Abstract
Congenital patients who lack natural killer (NK) cell activity experience repeated polymicrobial infections. NK cell activity varies significantly among normal people, but it is unknown whether this variation influences their ability to fight infections. This study examined this concern. NK cell activity and other variables, i.e. age, sex, performance status (PS), serum albumin value, lymphocyte and neutrophil counts, various lymphocyte subsets, etc. were determined for 108 immunologically normal elderly subjects who were in nursing homes due to an impaired PS. We analysed for correlations between these variables and the follow-up results of the subjects. Forty-eight subjects developed infection(s) during the first year of follow-up. A low NK cell activity was associated with the development of infection (P = 0·0105, multivariate logistic regression analysis). The relative risk for the development of infection increased in accordance with the decrease in the NK cell activity. Eleven subjects died of infection during the study period. A low NK cell activity was associated with short survival due to infection (P = 0·0056, multivariate Cox's proportional-hazards regression analysis). Our data indicate that low NK cell activity is associated with development of infections and death due to infection in immunologically normal elderly subjects with an impaired PS.
Keywords: ageing, infection, natural killer cells
INTRODUCTION
Natural killer (NK) cell-mediated innate immunity is important for fighting infections. It has been shown that NK cells can kill not only viruses but also other pathogens, such as bacteria and fungi, in vitro [1–4]. The in vivo importance of NK cells for fighting infections in humans is supported by reports that rare patients who almost totally lacked NK cell activity, but had no derangements of other immunological functions, suffered repeated viral and/or bacterial infections [5,6]. NK cell activity does not show an age-related decline even in centenarians [7–9], which contrasts with the reduced T- and B-cell functions in the elderly [10,11]. Meanwhile, NK cell activity varies significantly among normal people of all ages, but it remains unknown whether this variation is reflected in the ability to fight infections. In mice, it was reported that decreased NK cell activity was associated with mortality [12]. Our previous small-scale retrospective study using univariate analysis suggested that low NK cell activity might be related to a susceptibility to common infectious diseases, such as respiratory infections, in immunologically normal elderly people [7]. Other immunological parameters, such as the number and function of T cells and neutrophils and the γ-globulin value, showed no correlation with susceptibility to infections. Common infectious diseases cause significant morbidity and mortality in the elderly and may be leading causes of death in very old people in some countries [13]. Thus, it is important to clarify whether a relationship between low NK cell activity and susceptibility to infections exists in elderly people.
To address this question, we conducted this prospective cohort study of immunologically normal elderly people.
SUBJECTS AND METHODS
Subjects
Immunologically healthy subjects were selected from 395 elderly Japanese subjects who were not fully self-supporting and were being cared for in three nursing homes. We focused on this cohort of subjects because (1) reliable follow-up data can be obtained, (2) the subjects live in similar environments and receive similar medical support and (3) death due to infection was frequent in our preliminary study of such subjects. The criteria for selection were the admission criteria for immunogerontological studies (SENIEUR protocol) [14], a World Health Organization (WHO) performance status (PS) [15] of grade 2 or 3, and informed consent. The SENIEUR protocol strictly defines the exclusion criteria for immunological evaluations, which include clinical information indicating the presence and/or history of certain diseases (recent infection, inflammatory diseases, malignancy, etc.), abnormal laboratory data (erythrocyte sedimentation rate, blood cell count, blood chemistry tests, urinalysis, etc.), drug treatment of a defined disorder and drug treatment known to influence the immune system. One-hundred and twenty-eight subjects met the above selection criteria. The main reasons for excluding subjects were a PS of grade 4, drug treatment for a defined disease, dementia and the presence/history of the following diseases: neoplasia, collagen vascular disease, diabetes mellitus, chronic renal failure, chronic hepatitis B or C virus infection including carriers of either of these viruses, and decubitus ulcers. Only subjects with a PS of 2 or 3 were included, because subjects with a PS of 0 or 1 were very few in these nursing homes and subjects with a PS of 4 usually had a condition (such as decubitus ulcers and a defined disease requiring drug treatment) which violates the SENIEUR protocol. A few subjects in this cohort had smoking habits. These subjects were also excluded, because we expected that respiratory infections would be the main infections occurring during the follow-up period. Vaccination for influenza virus was not performed for the selected subjects, because no vaccination policy was in place in the nursing homes during the study period. This study was approved by our institution.
