kaolin clotting test (dKCT) was performed with four parts control plasma and one part patient plasma or purified antibody diluted in Tris-buffered saline (TBS) pH 7·4 to the desired concentrations. All the MoAbs were used at 100 µg/ml final concentration, after preliminary experiments showed no effect at 12·5 µg/ml (data not shown). The concentrations of kaolin used was 0·5% and calcium chloride solution 0·025 m. The ACL-3000 machine was set in research mode and acquisition times set at 300 s or 600 s as required by the clotting time results. The final results were reported as control plasma/patient plasma mixture clotting time divided by the control plasma clotting time, or for the MoAbs, the control plasma/antibody mixture clotting time divided by the control plasma/TBS clotting time. The dilute Russell's Viper Venom Time (dRVVT) was performed using the LA screen and confirm reagents obtained from Gradipore, North Ryde, Australia. The reagents have been simplified and standardized from the original methods of Thiagarajan et al. [14]. The ACL-3000 machine was set to thrombin clotting time mode and performed in a 1 : 1 ratio of control plasma/patient plasma or control plasma/(antibody in TBS) mixture, and results are reported as for the dKCT. Both test results are considered abnormal if the prolongation of the clotting time was at least 20% of the controls. This was reported as a ratio > 1·2. Diagnosis of LA activity in this study satisfied the established criteria including a neutralization procedure and the appropriate checks for inhibitors in clinical samples [15].
Standard ELISA for aPL antibodies
Patient plasma IgG and IgM aCL levels were performed using the aCL antibody ELISA test kit (Medical Innovations Ltd, Artarmon, Australia) according to the manufacturer's instructions.
ELISA for anti‐β2GPI antibodies
One half of 10 kGy irradiated 96-well ELISA plates (Titertek) were coated with 50 µl/well of β2GPI (10 µg/ml) in carbonate buffer pH 9·6 overnight at 4°C. The other half of the plates were coated with carbonate buffer only. Plates were washed three times with 0·15 m PBS, 0·1% Tween-20 and blocked with 0·15 m PBS, 0·1% Tween-20, 1% BSA for 1 h. Test samples of murine IgG and human IgM MoAbs were diluted in blocking buffer and incubated concurrently in triplicate in the β2GPI coated and uncoated wells for 3 h. After washing, the plates were incubated with alkaline phosphatase conjugated anti-mouse IgG (or anti-human IgM for the human MoAbs) in blocking buffer for 1·5 h, and washed again prior to the addition of enzyme substrate. The optical density (OD) was determined at 405 nm and results expressed as the mean OD of the triplicate after subtracting the mean OD of the uncoated wells. Isotype matched control antibodies were used as controls. A Quanta Lite ELISA kit (Inova, San Diego, CA, USA) was used for the determination of patient plasma IgG and IgM anti-β2GPI according to the manufacturer's instructions. A positive result was > 20 standard IgG or IgM anti-β2GPI units.
ELISA for antiprothrombin antibodies
Prothrombin ELISA (anti-PT) was performed according to [16] with slight modification. Human prothrombin (Sigma Chemical Co., USA) was coated onto a Nunc Maxisorp microtitre plate (Roskilde, Denmark) overnight at 4°C, at a concentration of 2·5 µg/well in bicarbonate buffer. Plates were blocked with 0·15 m PBS, 0·3% gelatin and 1% milk powder and washed three times with 0·15 m PBS/0·05% Tween 20. Test samples diluted 1/25 in 0·15 m PBS, 0·3% gelatin were incubated in duplicate for 1·5 h at 37°C. After washing, the plates were incubated with alkaline phosphatase conjugated anti-human IgG or anti-mouse IgG or anti-human IgM for 1 h at 37°C, and washed again prior to the addition of enzyme substrate. The OD was determined at 405 nm and results expressed as the mean OD of the duplicate after subtracting the mean OD of a normal control group. A reagent blank, PBS/Tween and control sera were included on each plate.
