Abstract
Growing evidence has supported the conclusion that melatonin, a pineal hormone, modulates the immune function. In our previous study, we evaluated in vivo the potential role of melatonin in the regulation of the antigen specific T and B cells. In the present study, we observe that melatonin down-regulated the expression of the co-stimulatory molecule B7-1 but not B7-2 on macrophages. Further, melatonin encouraged the proliferation of anti-CD3 antibody activated CD4+ T cells only in the presence of antigen-presenting cells and promoted the production of Th2-like cytokines. Furthermore, it failed to influence the activity of B cells in a T-independent manner. Melatonin suppressed the release of TNF-α by LPS or IFN-γ activated macrophages but failed to inhibit nitric oxide (NO) release. Thus the study shows that melatonin can engineer the growth of unprimed CD4+ T cells if both the signals are provided by antigen-presenting cells. However, it could not regulate the function of B cells.
Keywords: B cells and macrophages, CD4+ T cells, melatonin, unprimed Th1 and Th2 cells
INTRODUCTION
The induction of cell-mediated immunity (CMI) to protein antigens is dependent on the activation of CD4+ T-helper cells. The optimum activation of primary T cells requires not only TCR occupancy by the MHC–Ag complex, but also a set of secondary signals provided by APC (antigen-presenting cells) in the form of co-stimulatory molecules [1–3]. These molecules have been shown to play a major role in stimulating T cells, leading to their proliferation, in cytokines production and in the development of effectors functions.
On the basis of distinct patterns of lymphokine production, Th cells have been subdivided into Th1 and Th2 cells. Th1 cells secrete mainly IL-2, IFN-γ, lymphotoxin, etc. and are responsible for the generation of CMI responses; Th2 cells produce mainly IL-4, IL-5, IL-6, etc. and are generally involved in humoral immunity [4]. Both the subsets recognize foreign antigens in association with MHC-class II molecules. It appears that these two distinct Th cells are not only functionally different but also require discrete signals for their optimum activation [1–3].
The pineal hormone melatonin, in addition to its well-known circadian regulation, is also believed to play an important role in neuroimmunomodulation [5]. Specific binding sites for melatonin in the immune cells indicate a direct effect of melatonin on the immune system [6,7]. It has been shown that melatonin treatment of both normal and immunocompromised mice increase antibody responses and enhance impaired Th cell activity [5,8]. However, a connection between melatonin and activation of lymphocytes has not yet been precisely determined. Moreover, a majority of functional studies with melatonin have analysed cytokine and immunoglobulin production in an in vivo system [7,9,10]. Therefore it is difficult to predict a possible straightforward functional interaction between the immune cells (i.e. macrophages, T and B cells) and melatonin.
In our earlier in vivo study we have shown that melatonin acts on antigen specific Th2 cells, as evidenced by a predominant secretion of IL-4 and the IgG1-antibody and decreased production of IL-2, IFN-γ and IgG2a-subtype [10]. In the present study, we have demonstrated in vitro that the melatonin can influence successfully the immunological behaviour of macrophages and unprimed CD4+ T cells but not of B cells.
MATERIALS AND METHODS
Animals
Inbred female Balb/c mice, 6–10 weeks, were obtained from the National Institute of Immunology, New Delhi. During the experiments, the mice were kept at the Institute's Animal House under a 13/11-h light/dark cycle (lights on at 0600 h) in standard laboratory conditions with food and water ad libitum. The Institutional Animal Ethics Committee approved the experimental protocol.
Drug, antigen and antibodies
Melatonin (Morepen Laboratories, Parwanoo, India), ovalbumin (OVA) and goat antimouse IgM, IgA, IgG1 and IgG2a (Sigma, St Louis, USA) and biotinylated antimouse IgM, IgA, IgG1 and IgG2a and streptavidin-HRP were procured from Sera Labs, Crawley Down, UK. Recombinant murine IL-2, IL-4, IL-10, IFN-γ and TNF-α, anti-IL-10 and anti-IL-10 biotinylated antibodies were purchased from Genzyme (Cambridge, MA, USA). Antibodies to IL-4 (11B11) and IL-2 (Cocktail of TIB 222, HB 8794 and CRL 1698) were used as a culture SN.
Cell lines and hybridomas
The cell lines and hybridomas used in this study, namely EL-4, WEHI-164, WEHI-279, HT-2 (CRL-1841), TIB222 (PC61·5.3), CRL 1698 (7D4) and HB 8794) (S4B6), were procured from ATCC (Rockville, MD, USA). Th1 hybridoma (3DO.54·8) was a kind gift from Dr P. Marrack, Denver, CO, USA.
Immunization protocol
OVA (2 mg/ml) was dissolved in PBS (0·01 m, pH 7·2) and emulsified in Freund's complete adjuvant (FCA). Emulsion (100 µl) was then injected intraperitoneally into female Balb/c mice divided into groups of five. After 1 week, a booster dose of the antigen was repeated. For 5 days before bleeding, the animals were injected subcutaneously daily with melatonin (10 and 20 mg/kg body weight of mice). The control animals were immunized intraperitoneally with 100 µl each of a placebo (PBS) and 1% ethanol-PBS (a vehicle for melatonin). The selection of the melatonin doses was based on previous reports [9,10]. Splenocytes from each group were pooled for studying the expression of B7-1 and B7-2 by FACScan.