Cell preparation
Heparinized blood (10 ml) was obtained from each subject early in the morning. The white blood cell count was determined with a Coulter Counter (Coulter Electronics, Beds, UK). The leucocyte differentials in the peripheral blood were determined microscopically from Romanowsky-stained smear preparations. Mononuclear cells (MNCs) were separated from the blood by gradient centrifugation using Histopaque-1077 (Sigma, St Louis, MO, USA).
Determination of NK cell activity
A 51Cr-release assay was used to examine NK cell activity, as described previously [7,16]. Briefly, freshly isolated MNCs were used as effectors, and K562 cells labelled with 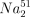 CrO4 (New England Nuclear, Boston, MA, USA) were used as targets. The labelled cells were plated at 5 × 103 cells per microtitre well. The effector-to-target (E:T) ratios were 2 : 1, 8 : 1, 32 : 1 and 96 : 1. All cultures were performed in duplicate. After a 4-h incubation, the radioactivity of the supernatant fluid was determined. The specific cytotoxicity was calculated using the following formula: (E-S)/(M-S) × 100 (where E = experimental 51Cr release; S = spontaneous 51Cr release; and M = maximum 51Cr release). The ratio of spontaneous to maximum 51Cr release was lower than 10% in all cases investigated in this study. The results are expressed as lytic units (LU) per 1 × 106 effector cells, with one LU defined as the number of effector cells required to lyse 20% of 5 × 103 target cells.
CrO4 (New England Nuclear, Boston, MA, USA) were used as targets. The labelled cells were plated at 5 × 103 cells per microtitre well. The effector-to-target (E:T) ratios were 2 : 1, 8 : 1, 32 : 1 and 96 : 1. All cultures were performed in duplicate. After a 4-h incubation, the radioactivity of the supernatant fluid was determined. The specific cytotoxicity was calculated using the following formula: (E-S)/(M-S) × 100 (where E = experimental 51Cr release; S = spontaneous 51Cr release; and M = maximum 51Cr release). The ratio of spontaneous to maximum 51Cr release was lower than 10% in all cases investigated in this study. The results are expressed as lytic units (LU) per 1 × 106 effector cells, with one LU defined as the number of effector cells required to lyse 20% of 5 × 103 target cells.
It is known that the NK cell activity (LU) differs even in the same subject when analysed on different days [17]. This interassay variation in NK cell activity is thought to be due to assay-related variability (for instance, variance in the counting of target cells), rather than a true biological variability [17]. To solve this problem, we determined the interassay variation of LU in one normal subject, who was not included in the study population, for 11 assays performed on different days; the result was 1·06 ± 0·23 × 105 (mean ±s.d.). In this study, the NK cell activity of this normal subject was determined concurrently in every NK cell activity assay as an internal control. When the activity of this subject fell outside 1·06 ± 0·23 × 105 LU, the results of the assay were rejected. To further minimize the variance induced by different NK assays, the LU of this normal subject was defined as 100, and the relative potency of LU of each examined subject (corrected LU, CLU) was calculated in each NK assay. The CLU was able to be determined for 108 subjects. This was done within 2–3 months (same season) for the subjects at each nursing home.
Lymphocyte phenotype analysis
The lymphocyte phenotype of the subjects was determined by dual colour analysis using a flow cytometer (FACScan; Becton Dickinson, Mountain View, CA, USA), as described previously [7,16]. Monoclonal antibodies directed to CD3 (Leu4), CD4 (Leu3a), CD8 (Leu2a) (Becton Dickinson), CD25 (IL-2R1), CD56 (NKH-1) (Coulter Immunology, Hialeah, FL, USA) and HLA-DR (IOT2a) (Immunotech, Marseille, France) were used.
Clinical assessment
After blood was collected for immunological analysis, the subjects were followed up in the nursing homes by nurses and physicians ignorant of the subjects' immunological data. The clinical assessment during the follow-up period included (1) occurrence of a definitive infection, such as an upper airway infection, pneumonia and urinary tract infection, (2) occurrence of a severe infection, i.e. an infection which might be fatal if untreated and (3) death. The cause of death was recorded according to the rules of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) [18]. All events counted as a severe infection were also counted as a definitive infection.