ELISA for direct antibody binding to CL
Purified murine IgG and human IgM MoAbs were tested in a modified CL-ELISA in the absence of any exogenous source of β2GPI [4]. Briefly, non-irradiated 96-well ELISA plates (Titertek) were coated with CL (30 µg/ml in ethanol) and dried under vacuum. Plates were blocked with 0·15 m PBS, 0·3% gelatin and 1% milk powder and washed three times with 0·15 m PBS. Test samples were diluted in 0·15 m PBS, 0·3% gelatin and incubated in triplicate for 3 h at room temperature. After washing, the plates were incubated with alkaline phosphatase conjugated anti-mouse IgG (or anti-human IgM for the human MoAbs) for 90 min at room temperature, and washed again prior to the addition of enzyme substrate. The OD was determined at 405 nm and the results expressed as the mean OD of the triplicate after subtracting the mean OD of the uncoated wells. Isotype matched irrelevant MoAbs were used as controls.
Production of anti-β2GPI moabs
Group 1
Twelve IgG MoAbs were obtained from BALB/c mice by intraperitoneal injection with 40 µg of purified human β2GPI emulsified in complete Freund's adjuvant. Booster injections were repeated three times at 6-week intervals using the same amount of antigen with incomplete Freund's adjuvant. Serum was evaluated for anti-β2GPI by ELISA, and spleen cells from mice with the highest titres were isolated and fused with the mouse myeloma cell line NS-1 using 40% polyethylene glycol. The fusion cells were selected by growth in hypoxanthine–aminopterin–thymidine medium. Positive clones were identified by ELISA screening for binding activity to β2GPI. Single cell cloning was carried out by limiting dilution. For some of the antibodies, hybridoma cells were injected into mouse peritoneum and ascites fluid collected, purified using a Protein A agarose column (Biorad) following the manufacturer's directions and exchange concentrated by ultrafiltration (10 000 MW cut-off; Amicon, MA, USA) using PBS pH 7·2. Six similar MoAbs (Cof no. 18–23) were generated as described previously [17]
Group 2
17 IgG MoAbs were derived from NZW × BXSB F1 mice as described previously [18]. These animals develop a systemic autoimmune lupus-like syndrome, which includes coronary artery disease and myocardial infarction.
Group 3
Five IgM MoAbs were obtained from three patients with APS as described previously [19]. Prior studies suggest that these autoantibodies recognize the same or closely related epitopes to polyclonal human IgG aCL autoantibodies from patients with APS [19]. The human IgM monoclonal TH1B9 derived from one of the patients but without binding to β2GPI was used as a negative control.
Human plasma samples and polyclonal affinity purified antibodies
Plasma samples were collected 9 : 1 in 3·8% sodium citrate from the following groups, and aliquots heat defibrinated as described previously. Group A: eight healthy subjects. Group B: 12 patients with aPL antibodies, a variety of connective tissue diseases or a history of fetal loss (which under some classifications could be diagnosed as APS) but no history of thrombosis. Group C: 13 patients with aPL antibodies, SLE or primary APS and a history of venous or arterial thrombosis (eight receiving warfarin, five receiving aspririn or no therapy). Group D: five patients with a lupus anticoagulant in association with infection or exposure to certain medications (chlorpromazine, atypical pneumonia, quinine, typhoid fever, TB therapy with lung adenocarcinoma). Group E: eight patients with mechanical heart valve prostheses who were receiving coumadin with a mean INR ± s.d. of 3·9 ± 1·0.
Total IgG was purified from four healthy subjects in Group A, three patients in Group C and from 10 patients with syphilis by passage of diluted plasma through protein A agarose (BioRad) as previously described.
Construction and expression of the β2GPI mutant gene
Mutant forms of β2GPI were generated as described previously [20]. Type 1 domain deleted mutants containing domain(s) I, I-II, I-III, I-IV genes, lacked domain(s) positioned in the C-terminal region and type 2 mutants containing II-V, III-V, IV-V, V genes lacked domain(s) positioned in the N-terminal region. Domain mutant forms of β2GPI were used in the anti-β2GPI ELISA as described above with the anti-β2GPI MoAbs [21]. Binding to domain mutants was determined by comparison of binding to the wildtype and mutant forms of β2GPI.
Statistical analysis
The association between anti-β2-GPI and CL-ELISA (direct) antibody levels and in vitro thrombin inhibition was examined by Pearson correlation coefficient. Differences in proportions between groups were determined by chi-square analysis and differences in thrombin generation between groups were compared by a one-way analysis of variance (anova).