Isolation of macrophages, T and B cells from the splenocytes
Splenic cells from the unprimed and OVA injected Balb/c mice were suspended in RPMI-1640 medium and exposed to Gey's solution to remove red blood cells. The cells were washed three times and incubated (1 × 107 cells/ml) for 1 h at 37°C on plastic Petri plates. After removing the floating cells (T and B cells), adherent cells (macrophages) were collected by gently scraping the plates with a rubber policeman. The macrophages were washed three times with HBSS, suspended in RPMI-10% FCS, and were used for the experiments. The purity of the cells was more than 97%.
The plastic non-adherent cells were loaded (1 × 106 cells/ml) onto the nylon wool column and incubated for 90 min at 37°C. After the incubation period, cold HBSS was passed into the column to elute T-lymphocytes. The cells were washed with an RPMI-1640 medium and incubated with anti-Mac3 (TIB 168), anti-IAd (MKD6) and anti‐dendritic cell (TIB 227) antibodies at 4°C with the RPMI-1640 medium. The cells were then treated with anti-IgM and anti-Lyt 2·2 antibodies for 45 min at 4°C. The cells were washed with the RPMI-1640 medium and incubated with the baby rabbit complement for 30 min at 37°C. The cells were washed three times with the RPMI-1640 medium and used for the proliferation assay. The purity of the cells as revealed by staining with anti-CD3 and CD4 antibodies was more than 95%.
After eluting T cells, the column was gently squeezed to isolate B cells. This process was repeated three times after passing warm medium. The B cell preparation was enriched by treating with anti-Thy 1·2 antibody and baby rabbit complement. The purity of stained B cells as analysed by FACS (Beckton Dickinson) was over 85%.
Expression of B7-1 and B7-2 on macrophages and B cells
The staining of B7-1 and B7-2 was performed as described elsewhere [3]. Briefly, 1 million macrophages or B cells, isolated from OVA primed mice were incubated with anti-B7-1 or anti-B7-2 antibodies (1·0 µg/100 µl) in PBS-FCS-2% for 1 h at 4°C. The cells were washed three times in PBS-1% FCS. Anti-rat-Ig-FITC (1 : 100 dilution) was added and the cells were further incubated for 1 h at 4°C. The cells were then washed five times with PBS and fixed in 1% paraformaldehyde and analysed by FACS for the expression of B7-1 and B7-2. The control groups were also maintained, using the cells isolated from the mice inoculated with PBS. As a control for anti-B7-1 and B7-2 antibodies, the cells were also incubated with RtIg. The cells from each suspension were acquired on Lysis II software of FACScan (Becton Dickinson). The exclusion of debris from the cell suspension by suitable gating allowed analysis of only those-scattering events (i.e. cells) of a size consistent with the macrophages and B cells. The analysis for the mean fluorescence intensity (MFI) was performed on histograms in which the abscissa and the ordinate denote log FITC fluorescence and relative cell count, respectively.
T cell proliferation assay
The 96-well microtitre plates (Costar, Cambridge, UK) were coated overnight at 4°C with 50 µl of anti-CD3 MoAb (10 µg/ml) in carbonate-bicarbonate buffer, pH 9·2. The wells were washed three times with HBSS. The CD4+ T cells (2 × 104/well) were cultured in wells in triplicate coated with anti-CD3 antibodies, with or without mitomycin C (50 µg/ml) treated splenocytes (1 × 105/well) as a source of costimulator cells. Varying concentrations (0·0001–100 µg/ml) of melatonin in complete RPMI-1640 medium, containing 10% FCS, was added to the cultures. To study the direct effect of melatonin, T cells were cultured, in the absence of anti-CD3 antibodies, with medium or SPC and varying concentrations of melatonin (0·0001–100 µg/ml). The control cultures were also set where the anti-CD3 antibody-stimulated Th cells were cultured, without SPC but with melatonin. Similarly, the effect of melatonin on the proliferation of 3DO.54·8 Th1-hybridoma cells was also monitored in the presence or absence of anti-CD3 antibodies. The cultures were incubated at 37°C in humidified atmosphere containing 7% CO2. After 72 h, the cultures were pulsed with 0·5 µCi of [3H]-thymidine and 16 h later the cells were harvested and the incorporation of thymidine was determined by using an automatic cell harvester (Skatron, Norway) and liquid scintillation counting.
Measurement of IL-2, IL-4, IFN-γ, TNF-α and IL-10
Cultures using CD4+ T cells and Th1-hybridoma were set as described in the case of the T cell proliferation assay. The IL-2 and IL-4 were measured using HT-2 cells. The culture supernatants (SN) from the experimental and control wells were collected 24 h later for the estimation of IL-2 and 48 h later for IFN‐γ and IL-4, respectively. The SNs were separated and levels of IL-2 and IL-4 were measured by their respective abilities to induce the proliferation of HT-2 cells, as described earlier [3]. All data are calculated from the mean ct/min of triplicate determinations as pg/ml of IL-2 and IL-4, by comparison with the standard curve for recombinant IL-2 or IL-4 (Genzyme).
IFN-γ and TNF-α were assayed by their ability to inhibit the proliferation of WEHI-279 [3] and WEHI-164 [11,12] cells, respectively. All the data are calculated from the mean ct/min of triplicate determinations as pg/ml, by comparison with the standard curve for recombinant IFN-γ and TNF‐α (Genzyme). IL-10 was estimated according to the manufacturer's (Genzyme) protocols by ELISA.
B cell functional assay
B cells (1 × 105/well) were cultured in triplicate in a 96-well plate (Costar, Cambridge, MA, USA) with varying concentrations of melatonin (0·0001–100 µg/ml) in the absence or presence of LPS (10 µg/ml). The plates were incubated at 37°C in a 7% CO2 atmosphere. After 72 h of incubation, [3H]-thymidine was added to the cultures and 16 h later the cells were harvested and the incorporation of thymidine was determined by using an automatic cell harvester (Skatron, Norway) and liquid scintillation counting.