Statistical analysis
The correlation between the age and other variables was examined by Spearman's rank correlation test. The association between the predetermined parameters (all variables listed in Table 1) and occurrence of infection during the first 12 months of follow-up was examined by univariate and multivariate logistic regression analyses. The multivariate analysis included only selected variables. The data of subjects who were either discharged from the nursing home or died before the end of the 12 months were omitted from the logistic regression analyses, regardless of the findings regarding infection. This is because these subjects included both those who did and those who did not develop an infection, and thus inclusion of all or part of these subjects might bias the results. The association between the predetermined parameters (all variables listed in Table 1) and the time to death due to infection during the total follow-up period was examined by univariate and multivariate Cox's proportional-hazards regression analyses. The multivariate analysis included only selected variables. Data were censored at the last follow-up date or the date of death due to a non-infectious cause.
Table 1.
Characteristics and immunological parameters of 108 subjects whose CLU could be determined
| Age (years) | 81 ± 8 (range 63–99) |
| 63–69 | 12 |
| 70–79 | 34 |
| 80–89 | 44 |
| 90–99 | 18 |
| Male/female | 25/83 |
| PS (2/3)* | 69/39 |
| Nursing home† | 48/24/36 |
| Serum albumin (g/dl) | 3·8 ± 0·5 |
| Neutrophils (/µl) | 3437 ± 1819 |
| Monocytes (/µl) | 288 ± 221 |
| Lymphocytes (/µl) | 1619 ± 956 |
| Lymphocyte subsets | |
| CD3+ | 54·3 ± 16·3 (952 ± 647) |
| CD4+ | 39·1 ± 15·7 (690 ± 475) |
| CD8+ | 27·7 ± 13·2 (499 ± 432) |
| CD25+ | 19·0 ± 10·3 (333 ± 248) |
| CD56+ | 27·6 ± 15·2 (493 ± 445) |
| CD3+HLADR+ | 0·6 ± 0·5 (9 ± 9) |
| CD3+CD56+ | 3·3 ± 3·3 (63 ± 89) |
| CD3−CD56+ | 24·3 ± 15·0 (430 ± 416) |
| CLU | 165·9 ± 535·2 |
Data are the mean ± s.d. or the number of cases. Data on lymphocyte subsets are expressed as percentages, with the absolute numbers in parentheses (/µl).
WHO grade 2 or 3.
Number of cases at each nursing home.
RESULTS
Characteristics and immunological data of subjects
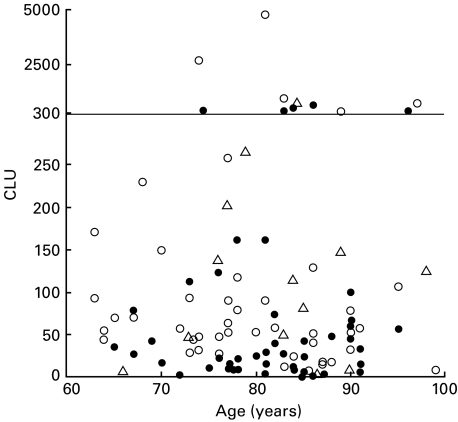
Table 1 shows the characteristics and immunological data of the 108 subjects for whom the CLU was able to be determined. We and others reported that some immunological parameters, such as the B- and T-cell counts, were decreased in immunologically healthy elderly subjects compared with young subjects [7,8]. However, in the elderly population, such age-related immunological changes are not clear. In our cohort of 108 elderly subjects, none of the studied immunological parameters, including the percentage and count of T cells, or the other continuous variables presented in Table 1 correlated with the age (P > 0·13 and − 0·11 < ρ < 0·16 for all variables). The distribution of CLU in association with the age is shown in Fig. 1.
Fig. 1.
The distribution of NK cell activity (CLU) in association with age in all subjects. Note that CLU values over 300 are plotted using a different scale for the y-axis. There is no correlation between the CLU and the age (P = 0·4801, ρ = − 0·068). The closed and open circles indicate subjects with and without an infection(s) during the first 12 months of follow-up, respectively. The triangles indicate subjects who were either discharged from the nursing homes or died before the end of these 12 months.