RESULTS
Plasma samples and affinity purified polyclonal antibodies from patients with APS
Plasma samples from healthy subjects (Group A) had a more rapid rate of thrombin generation compared to plasma from all other patient groups (P < 0·01) (Table 1). However, there was significantly greater inhibition of thrombin generation by plasma from patients who had a history of thrombosis (Group C) compared to patients with aPL with no history of thrombosis (Group B; P < 0·01) and compared to patients with aPL related to infection or drug therapy (Group D; P < 0·001). All patients with thrombotic APS (Group C) had a thrombin generation less than 60% of normal. Within Group C patients anticoagulated with warfarin had a mean thrombin generation of 23% (n = 8, range 7–48%), the remaining five patients taking aspirin or no therapy having a mean thrombin generation of 30% (range 14–58%). The thrombin generation was inhibited whether anti-β2GPI or antiprothrombin antibodies were present, as shown in Table 2.
Table 1.
Effect of patient plasma on in-vitro thrombin generation
| aCL | Anti-β2GPI | |||||||
|---|---|---|---|---|---|---|---|---|
| Group* | Thrombosis | LA | IgG | IgM | IgG | IgM | Anti-PT IgG | % unaltered thrombin generation, mean ± s.d. |
| A (n = 8) | – | n.d. | 0/8 | 0/8 | n.d. | n.d. | n.d. | 135 ± 37 |
| B (n = 12) | – | 10/12 | 7/12 | 4/12 | 2/10 | 2/8 | 3/12 | 63 ± 36 |
| C (n = 13) | + | 13/13 | 8/13 | 4/12 | 7/13 | 6/11 | 6/12 | 26 ± 16 |
| D (n = 5) | – | 5/5 | 0/5 | 0/5 | 0/5 | 1/3 | 3/5 | 49 ± 14 |
| E (n = 8) | – | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 79 ± 15 |
Group A: healthy subjects. Group B: antiphospholipid (aPL) antibodies without thrombosis. Group C: aPL antibodies with thrombosis. Group D: aPL antibodies in patients secondary to infection or medication. Group E: Patients on warfarin Results of anova: P < 0·001 for A versus B, A versus C, A versus E and C versus D. P < 0·01 for A versus D, B versus C and D versus E. P < 0·05 for C versus E. P > 0·05 for B versus D and B versus E. n.d. test not done.
Table 2.
Antigen-specific autoantibodises present in patient groups. The mean thrombin generation for each subgroup is given adjacent in italics
| Subjects | Anti-β2GPI alone (IgG or IgM) | Anti-PT alone (IgG) | Both anti-β2GPI and anti-PT | Neither | ||||
|---|---|---|---|---|---|---|---|---|
| Group B | 3/12 | 59·3% | 2/12 | 62·5% | 1/12 | 20% | 6/12 | 77·3% |
| Group C | 5/13 | 22% | 2/13 | 28·5% | 4/13 | 21·7% | 2/13 | 42·5% |
| Group D | 1/5 | 45% | 3/5 | 53% | Nil | 1/5 | 42% | |
The presence of anti-β2-glycoprotein I (anti-β2GPI) and antiprothrombin (anti-PT) antibodies has been shown for each of the three aPL patient groups.
Several of the patients with thrombosis were receiving anti-coagulant therapy. Therefore patients on warfarin prophylaxis for cardiac valve disease (Group E) were examined to determine whether the observed inhibition of thrombin generation was due to the effects of concurrent anticoagulation. Plasma samples from patients receiving warfarin (Group E) significantly inhibited the rate of thrombin generation compared to normal controls (Group A) (P = 0·001) but was comparable to that seen in patients with aPL antibodies without a history of thrombosis (Group B) (P > 0·05).
As an additional control, protein A purified IgG from three patients with aPL antibodies and a history of thrombosis was compared to IgG from four normal plasmas at a uniform concentration of 10 µg/ml. In three separate experiments, IgG from patients with thrombosis significantly inhibited thrombin generation compared to normal IgG (mean ± s.d.) 56·4 ± 9·5% versus 102 ± 7·6%, respectively, P = 0·017). Total IgG purified from 10 patients with syphilis and aCL antibodies did not significantly affect thrombin generation (98·4 ± 7·9%) compared to normal IgG (102 ± 7·6%) (P = 1·0).