Measurement of Ig-subtypes by ELISA
The cultures were set as described in the case of B cell proliferation. The production of IgM, IgA, IgG1 and IgG2a antibodies were measured in the SN collected after 6 days of culture, as described earlier [3]. Briefly, 96-well plates (Costar, Cambridge, MA, USA) were coated overnight with 5 µg/ml of goat antimouse IgM, IgA, IgG1 or IgG2a isotypes in carbonate–bicarbonate buffer (0·05 m, pH 9·6) at 4°C. Blocking was performed using 3% skimmed milk in PBS-Tween 20 for 90 min at 37°C. After incubation, log2 dilutions of test and control SN were added. The reaction was allowed to proceed at 37°C for 2 h. Microplates were then incubated at 37°C for 1 h with 50 µl of biotinylated goat antimouse IgM, IgA, IgG1 or IgG2a antibodies, and later with 50 µl of streptavidin-HRP. Finally, 50 µl of OPD.2HCl was added and incubated at 37°C for 20 min and the reaction was terminated by the addition of 50 µl of 7% H2SO4. The usual washing steps were maintained at each stage. The absorbance was read at 492 nm with a microplate reader (Eurogenetics, Torino, Italy). The concentration of antibody was expressed as microgram/ml, computed from the curves plotted for standard IgM, IgA, IgG1 and IgG2a subtypes (Sigma Co).
Macrophages functional assay
The macrophages (1 × 105 cells/well) were activated with LPS (1 µg/ml) or IFN-γ (10 U/ml) and cultured in triplicate wells with or without melatonin (0·0001–100 µg/ml) in the complete medium at 37°C in a humidified atmosphere containing 7% CO2. The culture SNs were collected after 14 h of incubation for the estimation of TNF-α, and 14 and 28 h later for measuring the NO.
Estimation of nitric oxide
Nitrite concentrations in the culture medium were measured by a microplate assay method based on the Griess reaction [12,13] and data are expressed in µm/1 × 105 cells.
Statistical analysis
The data were analysed by one-way analysis of variance (anova) followed by Dunnett's t-test. P < 0·05 was considered statistically significant.
RESULTS
Melatonin down-regulates the expression of B7-1 but not B7-2 molecules on the surface of macrophages
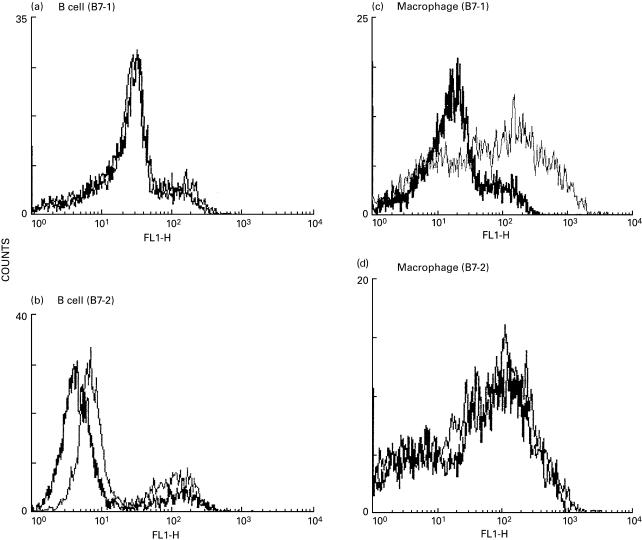
The administration of melatonin (10 mg/kg) daily for a period of 5 days to animals primed with OVA-CFA showed decreased expression of B7-1 but not of B7-2 molecules on macrophages. However, melatonin failed to influence the expression of B7-1 and B7-2 on B cells (Fig. 1). The B cells or macrophages isolated from unprimed mice, mice immunized with OVA-CFA (not treated with melatonin) or mice inoculated only with PBS or melatonin (10 mg/kg, 5 days) did not express any alteration in B7-1 and B7-2 molecules. The control cultures containing isotype-matched antibody did not produce any shift in the mean fluorescence intensity (MFI).
Fig. 1.
Expression of B7–1 (a, c) and B7–2 (b, d) molecules on B cells (a, b) and macrophages (c, d). The cells were isolated from the splenocytes of the group of OVA-immunized mice, injected with melatonin. The unprimed control group of animals, and animals inoculated with PBS did not show any change in the expression of B7 molecules. The values shown are the mean fluorescence intensity (MFI) for different groups of mice. —, PBS; —, MLT.
Melatonin induces in vitro proliferation of CD4+ T cells which are stimulated with anti-CD3 antibodies in the presence of mitomycin C treated splenocytes
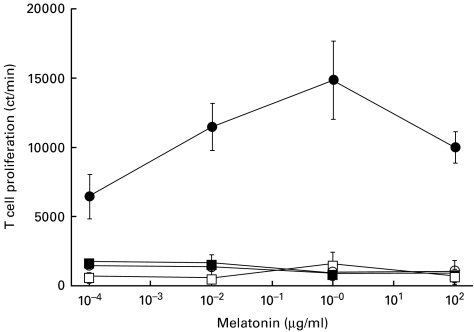
Melatonin (0·0001–100 µg/ml) failed to induce the proliferation of CD4+ T cells isolated from unprimed mice when the first signal was provided by anti-CD3 antibodies. When the mitomycin C treated splenocytes were added to the cultures of T cells stimulated with anti-CD3 antibodies, melatonin induced a statistically significant (P < 0·05) increase in the proliferation. Melatonin facilitated Th cell proliferation in a dose-dependent fashion. A maximum 9·5-fold increase in Th cells proliferation was observed at a concentration of 1 µg/ml of melatonin compared to Th cells stimulated with only anti-CD3 antibodies in the presence of mitomycin C treated splenocytes. However, melatonin could not modify the growth of T cells when mitomycin C treated splenocytes were added to the cultures of Th cells in the absence of anti-CD3-antibodies (Fig. 2).