Diagnosed infections and causes of death of subjects
The occurrence of infection during the first four seasons (12 months) after blood collection was analysed for 95 of the 108 subjects. The data of 13 subjects who were either discharged from the nursing homes (n = 3) or died (n = 10) before the end of the 12 months were omitted. Forty-eight of the 95 subjects developed an infection(s) during the 12 months. The kinds of infection recorded in these 48 subjects are summarized in Table 2. Twelve of these 48 subjects developed two kinds of infection, thus a total of 60 infections are shown. Ten of the 48 subjects developed the same kind of infection repeatedly during the 12 months (such recurrences are shown as one event in Table 2). Among the infections shown in Table 2, pneumonia, pyelonephritis and sepsis were categorized as severe infections. These severe infections developed in 20 of the 48 subjects.
Table 2.
Infections developed during the first 12 months of follow-up in 95 subjects and cause of death during entire follow-up period in 108 subjects
| Diagnosed infection | |
| Respiratory infections | |
| Common cold | 18 |
| Acute bronchitis | 3 |
| Pneumonia | 15 |
| Urinary tract infections | |
| Cystitis | 13 |
| Pyelonephritis | 4 |
| Enterocolitis | 2 |
| Cellulitis | 2 |
| Others* | 3 |
| Cause of death | |
| Pneumonia (J13–J15) | 11 |
| Heart disease (I21 and I50) | 5 |
| Malignant neoplasms (C16 and C25) | 2 |
| Cerebrovascular disease (I61 and I63) | 2 |
| Asphyxia due to food (T17) | 2 |
| Senility (R54) | 3 |
| Acute respiratory failure (J96·0) | 1 |
| Unspecified gastrointestinal haemorrhage (K92·2) | 1 |
The numbers of cases who developed each kind of infection and who died of each cause are shown.
Some subjects developed multiple kinds of infection. The code numbers of ICD-10 are shown in the parentheses.
Herpes zoster, perianal abscess and sepsis.
Twenty-seven of the 108 subjects died during the follow-up period. The median follow-up time for the subjects who were alive at the last follow-up was 23 months (range 4–27 months). Eleven subjects died of bacterial pneumonia (Table 2). Severe infections and death due to pneumonia were frequent in this cohort, which was thought to be due to the poor PS of the subjects (see Discussion).
Correlation between the predetermined parameters and development of infection and time to death due to infection
We then analysed the correlation between the predetermined parameters (all variables listed in Table 1) and infection(s) during the first 12 months of follow-up (Table 3). By univariate logistic regression analysis, in which continuous variables were divided into 2–5 groups using various cut-off points, a poor PS, low Alb value, high age and low NK cell activity (low CLU) correlated significantly with the development of infection. The association between NK cell activity and infection(s) during the first 12 months of follow-up is illustrated in Fig. 1. Among the other variables, only a low NK cell count tended to correlate with the development of infection (P = 0·1146 for low NK cell count; P > 0·2 for other variables). Multivariate logistic regression analysis showed that a low NK cell activity was an independent variable associated with the development of infection. The odds ratio for the development of infection increased in accordance with the decrease in the NK cell activity. Other independent variables associated with the development of infection were a poor PS and high age.
Table 3.
Associations between predetermined parameters and the development of infection during the first 12 months of follow-up (n = 95)
| Univariate analysis | Multivariate analysis† | ||||
|---|---|---|---|---|---|
| Infection* Yes/no | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Age < 75 years | 7/15 | 1 | 1 | ||
| Age ≥ 75 years | 41/32 | 2·75 (1·00–7·53) | 0·0498 | 3·90 (1·03–14·72) | 0·0449 |
| PS = 2 | 21/40 | 1 | 1 | ||
| PS = 3 | 27/7 | 7·35 (2·74–19·67) | < 0·0001 | 9·98 (2·97–33·54) | 0·0002 |
| Alb ≥ 3·5 g/dl | 32/41 | 1 | 1 | ||
| Alb < 3·5 g/dl | 16/6 | 3·42 (1·20–9·73) | 0·0213 | 1·90 (0·52–6·97) | 0·3331 |
| CLU ≥ 50 | 15/29 | 1 | 1 | ||
| CLU ≥ 30 to < 50 | 8/7 | 2·21 (0·67–7·27) | 0·1919 | 2·09 (0·50–8·74) | 0·3109 |
| CLU ≥ 15 to < 30 | 10/6 | 3·22 (0·98–10·58) | 0·0537 | 4·18 (1·00–17·39) | 0·0494 |
| CLU < 15 | 15/5 | 5·80 (1·77–19·04) | 0·0037 | 5·85 (1·51–22·67) | 0·0105 |
CI, confidence interval. Low CLU indicates low NK cell activity.