Characterization of MoAbs
All MoAbs were examined for direct binding to β2GPI and CL at a uniform concentration of 1 µg/ml (Table 3). Of the 18 MoAbs in Group 1, 18 (100%) (nos 1, 2, 3, 4, 5, 7, 11, 13, 14, 15, 16, 17, Cof18, Cof19, Cof-20, Cof-21, Cof-22, Cof-23) demonstrated binding to β2GPI, three (16·6%) (nos 13, 14, 15) also showed binding to CL (almost certainly as a result of β2GPI contaminating the preparations as detected on SDS-PAGE and Western blot; data not shown) and none of them bound to prothrombin. In addition, MoAbs showed domain-specific binding: MoAbs 1, 7, 11, 16 and 17 only bound to mutant proteins having domain I; MoAbs Cof-20, and Cof-22 bound to domain III; MoAbs 2, 3, 4, 5, Cof-21, Cof-23 bound to domain IV; and MoAbs Cof-18, and Cof-19 bound to those having domain V. A domain specificity for MoAbs 13, 14 and 15 was not able to be established. In a control study, a MoAb against human mast cells bound neither to β2GPI nor to any of the mutant proteins (data not shown).
Table 3.
Characteristics of anti-β2GPI monoclonal antibodies and the examination of the effects of these MoAbs on the in vitro thrombin generation and lupus anticoagulant tests
| Antibody no. | Epitopes | Inhibition of thrombin generation | dRVVT* | dKCT* |
|---|---|---|---|---|
| 1 | Domain I | 73·3 ± 2·3% | + | + |
| 7 | Domain I | 72 ± 1·9% | + | + |
| 11 | Domain I | 74·8 ± 1·2% | + | + |
| 16 | Domain I | 74 ± 2% | + | + |
| 17 | Domain I | 77 ± 4% | + | + |
| Cof-20 | Domain III | 72 ± 2·1% | + | – |
| Cof-22 | Domain III | 65 ± 2·5% | + | – |
| 2 | Domain IV | 69·3 ± 6·3% | – | – |
| 3 | Domain IV | 59·3 ± 12·6% | – | – |
| 4 | Domain IV | 57·7 ± 11·4 | – | – |
| 5 | Domain IV | 59·9 ± 15·8% | – | – |
| Cof-21 | Domain IV | 58·8 ± 4·5% | – | – |
| Cof-23 | Domain IV | 22 ± 2·5% | – | – |
| Cof-18 | Domain V | 22 ± 2·5% | – | – |
| Cof-19 | Domain V | 67·9 ± 3% | – | – |
| 13 | ? | 73 ± 10·2% | n.d. | n.d. |
| 14 | ? | 66 ± 11·2% | – | – |
| 15 | ? | 74·8 ± 24% | – | – |
Both test results are considered abnormal if the prolongation of the clotting time was at least 20% of the controls. This was reported as a ratio > 1·2. Abnormal results are indicated by +, results within the normal range by -, test not performed by n.d.
In Group 2, FC1 was the only (6%) antibody of the 17 MoAbs which demonstrated binding to β2GPI,10 (59%) (FD5, FA1, FD3, FC3, FB1, FB2, FB3, FB4, FF2, FG1) bound directly to CL and none of them bound to prothrombin. Four (80%) (TM1G2, GR1D5, EY1C8, EY1B1) of the five human IgM MoAbs in Group 3 bound to β2GPI, 2/5 (40%) (GRID5, EY1B1) also bound to CL and 1/4-tested (TM1B3) bound to prothrombin.
LA activity was determined by prolongation of either the dRVVT or dKCT and analysis was limited to those samples for which there was a sufficient amount of antibody (Tables 3 and 4). Of the 18 MoAbs in Group 1, five out of five MoAbs against domain I had LA activity (both dKCT and dRVVT), two out of two MoAbs against domain III had LA activity (only dRVVT, not dKCT), while no MoAbs to domain IV had LA activity (Table 3). Additionally, FC1 (shown elsewhere to react against domain I) from Group 2 and TM1G2 from Group 3 also induced prolongation of the dKCT. Thus LA activity was detected in 7/18 (38%) MoAbs in Group 1, in 1/14 (7%) in Group 2 and in 1/4 (25%) in Group 3 (Tables 1 and 3).
Table 4.