Fig. 2.
In vitro effect of melatonin on anti-CD3 antibodies stimulated Th cell proliferation in the presence of APC. Anti-CD3 antibodies elicited CD4+ T cells were cultured with melatonin and splenocytes. CD4+ T cells cultured with anti-CD3 antibodies in the presence of APC induce 6395 ± 572 cpm. Cells incubated with the medium alone or in the presence of APC showed < 2000 cpm. Data are the mean + s.d. of ct/m for three experiments. • αCD3 antibodies + SPC; ○ αCD3 antibodies; ▪ SPC; □, medium.
Melatonin induces the secretion of IL-4 and IL-10 but decreases IL-2 and IFN-γ production by Th cells stimulated with anti-CD3 antibodies in presence of APC
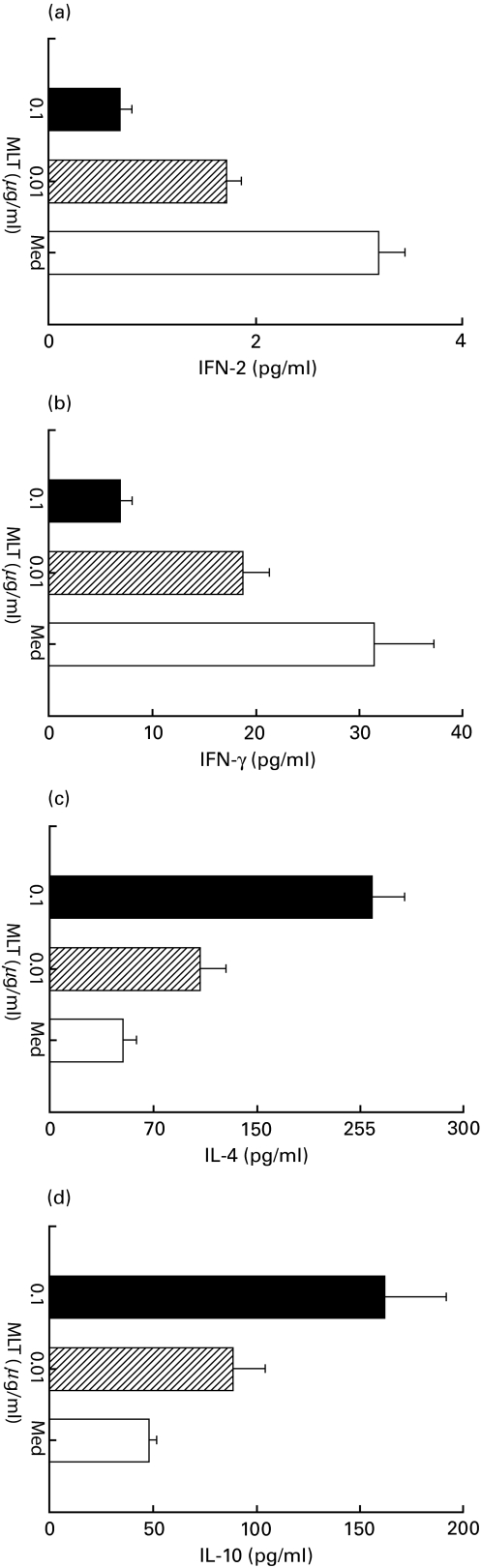
Melatonin at a concentration of 0·01 and 1 µg/ml induced a statistically significant fall (P < 0·05) in the levels of IL-2 and IFN-γ in the culture SN of Th cells stimulated with anti-CD3 antibodies in the presence of mitomycin C treated splenocytes. The maximum of 76·7% and 78·1% inhibition in the production of IL-2 and IFN-γ, respectively, was observed in the presence of 1 µg/ml of melatonin. In contrast, the secretion of IL-4 and IL-10 was significantly enhanced by melatonin. The yield of IL-4 and IL-10 was enhanced to a maximum of 340% and 238%, respectively, by 1 µg/ml of melatonin (Fig. 3). In the control cultures no detectable change in the output of lymphokines was observed in the case of anti-CD3 antibodies elicited Th cells stimulated with melatonin in the absence of splenocytes. Also, melatonin failed to induce lymphokine production by Th cells in the absence of anti-CD3 antibodies.
Fig. 3.
In vitro effect of melatonin on the secretion of (a)IL-2, (b) IFN-γ, (c) IL-4 and (d) IL-10 from anti-CD3 antibodies stimulated Th cells in the presence of APC. IL-2 and IL-4 activity was assayed using HT-2 cells in the presence of anti-IL-4 antibodies (1 µg/ml) and anti-IL-2 + IL-2R antibodies, respectively. The production of IFN-γ was measured by WEHI-279 cells and IL-10 secretion by ELISA. No significant levels of cytokines were detected in the case of Th cells cultured with medium alone or stimulated with melatonin in the absence of splenocytes. All the data obtained were as a result of three determinations and expressed as mean ± s.d. as computed by comparison with the standard curves for recombinant cytokines (Genzyme). □ Medium; MLT (0·01 μg/ml); ▪ MLT (1·0 μg/ml).