Number of cases who developed infection (yes) and who did not (no).
CLU, age, PS and serum Alb value were analysed by multivariate analysis.
When possible correlations between the predetermined parameters and the subjects who developed a severe infection were analysed in the 95 subjects, only a poor PS (PS = 3) was a significant independent variable for the development of a severe infection (adjusted odds ratio 6·61 [95% CI, 1·99–21·98], P = 0·0021). A low NK cell activity (CLU < 15) was an independent variable for the development of a severe infection with marginal significance (adjusted odds ratio 2·89 [95% CI, 0·82–10·17], P = 0·0977).
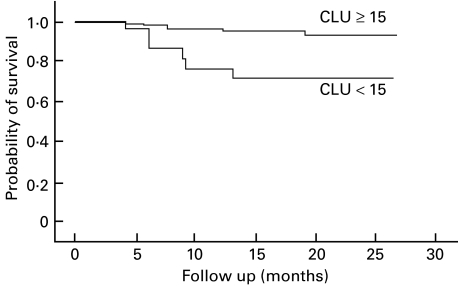
We next analysed for correlations between the predetermined parameters and the time to death due to infection in the 108 subjects (Table 4). Eleven subjects died of an infection (all due to pneumonia) as shown in Table 2. Univariate Cox's proportional-hazards regression analysis showed that a poor PS, high CD8 cell count and low NK cell activity correlated significantly with a short survival due to infection. The time to death due to infection as a function of NK cell activity is illustrated in Fig. 2. Among the other variables, only a low CD3+ CD56+ cell percentage tended to correlate with a short survival due to infection (P > 0·2 for all other variables). Multivariate Cox's proportional-hazards regression analysis showed that a low NK cell activity was an independent variable associated with a short survival due to infection. Other independent variables for a short survival due to infection were a poor PS and a high CD8 cell count.
Table 4.
Associations between predetermined parameters and the time to death due to infection (n = 108)
| Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Age < 75 years | 1 | 1 | ||
| Age ≥ 75 years | 0·77 (0·20–2·90) | 0·6967 | 0·91 (0·20–4·14) | 0·8971 |
| PS = 2 | 1 | 1 | ||
| PS = 3 | 5·34 (1·42–20·16) | 0·0134 | 4·90 (1·12–21·45) | 0·0351 |
| Alb ≥ 3·5 g/dl | 1 | 1 | ||
| Alb < 3·5 g/dl | 2·13 (0·63–7·29) | 0·2266 | 0·96 (0·21–4·46) | 0·9592 |
| CD3+CD56+ cells ≥ 1% | 1 | 1 | ||
| CD3+CD56+ cells < 1% | 3·04 (0·80–11·59) | 0·1029 | 3·11 (0·62–15·48) | 0·1668 |
| CD8+ cells < 1000/µl | 1 | 1 | ||
| CD8+ cells ≥ 1000/µl | 6·32 (1·66–24·04) | 0·0068 | 21·35 (3·04–150·19) | 0·0021 |
| CLU ≥ 15 | 1 | 1 | ||
| CLU < 15 | 4·90 (1·49–16·07) | 0·0088 | 10·44 (1·98–54·95) | 0·0056 |
CLU, age, PS, serum Alb value, CD3+CD56+ cell percentage and CD8+ cell count were analysed by multivariate analysis.
Fig. 2.
Kaplan–Meier curves for the time to death due to infection according to NK cell activity.
DISCUSSION
Common infectious diseases cause significant morbidity and mortality in ageing populations. However, the immunological features of elderly people who are immunologically healthy but are prone to develop infections or to die due to infections have not yet been clarified. The role of NK cells in fighting viral infections has been extensively studied. A typical viral infection activates NK cells, and increased NK-cell responses are observed during the first 1–3 days of infection [19,20]. This is replaced gradually with viral antigen-specific T-cell responses. When T-cell responses do not occur, as in severe combined immunodeficient mice and athymic nude mice, the increased NK-cell response is maintained for a prolonged period to defend the host [20–22]. Besides the antiviral function of NK cells, NK cells may play a role in eliminating various other pathogens. This is supported by the findings that NK cells kill such pathogens as bacteria and fungi in vitro [2,3] and that NK-cell-deficient humans suffer life-threatening viral and bacterial infections [5,6]. It has been reported that the count and function of T cells are reduced in healthy elderly subjects compared with young people [7,8,10,11]. Considering the functional link between NK and T cells, the role of NK cells against infections may be more important in the elderly than in young people. Our prior study showed that so-called NK cell activity, i.e. the activity of freshly isolated MNCs from peripheral blood, correlated well with the activity of NK cells which are stimulated in vitro with interleukin-2 [7]. Thus, the magnitude of the so-called NK cell activity may correlate with the magnitude of the activity of NK cells which are activated in vivo by infections. Based on this background and the results of our previous small-scale study described in the Introduction, we conducted this prospective cohort study, which paid special attention to the NK cell activity.