Antibody binding characteristics of the three groups of monoclonal antibodies and their effects on in vitro coagulation
| Anti-β2GPI | Anticardiolipin (direct ELISA) | Anti- prothrombin | Lupus anticoagulant | Thrombin inhibition | |
|---|---|---|---|---|---|
| Group 1 | 11/13 (85%) | 3/18 (16%) | 0/13 (0%) | 7/15 (46%) | 18/18 (100%) |
| Group 2 | 1/17 (6%) | 10/17 (59%) | 0/7 (0%) | 1/14 (7%) | 1/17 (6%) |
| Group 3 | 4/5 (80%) | 2/5 (40%) | 1/4 (25%) | 1/4 (25%) | 4/5 (80%) |
Group 1: 18 IgG monoclonal antibodies from BALB/c mice immunized with human β2GPI. Group 2: 17 IgG autoimmune monoclonal antibodies from NZW × BXSB F1 mice. Group 3 : five IgM autoimmune monoclonal antibodies from three patients with antiphospholipid syndrome. Note that not all tests were able to be performed with all MoAbs as the quantities available were limited.
Effect of MoAbs on in vitro thrombin generation
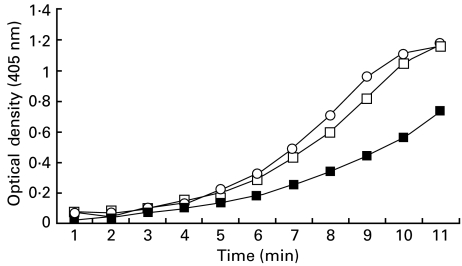
All MoAbs were initially examined at a uniform concentration of 12·5 µg/ml for their effect on in vitro thrombin generation. Of the 18 MoAbs in Group 1, the mean percentage inhibition of thrombin generation (Table 3) for MoAbs 1, 7, 11, 16 and 17 which are directed against domain I was 74·26% (range, 72–77%, n = 5) versus 68·5% (range 65–72%, n = 2), for Cof-20 and Cof-22 which are directed against domain III, versus 54·5% (range 22–69·3%, n = 6) for MoAbs 2, 3, 4, 5, Cof-21, Cof-23 which are directed against domain IV, versus 44·99% (range 22–67·9%, n = 2) for MoAbs Cof-18 and Cof-19 which are directed against domain V. As shown in Table 3, all MoAbs inhibited thrombin generation to varying degrees in a dose-dependent manner; however, the MoAbs directed against domain I show the highest inhibition of thrombin generation. In Group 2 only FC1 inhibited thrombin generation at an antibody concentration of 12·5 µg/ml. When the entire group of antibodies was re-examined at a higher concentration of 75 µg/ml only FC1 caused further inhibition of thrombin generation (77–49%). Comparison of the effect on thrombin generation by MoAbs FC1 and FC3, which were derived from the same host mouse, showed that only FC1 induced a dose-dependent inhibition (Fig. 2). The predominant reactivity of FC1 was against β2GPI while FC3 was against cardiolipin. In Group 3, four of the five (80%) human MoAbs (TM1B3, EY1C8, GRID5, EY1B1) induced variable degrees of inhibition in thrombin generation.
Fig. 2.
Effect of monoclonal antibodies against β2-glycoprotein I and cardiolipin (direct) on in-vitro thrombin generation. Two murine MoAbs were derived from the same host. The predominant reactivity of MoAb FC1 was directed against β2-glycoprotein I and inhibited in vitro thrombin generation by 49% at a concentration of 75 µg/ml. In contrast the predominant reactivity of MoAb FC3 was against cardiolipin and had a negligible impact on in-vitro thrombin generation at the same antibody concentration. ○, Spontaneous; ▪, FC1; □, FC3.