Melatonin inhibits the proliferation and secretion of IL-2 by Th1 hybridoma
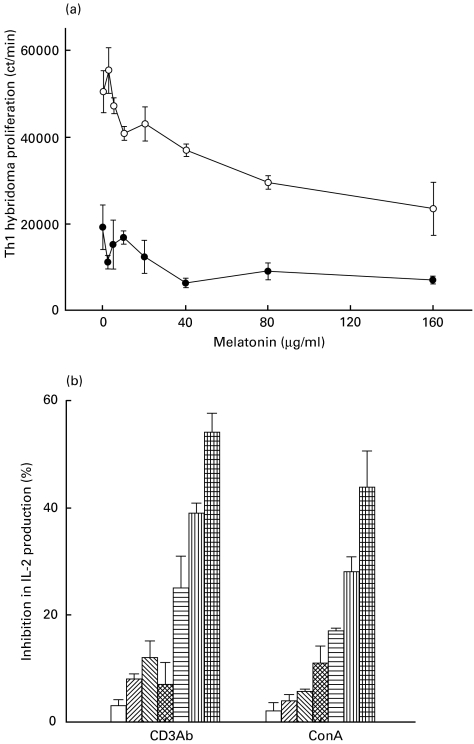
To support our previous observation that melatonin down-regulates the activity of Th1 cells, we further evaluated its effect on 3DO.54·8-Th1-hybridoma. Melatonin alone significantly obstructed the proliferation of Th1 hybridoma cells. In the concentration range of melatonin we studied, the maximum suppression of cell proliferation (27%) was observed at a dose of 40 µg/ml. A similar concentration of melatonin (40 µg/ml) in conjunction with anti-CD3 antibodies (2 µg/ml) significantly (P < 0·05) enhanced its inhibitory (88%) activity. The Th1 hybridoma incubated with anti-CD3 antibodies showed a 19154 ± 5076 cpm compared to a 50433 ± 4827 cpm of cells cultured with medium alone (in the absence of antibody) (Fig. 4a). Further, melatonin also inhibited the production of IL-2 by Th1 hybridoma in a dose-dependent manner. The highest inhibition by 100 µg/ml of melatonin in the levels of IL-2 in the presence of anti CD3 antibodies or ConA activated Th1 hybridoma were 54% and 44%, respectively (Fig. 4b).
Fig. 4.
(a) Melatonin inhibits the proliferation of 3DO.54·8-Th1-hybridoma. Th1-hybridoma cells were cultured with anti-CD3 antibodies in the presence of melatonin. The proliferation was measured by incorporation of 3H-thymidine. The data are represented as the mean ± s.d. of the cpm. •, αCD3 antibodies + MLT; ○, MLT. (b) Melatonin inhibits the secretion of IL-2 by Th1 hybridoma. The cultures were set as in (a). After 24 h of incubation of Th1-hybridoma, SN were collected and IL-2 levels were checked using HT-2 cells. The results expressed as the percentage inhibition in the IL-2 production were calculated by comparing the secretion of IL-2 by Th1-hybridoma incubated with anti-CD3 antibodies/ConA alone with the secretion from cells cultured with anti-CD3 antibodies/ConA and melatonin. □, 1·0 μg/ml; , 10 μg/ml;
, 10 μg/ml; , 20 μg/ml;
, 20 μg/ml; , 40 μg/ml;
, 40 μg/ml; , 60 μg/ml;
, 60 μg/ml; , 80 μg/ml; ⊞, 100·00 μg/ml.
, 80 μg/ml; ⊞, 100·00 μg/ml.
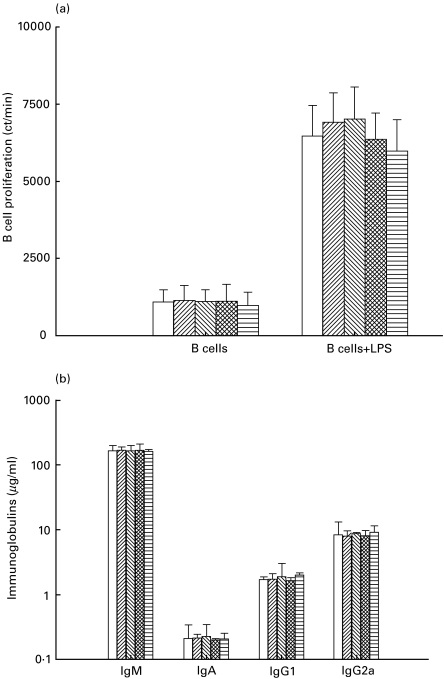
Melatonin failed to promote the LPS-stimulated B-cells proliferation and antibody secretion
Melatonin (0·0001–100 µg/ml) failed to show any significant changes in either proliferation or secretion of immunoglobulins (IgA, IgM, IgG1 and IgG2a) by B cells isolated from splenocytes of unprimed mice. Melatonin also failed to modulate the proliferation or secretion of Ig-isotypes by LPS (10 µg/ml) stimulated B cells (Fig. 5a,b).
Fig. 5.
Melatonin failed to modulate the activity of LPS activated B cells. The B cells were cultured with LPS and melatonin. The proliferation of the cells was monitored by scintillation spectroscopy (a). The cultures SN were collected from triplicate wells of control and experimental cultures on day 6, pooled and analysed for Igs production. Melatonin, in the absence of LPS, failed to induce secretion of Igs by B cells. Antibody concentration is expressed in microgram/ml as computed from the standard curves plotted for standard murine IgM, IgA, IgG1 and IgG2a (Sigma Co) (b). All the values are expressed in mean ± s.d. from triplicate samples. □, No MLT; , 0·0001 μg/ml;
, 0·0001 μg/ml; , 0·01 μg/ml;
, 0·01 μg/ml; , 1·0 μg/ml;
, 1·0 μg/ml; , 100·00 μg/ml.