In our cohort, development of a severe infection (pneumonia was most prevalent) and death due to pneumonia were frequent, which contradicts the data for the general Japanese population. A survey by the Ministry of Health and Welfare of Japan showed that in Japanese at age 80, similar numbers of deaths were caused by pneumonia, heart disease, malignant neoplasms and cerebrovascular diseases [23]. We believe that this discrepancy is attributable, at least in part, to the poor PS of our subjects. This is because our analysis showed that a worse PS was an independent variable for the development of a severe infection and short survival due to pneumonia.
Our multivariate analyses showed that low NK cell activity is an independent variable associated with the development of infection and short survival due to infection in our cohort. The developed infections were due to viruses (the common cold and herpes zoster) and bacteria, while infectious death was due to bacterial pneumonia. These data on pathogens are consistent with the reports of patients who lacked NK cell activity [5,6]. It should be noted that the odds ratio for development of an infection increased in accordance with the decrease in the NK cell activity (the adjusted odds ratios were 2·09, 4·18 and 5·85 for subjects with CLU ≥ 30 to < 50, CLU ≥ 15 to < 30 and CLU < 15, respectively). Moreover, only the lowest NK cell activity (CLU < 15) was an independent variable for development of a severe infection, with marginal significance (adjusted odds ratio: 2·89), as well as for short survival due to an infection, with strong statistical significance (adjusted hazard ratio: 10·44). These findings indicate that the NK cell activity is important for fighting infections in our cohort. Nevertheless, because a small number of subjects in our cohort died due to infection, it is better to confirm the association between a low NK cell activity and death due to infection in larger cohorts of subjects. Another point to be considered is possible fluctuation in NK cell activity. Although we adjusted for interassay variation in the NK cell activity, this adjustment does not guarantee that the NK cell activity of each elderly subject is stable over a long period of time. We currently have no data on whether the ability to fight infections changes with a fluctuation in NK cell activity. Our analyses also showed that, besides the NK cell activity, a poor PS, high CD8+ cell count and high age were independent variables associated with the development of infection or short survival due to infection. Among these, the association between a high CD8+ cell count and short survival due to infection is poorly understood. One proposed explanation is that clonal expansions of CD8+ cells, which are often observed in healthy elderly people [24], are exaggerated in the subjects with a high CD8+ cell count. These clonal expansions probably reduce the naive repertoire of CD8+ cells, which impairs the subjects' T-cell responses. Nevertheless, even if this speculation is true, the reduced naive repertoire of CD8+ cells cannot clearly explain the short survival due to bacterial pneumonia in our cohort. Besides our data, an association between a high CD8+ cell percentage and high mortality in elderly people was also suggested by an other study [25]. The underlying mechanism and significance of a high CD8+ cell count in the elderly population should be investigated in future studies.
Recently, a subset of T cells sharing characteristics of NK cells (NKT cells) has been vigorously studied. Although the physiological role of NKT cells remains elusive, these cells have an immunoregulatory function [26,27]. CD3+CD56+ cells are a subset of human NKT cells, and the proportion of CD3+CD56+ cells may increase in the peripheral blood of elderly people [28,29]. Our analyses did not show a significant association between CD3+CD56+ cells in the peripheral blood and the time to death due to infection (Table 4). However, because a large number of CD3+CD56+ cells reside in the human liver [30], our results do not exclude a possible association between CD3+CD56+ cells and infection in the elderly population.
In summary, our data indicate that, in immunologically normal elderly subjects with an impaired PS, low NK cell activity relates to the development of infections and short survival due to infection. Subsequent questions to be investigated are whether this finding is also true in other populations, such as normal elderly subjects with a good PS, and whether intervention for subjects with low NK cell activity can change the outcome.