Monoclonal reactivity to β2GPI and CL, LA activity and in vitro thrombin generation
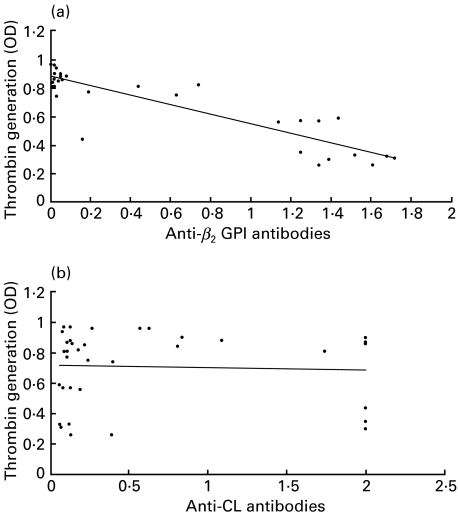
Comparison between the antibody binding characteristics of the MoAbs and their effects on in vitro coagulation are summarized in Table 4. Combining all antibodies the thrombin generation assay was more sensitive to inhibition by MoAbs than the effect of these on the combination of dKCT and dRVVT coagulation assays for LA (57·5% versus 27%). There was a highly significant association between the level of anti-β2GPI antibodies and inhibition of in vitro thrombin generation (r = 0·90; P < 0·001) (Fig. 3a). Similarly, those MoAbs which had LA activity induced a greater inhibition of thrombin generation compared to those without LA activity (mean ± s.d.): 48% ± 23% versus 78% ± 17%; P < 0·01). In contrast there was no association between aCL antibody levels and thrombin generation (r = 0·06; P = 0·76) (Fig. 3b).
Fig. 3.
Correlation between in-vitro thrombin generation and reactivity to β2-glycoprotein I and cardiolipin. There was a highly significant association (r = 0·90; P < 0·001) between the level of monoclonal anti-β2GPI antibodies and inhibition of in-vitro thrombin generation (a). In contrast there was no association (r = 0·06; P = 0·76) between the level of monoclonal anticardiolipin antibody levels and in-vitro thrombin generation (b).
DISCUSSION
Since the antiphospholipid syndrome (APS) was described in 1983 [22], advances have been made in the detection and characterization of the autoantibodies that are found in this condition. The two main groups of autoantibodies that have been extensively characterized are LA antibodies and antibodies detected in a CL-ELISA in the presence of β2GPI [23–26]. Anti-β2GPI antibodies may also be determined directly and have been shown to be associated with clinical manifestations of the APS, in particular thrombosis [23–26]. However, current assays do not reliably distinguish between this group of autoantibodies and the closely related but non-pathogenic family of aPL antibodies which occur following infection and exposure to certain medications. Most importantly, all current assays are of incomplete sensitivity and specificity.
In the present study, using a novel, sensitive and reproducible in vitro assay, we have examined the effects of human and murine monoclonal anti-β2GPI and CL-ELISA (direct) antibodies on the rate of thrombin generation. MoAbs with specificity for β2GPI but not those with direct binding to CL caused inhibition of thrombin generation, and at significantly lower concentration than that required to affect the LA tests. Furthermore, there was a significant correlation between inhibition of thrombin generation and the level of MoAb reactivity to β2GPI but not to CL. Comparable effects on thrombin generation were seen with plasma and with affinity purified polyclonal antibodies isolated from patients with thrombotic manifestations of APS, with a significantly lesser effect seen in samples from patients with aPL antibodies in the absence of thrombosis or occurring in association with infection or medication use. Although not evaluated in this study, it is likely that antiprothrombin MoAbs would have a similar effect, as a rabbit polyclonal antiprothrombin IgG showed dose-dependent inhibition of thrombin generation (data not shown), as did the human IgM antiprothrombin MoAb TM1B3. Compared to conventional assays such as the dKCT and dRVVT for the detection of LA, inhibition of thrombin generation was a more sensitive indicator of antibody perturbation of in vitro coagulation.
The monoclonal anti-β2GPI antibodies that we have generated show binding to specific domains. Interestingly, the effect of these anti-β2GPI MoAbs on the dRVVT and the dKCT appears to be domain-specific. MoAbs binding domain I cause considerable prolongation of the dRVVT and the dKCT, while MoAbs binding domain III cause a lesser prolongation, with a trend to affecting only the dRVVT (Table 3). Anti-β2GPI MoAbs binding other domains did not have an LA effect. This is in keeping with the finding that anti-β2GPI antibodies from patients with APS bind to domain I [21].