, 100·00 μg/ml.
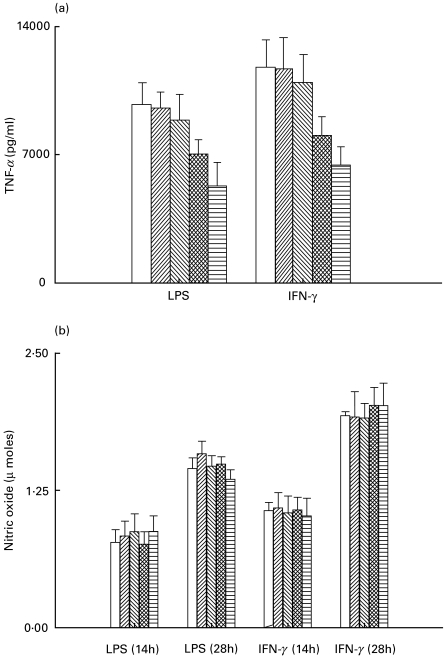
Melatonin suppressed the production of TNF-α but not the release of nitric oxide by LPS or IFN-γ elicited macrophages
Melatonin inhibited the LPS (1 µg/ml) or IFN-γ (10 U/ml) induced release of TNF-α by macrophages. A statistically significant (P < 0·05) suppression of TNF-α was observed at concentrations of 1 and 100 µg/ml of melatonin (Fig. 6a). However, the same concentrations of melatonin (0·0001–100 µg/ml) failed to inhibit LPS (1 µg/ml) or IFN-γ (10 U/ml) induced release of NO from macrophages at both the time intervals (14 and 28 h) studied (Fig. 6b).
Fig. 6.
Melatonin suppresses the secretion of TNF-α but not of nitric oxide production by the macrophages activated by either LPS or IFN-γ. For monitoring TNF-α and NO, the splenic macrophages were cultured with IFN-γ and LPS, and the SNs were collected from the triplicate cultures of control and experimental wells. The TNF-α was measured using WEHI-164 cells. The results expressed as pg/ml were computed by comparison with the standard curve for recombinant TNF-α (Genzyme) (a). Nitric oxide was estimated by microplate assay based on the Griess reaction (b). The data are the mean ± s.d. of three determinations. □, No MLT; , 0·0001 μg/ml;
, 0·0001 μg/ml; , 0·01 μg/ml;
, 0·01 μg/ml; , 1·0 μg/ml;
, 1·0 μg/ml; , 100·00 μg/ml.
, 100·00 μg/ml.
DISCUSSION
Melatonin is regarded as a major hormone of the pineal gland. It is also secreted by lymphocytes and plays an important role in the modulation of the immune system [5,14]. It has been reported that administration of melatonin increases antigen presentation by splenic macrophages and antibody response by B cells to various antigens [15]. It also restores the antibody production and Th cell activity in mice immunosuppressed by ageing, by acute stress or by corticosterone and cyclophosamide treatment [8–10,16].
We have demonstrated earlier that the daily administration of melatonin after a secondary antigen challenge augmented the Th2 activity and decreased the Th1 response. This is shown by the enhanced production of antigen-specific IgG1 antibody and secretion of IL-4 and by the decrease in the yields of IL-2 and IFN-γ and of the IgG2a antibody [10]. The main aim of the present study was to study whether melatonin can play the role of a co-stimulatory signal in activating CD4+ T cells. Here we report for the first time the effect of melatonin on purified populations of unprimed CD4+ T cells, LPS primed B cells and macrophages. Six major findings have emerged from this study. Melatonin: (1) down-regulated the expression of B7-1 but not of B7-2 on the surface of macrophages, but failed to induce any change with LPS-primed-B cells; (2) significantly enhanced the proliferation of anti-CD3 antibodies elicited naive CD4+ T cells in the presence of APC; (3) substantially inhibited secretion of IL-2 and IFN-γ, but enhanced production of IL-4 and IL-10; (4) suppressed the proliferation and secretion of IL-2 by Th1 hybridoma; (5) had no effect on the proliferation and differentiation of LPS stimulated B cells; and (6) inhibited secretion of TNF-α but had no effect on the release of NO by LPS and IFN-γ stimulated macrophages.
On the basis of distinct patterns of lymphokine profiles, CD4+ Th cells can be subdivided into Th1 and Th2 cells. Th1 cells are defined by the production of IL-2, IFN-γ, TNF-β, etc. and induce cell-mediated immunity, whereas Th2 cells secrete IL-4, IL-5, IL-10, etc. and are responsible for humoral immunity [4]. The differentiation of naive CD4+ T cells into Th1 and Th2 cells is influenced by a number of factors including antigen dose, the nature of the peptide/MHC complex, exposure to cytokines and co-stimulatory molecules and the kind of adjuvant and type of antigen-presenting cells. The cytokine milieu present during the priming of naive T cells determines the ultimate pathway of differentiation [1–3,17–19]. Moreover, it has been established that an encounter with an antigen may lead to two distinct outcomes: activation and anergy. Which outcome occurs depends on the delivery of appropriate co-stimulatory signals to the T cells. Hence, for the activation of naive T cells two signals are essential: (1) occupancy of TcR by peptide–MHC complex and (2) delivery of co-stimulatory signals (B7-1, B7-2, CD40, ICAM-1, etc.). The two signals together may be sufficient for the activation of T cells, but for enhancement of the growth of T cells may require a third signal (in the form of factors such as IL-1, IL-2, IL-6, TNF-α, and IL-7, etc.) [20–22]. In the present study, we have established categorically that melatonin alone cannot provide either signal 1 or signal 2 to naive CD4+ T cells but can only function when a bystander APC is also provided. It can only deliver signal 3 leading to the clonal expansion of Th cells and the predominant secretion of the Th2-like cytokines IL-4 and IL-10. Thus it is evident that melatonin can amplify clonally the Th2 cells which have already received the two signals and cannot substitute for the co-stimulatory molecules.