Acknowledgments
This study was funded by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 09670494).
REFERENCES
- 1.Welsh RM. Regulation of virus infections by natural killer cells. A Review. Nat Immun Cell Growth Regul. 1986;5:169–99. [PubMed] [Google Scholar]
- 2.Garcia PP, Koster FT, Kelley RO, McDowell TD, Bankhurst AD. Antibacterial activity of human natural killer cells. J Exp Med. 1989;169:99–113. doi: 10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidore MR, Murphy JW. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect Immun. 1989;57:1990–7. doi: 10.1128/iai.57.7.1990-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar MT. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Ann Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 5.Fleisher G, Starr S, Koven N, Kamiya H, Douglas SD, Henle W. A non x–linked syndrome with susceptibility to severe Epstein–Barr virus infections. J Pediatr. 1982;100:727–30. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- 6.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 7.Ogata K, Yokose N, Tamura H, et al. Natural killer cells in the late decades of human life. Clin Immunol Immunopathol. 1997;84:269–75. doi: 10.1006/clin.1997.4401. [DOI] [PubMed] [Google Scholar]
- 8.Sansoni P, Cossarizza A, Brianti V, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–73. [PubMed] [Google Scholar]
- 9.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–20. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi C, Monti D, Sansoni P, Cossarizza A. The immunology of exceptional individuals: the lesson of centenarians. Immunol Today. 1995;16:12–6. doi: 10.1016/0167-5699(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 11.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 12.Heller DA, Ahern FM, Stout JT, McClearn GE. Mortality and biomarkers of aging in heterogeneous stock (HS) mice. J Gerontol Series a Biol Sci Med Sci. 1998;53:B217–B230. doi: 10.1093/gerona/53a.3.b217. [DOI] [PubMed] [Google Scholar]
- 13.Pawelec G, Adibzadeh M, Pohla H, Schaudt K. Immunosenescence: ageing of the immune system. Immunol Today. 1995;16:420–2. doi: 10.1016/0167-5699(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 14.Ligthart GJ, Corberand JX, Fournier C, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979. [Google Scholar]
- 16.Ogata K, Tamura H, Yokose N, et al. Effects of interleukin-12 on natural killer cell cytotoxicity and the production of interferon-γ and tumour necrosis factor-α in patients with myelodysplastic syndromes. Br J Haematol. 1995;90:15–21. doi: 10.1111/j.1365-2141.1995.tb03375.x. [DOI] [PubMed] [Google Scholar]
- 17.Whiteside TL, Bryant J, Day R, Herberman RB. Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J Clin Lab Anal. 1990;4:102–14. doi: 10.1002/jcla.1860040207. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Geneva: World Health Organization; 1992. International statistical classification of diseases and related health problems, 10th revision (ICD-10) [Google Scholar]
- 19.Welsh RM. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–106. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- 20.Welsh RM, Vargas-Cortes M. Natural killer cells in viral infection. In: Lewis CE, McGee JO'D, editors. The natural killer cell. Oxford: Oxford University Press; 1992. pp. 107–50. [Google Scholar]
- 21.Welsh RM, Brubaker JO, Vargas CM, O'Donnell CL. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991;173:1053–63. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su HC, Ishikawa R, Biron CA. Transforming growth factor-β expression and natural killer cell responses during virus infection of normal, nude, and SCID mice. J Immunol. 1993;151:4874–90. [PubMed] [Google Scholar]
- 23. http://www.mhw.go.jp.
- 24.Wack A, Cossarizza A, Heltai S, et al. Age-related modifications of the human αβ T cell repertoire due to different clonal expansions in the CD4+ and CD8+ subsets. Int Immunol. 1998;10:1281–8. doi: 10.1093/intimm/10.9.1281. [DOI] [PubMed] [Google Scholar]
- 25.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102:187–98. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 26.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Ann Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 27.Joyce S. Natural T cells: cranking up the immune system by prompt cytokine secretion. Proc Natl Acad Sci USA. 2000;97:6933–5. doi: 10.1073/pnas.97.13.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrego F, Alonso MC, Galiani MD, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 29.Miyaji C, Watanabe H, Minagawa M, et al. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420–9. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 30.Norris S, Doherty DG, Collins C, et al. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Vα24-JαQ and γδ T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]