There are limitations recognized in this preliminary study. It was noted that the rate of thrombin generation with the plasma samples from patient Group A was actually faster than that achieved by the pooled, aliquoted standard plasma used in the patient experiments. This may reflect the presence of plasma from an individual not truly normal in the standard pooled batch, or some deterioration during preparation or storage of the larger batch of plasma, rather than a genuine acceleration in the normal controls; however, in any event the effect of this slowing of the normal pooled plasma would be to diminish the observed slowing of thrombin generation caused by the abnormal samples (Groups B–E). It should be noted that patients in Group B may conceivably have thrombotic APS but be yet to develop thrombosis, and that patients in Group C may have had a thrombosis for another, undiagnosed reason, despite the presence of aPL. Any test capable of suggesting a likelihood of thrombosis in APS might therefore be expected to show a degree of overlap between these clinically determined groups.
The study found an increased sensitivity for an abnormal result using the thrombin generation assay with various MoAbs; however, whether this correlates with an increased sensitivity using human samples remains unclear, as these samples were selected on the basis of their aPL abnormality. However, as no gold standard test for APS exists [3] the development of a reference test is likely to require an advance in our understanding of the pathophysiology. Even allowing for this limitation, this test appears very sensitive: it may therefore make an excellent single screening test for APS in that the diagnosis may be able to be ruled out in patients with a normal thrombin generation time, although this needs evaluation in a much larger group of clearly defined APS and control patients.
The physiological and clinical significance of in-vitro inhibition of thrombin formation is unclear at this time. The generation of thrombin is important for both thrombus formation and regulation of anticoagulation mediated via interaction with thrombomodulin, protein C and protein S and inactivation of coagulation factors V and VIII. Hence inhibition of thrombin generation in vivo may be prothrombotic by retarding the generation of activated protein C on endothelial cells [27]. Nevertheless, patients with APS appear in vivo to have increased rather than suppressed levels of circulating thrombin [28].
In the present study plasma samples from a small number of patients with thrombotic manifestations of APS demonstrated the greatest inhibition of thrombin generation. If these results are reproduced in a larger patient population, this novel assay may provide valuable information predictive of clinical thrombotic events in patients with this unique family of autoantibodies.
Acknowledgments
Supported in part by grants from the National Health and Medical Research Council of Australia grant numbers 990287 and 960779 and the Arthritis Society of Canada. Dr Hanly is a Clinical Associate of the Arthritis Society of Canada and completed this work during a sabbatical in Professor Krilis's laboratory while on leave from Dalhousie University, Halifax, Nova Scotia, Canada.
REFERENCES
- 1.McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 2.Kandiah DA, Sali A, Sheng Y, et al. Current insights into the ‘Antiphospholipid’ syndrome: clinical, immunological and molecular aspects. Adv Immunol. 1998;70:507–63. doi: 10.1016/s0065-2776(08)60393-4. [DOI] [PubMed] [Google Scholar]
- 3.Reddel SW, Krilis SA. Testing for and clinical significance of anticardiolipin antibodies. Clin Diagn Lab Immunol. 1999;6:775–82. doi: 10.1128/cdli.6.6.775-782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil HP, Simpson RJ, Chesterman CN, Krilis SA. Antiphospholipid antibodies are directed to a complex antigen that includes a lipid-binding inhibitor of coagulation: β2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli M, Comfurius P, Massen C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet. 1990;335:1544–7. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 6.Bevers EM, Galli M, Barbui T, Comfurius P, Zwaal RFA. Lupus anticoagulant IgGs (LA) are not directed to phospholipids only, but to a complex of lipid-bound human prothrombin. Thromb Haemost. 1991;66:629–32. [PubMed] [Google Scholar]