It is known that B7-1 preferably activates Th1 cells and B7-2 influences the activity of Th2 cells [3,23]. We thought that it would be of interest to see whether melatonin regulates Th2 activity by modulating the expression of B7-1 and B7-2 molecules. Interestingly, melatonin down-regulated the expression of B7-1 on macrophages. It may be mentioned here that melatonin also decreased the production of IFN-γ and IL-2 and enhanced the secretion of IL-4 and IL-10 by naive CD4+ T cells. Hence our study establishes emphatically down-regulation of B7-1 with decrease in Th1 activity and thus supports earlier findings [23]. Consequently, melatonin may be augmenting Th2 activity by decreasing the expression of B7-1. Furthermore, we have established using Th1 hybridoma that melatonin can directly inhibit the activity of Th1 cells. Therefore, it can be safely inferred that melatonin not only interacts with Th2 cells and boosts their performance but also controls the activity of Th1 cells.
In the present study, attention has also been focused on the direct effect of melatonin on B cell. To date, B cell functional studies using melatonin have analysed the production of Ig in response to antigens that require T cell participation [8,10,16]. The secretion of Ig isotypes by B cells is either independent of T cell help or is regulated by the interaction with Th2 clones [24]. Unlike the observation in vivo, melatonin did not alter the proliferation or differentiation of either unprimed B cells or B cells primed with LPS. Similarly, no change in the expression of B7-1 and B7-2 on B cells was noticed. It has been shown earlier that melatonin appears to have no immunomodulatory effect on rat lymphocytes [25]. To the best of our knowledge this is the first report showing an effect of melatonin on B cells in the absence of T cells. Therefore, it appears that melatonin mediates its effect on B cells through Th cells, leading to the production of IL-4, which in turn acts on B cells to promote the secretion of IgG1 antibodies. Melatonin may enable Th2 cells to provide enhanced help to B cells.
In the concentration range studied, melatonin significantly attenuated the production of TNF-α by LPS as well as IFN-γ elicited macrophages but failed to inhibit the secretion of nitric oxide. Activation of the nuclear factor kappa B (NFκB) is supposed to be one of the principal mechanisms by which LPS induces its cellular processes [26,27]. NFκB thus activated initiates the transcription of early response genes such as TNF-α, IL-1, GM-CSF, COX-2 and iNOS [28]. In fact, inhibition of NFκB has been proposed recently as a mechanism by which melatonin exerts its inhibitory effect on NO production [12,29]. However, our result shows that melatonin produces no significant change in NO release, but it was effective in suppressing LPS-induced TNF-α release from macrophages. The discrepancy in our result may be due to the low concentration of melatonin used (i.e @0·86 pm to 0·86 µm). However, Gilad et al. [29] observed the suppression of NO release subsequent to inhibition of iNOS enzyme expression at a higher concentration (i.e. 10 µm and above).
Preferential production of cytokines typical of Th2 cells and suppression of Th1-like cytokines, inhibition of TNF-α release and down-regulation of B7-1 co-stimulatory molecule are of particular interest in the regulation of inflammation and autoimmunity [30–32]. Moreover, melatonin has an ability to regulate these factors effectively. Accordingly, there may be beneficial effect in overcoming autoimmune and inflammatory diseases by decreasing the Th-1 activity and B7-1 expression and by enhancing Th2 cell performance. It is worth mentioning here that melatonin is effective in treating experimentally induced arthritis [33].
In conclusion, the present study has shown that melatonin cannot be substituted as a co-stimulatory signal. It cannot provide the second signal to anti-CD3 antibodies elicited naive CD4+ T cells. However, in the presence of APC, it helps in the clonal expansion of Th2 cells. Further, melatonin does not act directly on B cells to produce antibodies. It regulates the secretion of pro-inflammatory cytokine TNF-α by a macrophage and down-regulates the expression of the co-stimulatory molecule B7-1.
Acknowledgments
We are thankful to Dr S.K. Basu and Dr R. Anand, National Institute of Immunology, New Delhi for providing the animals and Mr Susmit Suvas for helping in FACS analysis. We are indebted to Dr Aldric L. Brown for a native English correction of our work. We also appreciate the secretarial help provided by Mr Dinesh Verma. The Senior Research Fellowship (V.R. and V.S.) of the Council of Scientific and Industrial (CSIR), New Delhi, is gratefully acknowledged.
