Abstract
The aim of the present study was to investigate whether the IL-1 family cytokines, in addition to IL-6 and IL-8, could be induced in normal human cortical epithelial cells in response to bacterial stimuli. Human renal tissue was obtained from 9 patients undergoing elective tumour nephrectomy. Renal cortical epithelial cells of tubular origin were prepared from the unaffected tissue. The proximal tubular cells were stimulated for 2, 6 and 24 h with a heat-inactivated pyelonephritogenic Escherichia coli strain DS-17. Cultured unstimulated tubular cells served as controls. IL-1α, IL-1β, IL-1 receptor antagonist, IL-6, IL-8, IL-10, TNF-α, G-CSF and GM-CSF were analysed using immunohistochemistry at the single cell level. The nonstimulated cells were found to express low levels of IL-6 and IL-8 (mean value < 3% of total cells). In contrast, E. coli exposure resulted in significantly increased incidences of IL-6 and IL-8 expressing cells (mean values ≈18% of total cells) peaking within two hours of stimulation (P < 0·008 and P < 0·02 versus non-stimulated cells, respectively). A gradual decrease was thereafter observed at 6 and 24 h, respectively, although persistently higher compared to controls. A different kinetic response was found for IL-1α, IL-1β and IL-1 receptor antagonist-expressing cells, which peaked 24 h after E. coli stimulation (mean values 3–10%) (P < 0·008, P < 0·02, P < 0·02 versus non-stimulated cells, respectively). Low levels of TNF-α and GM-CSF were found in 3 of the 9 donated epithelial cells, peaking at 2 h, and IL-10 and G-CSF producing cells in 1 patient each. In conclusion we found that heat-inactivated pyelonephritic E. coli induced a proinflammatory cytokine response in the normal human proximal tubular cells including the IL-1 family, IL-6 and IL-8.
Keywords: IL-1, IL-6, IL-8 human tubular epithelial cells
INTRODUCTION
Gram-negative infections rapidly induce an inflammatory response in which the cytokine network plays a major role. Lipopolysaccharides (LPS) released from these bacteria may be a major immunogen contributing to the cytokine burst by the LPS–LPS binding protein (BP)–CD14 complex formation [1]. Despite the fact that epithelial cells in the urinary mucosa lack CD14 expression, up-regulation of MHC class II and NO production through iNOS activation has been reported consistently following Escherichia coli infection. The altered NO production by renal cells may contribute to the tubulointerstitial inflammation found in these patients [2]. Most of these innate responses are mediated by pro-inflammatory cytokine production. Interleukin-1 (IL-1), IL-1 receptor antagonist (IL-1ra), tumour necrosis factor-α (TNF-α), IL-6 and IL-8 are predominantly produced in granulocytes and macrophages. IL-1α and IL-1β are both agonists and IL-1ra is an endogenously occurring competitive antagonist. They bind to the same receptors, but despite the similarity of IL-1α and IL-1β in size and function they have only 20% homology in the human amino acid sequences [3]. The activity of IL-1 overlaps greatly with that of TNF in the induction of the innate immune response [4]. IL-6, on the other hand, acts in both pro- and counterregulating fashion [5,6], while the major effect of the α-chemokine, IL-8, is chemoattractant on granulocytes [7]. Both IL-6 and IL-8 have been shown previously to be derived after stimulation with E. coli in transformed epithelial cell-lines [8,9].
The aim of the present study was to investigate whether the IL-1 family cytokines could be induced, in addition to IL-6 and IL-8, by normal human renal cortical epithelial cells of tubular origin in response to bacterial stimuli.
MATERIALS AND METHODS
Bacteria
E. coli DS-17 was used to stimulate the human renal tubular cells. The wild-type E. coli strain DS-17 was isolated originally during an epidemic outbreak of pyelonephritis in a neonatal ward. It is of serotype O6:K5:H-, expresses P-fimbriae with a Class II G-adhesin and type 1 fimbriae but lacks S-fimbriae and Afa-1 adhesin. The strain produces haemolysin and the siderophore aerobactin. It is resistant to ampicillin and trimethoprim-sulphonamide but sensitive to ciprofloxacin.