- 7.Hunt JE, McNeil HP, Morgan GJ, Crameri RM, Krilis SA. A phospholipid-β2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1:83–90. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AJ, Philips TM, Kessler CM. Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104:75–180. doi: 10.7326/0003-4819-104-2-175. [DOI] [PubMed] [Google Scholar]
- 9.Zarrabi MH, Zucker S, Miller F, et al. Immunologic and coagulation disorders in chlorpromazine-treated patients. Ann Intern Med. 1979;91:194–9. doi: 10.7326/0003-4819-91-2-194. [DOI] [PubMed] [Google Scholar]
- 10.Hemker HC, Beguin S. Phenotyping the clotting system. Thromb Haemost. 2000;84:747–51. [PubMed] [Google Scholar]
- 11.Lando P, Biazak C, Edginton T. Amidolytic assay for procoagulant activity of lymphoid and tumor cells. J Immunol Meth. 1986;89:131–9. doi: 10.1016/0022-1759(86)90041-4. [DOI] [PubMed] [Google Scholar]
- 12.Brandt JT. Antibodies to β2-glycoprotein I inhibit phospholipid dependent coagulation reactions. Thromb Haemost. 1993;70:598–602. [PubMed] [Google Scholar]
- 13.Mann KG. How much Factor V is enough? Thromb Haemost. 2000;83:3–4. [PubMed] [Google Scholar]
- 14.Thiagarajan P, Pengo V, Shapiro SS. The use of the dilute Russell's Viper Venom Time for the diagnosis of lupus anticoagulant. Blood. 1986;68:869–74. [PubMed] [Google Scholar]
- 15.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis and lupus anticoagulants: an update. Thromb Haemost. 1995;74:1185–90. [PubMed] [Google Scholar]
- 16.Guerin J, Smith O, White B, Sweetman G, Feighery C, Jackson J. Antibodies to prothrombin in antiphospholipid syndrome and inflammatory disorders. Br J Haemotol. 1998;102:896–902. doi: 10.1046/j.1365-2141.1998.00876.x. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi M, Matsuura E, Igarashi Y, et al. Human β2-glycoprotein I as an anticardiolipin cofactor determined using deleted mutants expressed by a baculovirus system. Blood. 1996;87:3262–70. [PubMed] [Google Scholar]
- 18.Monestier M, Kandiah DA, Kouts S, et al. Monoclonal antibodies from NZWxBXSB/F1 mice to β2-glycoprotein I and cardiolipin. Species specificity and charge-dependent binding. J Immunol. 1996;156:2631–41. [PubMed] [Google Scholar]
- 19.Ichikawa K, Khamashta M, Koike T, Matsuura E, Hughes GRV. β2-glycoprotein I reactivity of monoclonal anticardiolipin antibodies from patients with the antiphospholipid syndrome. Arthritis Rheum. 1994;37:1453–61. doi: 10.1002/art.1780371008. [DOI] [PubMed] [Google Scholar]
- 20.Sheng Y, Sali A, Herzog H, Lahnstein J, Krilis SA. Site-directed mutagenesis of recombinant human β2-glycoprotein I identifies a cluster of lysine residues that are critical for phospholipid binding and anti-cardiolipin antibody activity. J Immunol. 1996;157:3744–51. [PubMed] [Google Scholar]
- 21.Iverson GM, Victoria EJ, Marquis DM. Anti-β2 glycoprotein I (β2GPI) autoantibodies recognize an epitope on the first domain of β2GPI. Proc Natl Acad Sci USA. 1998;95:15542–6. doi: 10.1073/pnas.95.26.15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes GRV. Thrombosis, abortion, cerebral disease and the lupus anticoagulant. BMJ. 1983;287:1088–9. doi: 10.1136/bmj.287.6399.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roubey RAS. Immunology of the antiphospholipid antibody syndrome. Arthritis Rheum. 1996;39:1444–54. doi: 10.1002/art.1780390903. [DOI] [PubMed] [Google Scholar]
- 24.Roubey RAS, Maldonado MA, Byrd SN. Comparison of an enzyme-linked immunosorbent assay for antibodies to β2GPI and a conventional anticardiolipin immunoassay. Arthritis Rheum. 1996;39:1606–7. doi: 10.1002/art.1780390922. [DOI] [PubMed] [Google Scholar]
- 25.Viard JP, Amoura Z, Bach J-F. Association of anti-β2GPI antibodies with lupus-type circulating anticoagulant and thrombosis in systemic lupus erythematosus. Am J Med. 1992;93:181–6. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 26.Koike T. Anticardiolipin antibodies and β2GPI. Clin Immunol Immunopathol. 1994;72:187–92. doi: 10.1006/clin.1994.1128. [DOI] [PubMed] [Google Scholar]
- 27.Bombell T, Mueller M, Haeberli A. Anticoagulant properties of the vascular endothelium. Thromb Haemost. 1997;77:408–23. [PubMed] [Google Scholar]
- 28.Ginsberg JS, Demers C, Brill-Edwards P, et al. Increased thrombin generation and activity in patients with systemic lupus erythematosus and anticardiolipin antibodies: evidence for a prothrombotic state. Blood. 1993;81:2958–63. [PubMed] [Google Scholar]