REFERENCES
- 1.Janeway CA, Jr, Bottomly K. Signals and signs for lymphocyte response. Cell. 1994;76:275–85. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–9. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 3.Agrewala JN, Suvas S, Verma RK, et al. Differential effect of anti-B7–1 and anti-M150 antibodies in restricting the delivery of costimulatory signals from B cells and macrophages. J Immunol. 1998;160:1067–77. [PubMed] [Google Scholar]
- 4.Bottomly KA. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1998;9:268–74. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- 5.Liebmann PM, Wolfer A, Felsner P, et al. Melatonin and the immune system. Int Arch Allergy Immunol. 1997;112:203–11. doi: 10.1159/000237455. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Haba MG, Garcia-Maurino S, Calvo JR, et al. High-affinity binding of melatonin by human circulating T lymphocytes (CD4+) FASEB J. 1995;9:1331–5. doi: 10.1096/fasebj.9.13.7557023. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Perganda A, Guerrero JM, Rafii-El-Idrissi M, et al. Characterization of membrane melatonin receptor in mouse peritoneal macrophages: inhibition of adenylcyclase by a pertusis toxin-sensitive G protein. J Neuroimmunol. 1999;95:85–94. doi: 10.1016/s0165-5728(98)00268-9. [DOI] [PubMed] [Google Scholar]
- 8.Caroleo MC, Frasca D, Nistico G, et al. Melatonin as an immunomodulatory in immunodeficient mice. J Immunopharmacol. 1992;23:81–9. doi: 10.1016/0162-3109(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 9.Maestroni GJM, Conti A, Pierpaoli W. Role of pineal gland in immunity. 1. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986;13:19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- 10.Shaji AV, Kulkarni SK, Agrewala JN. Regulation of secretion of IL-4 and IgG1 isotype by melatonin-stimulated ovalbumin-specific T cells. Clin Exp Immunol. 1998;111:181–5. doi: 10.1046/j.1365-2249.1998.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaraman S, Martin CA, Dorf ME. Enhancement of in vivo cell-mediated immune responses by three distinct cytokines. J Immunol. 1990;144:942–51. [PubMed] [Google Scholar]
- 12.Raghavendara V, Agrewala JN, Kulakarni SK. Melatonin reversal of lipopolysacharides-induced thermal and behavioral hyperalgesia in mice. Eur J Pharmacol. 2000;395:15–21. doi: 10.1016/s0014-2999(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 13.Di Rosa M, Radomski M, Carnuccio R, et al. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990;172:1246–52. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- 14.Finocchiaro LME, Nahmod VE, Launay JM. Melatonin biosynthesis and metabolism in peripheral mononuclear leukocytes. Biochem J. 1991;280:727–31. doi: 10.1042/bj2800727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piloi C, Caroleo MC, Nistico G, et al. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int J Immunopharmacol. 1993;15:463–8. doi: 10.1016/0192-0561(93)90060-c. [DOI] [PubMed] [Google Scholar]
- 16.Caroleo MC, Doria G, Niatico G. Melatonin restores immunodepression in aged and cyclophosphamide-treated mice. Ann NY Acad Sci. 1994;719:343–52. doi: 10.1111/j.1749-6632.1994.tb56841.x. [DOI] [PubMed] [Google Scholar]
- 17.Paul WE, Seder RA. Lymphocytes responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 18.Weaver CT, Unanue ER. The co-stimulatory function of antigen presenting cells. Immun Today. 1990;11:49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- 19.Agrewala JN, Wilkinson RJ. Influence of HLA-DR on the phenotype of CD4+ T lymphocytes specific for an epitope of the 16-kDa α-crystalline antigen of Mycobacterium tuberculosis. Eur J Immunol. 1999;29:1753–61. doi: 10.1002/(SICI)1521-4141(199906)29:06<1753::AID-IMMU1753>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 21.Wingren AG, Parra E, Varga M, et al. T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles. Crit Rev Immunol. 1995;15:235–53. doi: 10.1615/critrevimmunol.v15.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 22.Sepulveda H, Cerwenka A, Morgan T, et al. CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation. J Immunol. 1999;163:1133–42. [PubMed] [Google Scholar]
- 23.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 24.Stevens TL, Bossie A, Sanders VM, et al. Regulation of antibody isotype secretion by subset of antigen specific helper T cells. Nature. 1988;334:2525–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 25.Pahlavani MA, Harris MD. In vitro effects of melatonin on mitogen-induced lymphocyte proliferation and cytokine expression in young and old rats. Immunopharmacol Immunotoxicol. 1997;19:327–37. doi: 10.3109/08923979709046979. [DOI] [PubMed] [Google Scholar]
- 26.Muller JM, Ziegler-Heitbrock HW, Baeucrle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–56. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- 27.Raghavendra V, Agrewala JN, Kulkarni SK. Role of centrally administered melatonin and inhibitors of COX and NOS in LPS- induced hyperthermia and adipsia. Prostagland Leukotrienes Essen Fatty Acids. 1999;60:249–53. doi: 10.1054/plef.1999.0032. [DOI] [PubMed] [Google Scholar]
- 28.Baeuerle PA, Henkel T. Functional and activation of NFkB in the immune system. Annu Rev Immunol. 1994;12:141–9. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 29.Gilad E, Wong HR, Zingarelli B, et al. Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: role of inhibition of NFkappaB activation. FASEB J. 1998;12:685–93. doi: 10.1096/fasebj.12.9.685. [DOI] [PubMed] [Google Scholar]
- 30.Guerder S, Picarella DE, Linsley PS, et al. Costimulator B7–1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor alpha leads to autoimmunity in transgenic mice. Proc Natl Acad Sci U S A. 1994;91:5138–42. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Current Opinion Immunol. 1996;8:837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 32.Haworth C, Brennan FM, Chantry D, et al. Expression of granulocyte-macrophage colony stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-α. Eur J Immunol. 1991;21:2575–9. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- 33.Missbach M, Jagher B, Sigg I, et al. Thiazolidine diones, specific ligands of the nuclear receptor retinoid Z receptor/Retinoid acid receptor-related orphan receptor α with potent antiarthritic activity. J Biol Chem. 1996;271:13515–22. doi: 10.1074/jbc.271.23.13515. [DOI] [PubMed] [Google Scholar]