The DS-17 strain was cultured overnight in 37°C on colony factor antigen agar. Bacterial colonies were suspended in phosphate buffered saline (PBS), washed three times by repeated cycles of centrifugation at 3500 r.p.m. (Wifug Lab centrifuge) for 15 min. Bacterial suspension was heat inactivated for 60 min at 70°C. The DS-17 bacteria were finally resuspended in PBS to a final concentration of 108 cells/ml. Efficacy of heat-inactivation was controlled by culturing the bacterial suspension and no bacterial growth was found.
Epithelial cell cultures
Human renal tissue was obtained from nine renal cell carcinoma patients (median age 70, range 40–81 years, 6 women and 3 men) undergoing elective tumour nephrectomy. Proximal tubular cells were prepared as described previously [10]. In short, parts of the outer cortex from morphologically unaffected tissue were excised, decapsulated and minced to 1 mm fragments with a scalpel. The parenchyma was incubated overnight in culture medium RPMI 1640 (Life Technologies, USA) supplemented with 2 mm l-glutamine, 10 mm N′-2-hydroxyetylpiperazine-N′-2-ethanesulphonic acid (HEPES) (Gibco BRL, Gaithersburg, MD, USA), bensylpenicillin (100 U/ml) and streptomycin (100 µ;g/ml), AB serum 10% and during the first passage collagenase VIII (1 mg/ml) (Sigma, St Louis, MO, USA) in 75 cm2 culture flasks (Nunc, Roskilde, Denmark). Half of the medium was changed every second day during the first week. The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2−95% O2. After the first week, the cell cultures were fed with fresh culture medium every third day and reached confluence in 10–14 days. The cells exhibited an epithelial morphology with a central nucleus, granular cytoplasm and cobblestone appearance in light microscopy. In 3 weeks the monolayers formed domes, indicating vectorial transport. In electron microscopy, numerous apical microvilli were observed. Ninety-five per cent of the cells showed positive cytokeratin staining (antipan cytokeratin clone C-11) (Sigma). The renal cells were negative for factor VIII staining (antivwf clone f8/86, Dako A/S, Glostrup, Denmark) indicating no significant contamination by endothelial or glomerular cells (< 5%).
Confluent monolayers of human proximal tubular cells were washed in calcium-free phosphate buffer (pH 7·4) and passaged by trypsinization with 0·25% trypsin and 0·02% versene (Life Technologies). The trypsin was inactivated by addition of RPMI 1640 with 10% human AB serum. Detached cells were then washed once in centrifugation tubes in RPMI 1640, counted in a Bürker chamber and resuspended in culture medium (5 × 105 cells/ml). For cryopreservation, the cells were resuspended in culture medium supplemented with 10% DMSO (dimethylsulphoxide) (Sigma), gradually frozen and stored at − 135°C. For recovery, the vials were thawed at 37°C and washed once in RPMI 1640 with 10% human AB serum before reseeding. Viability was assessed by trypan blue exclusion and by the CFDA method, described elsewhere [11]. Briefly, cells were incubated with carboxy fluorescein diacetate (CFDA)(10 µg/ml) (Becton and Dickinson Immunocytometry Systems) for 15 min at room temperature, followed by one wash in PBS. CFDA is retained and hydrolysed by intracellular esterases, yielding green fluorescence. The fluorescence intensity was analysed with flow cytometry (Epics XL, Beckman Coulter Inc., Hialeah, FL, USA), and results are expressed as the percentage of CFDA positive cells. Unlabelled cells were run in parallel to define the cut-off for positive staining.
Bacterial stimulation of epithelial cells
The epithelial cells were cultured in 150 cm2 culture flasks (Nunc) as above until subconfluenced, trypzinated, washed twice in calcium-free phosphate buffer (pH 7·4) and resuspended into RPMI 1640 with 10% human AB serum to a concentration of 106. Heat inactivated E. coli strain DS-17, in a ratio of 100 bacteria per cell, was added as stimuli to the epithelial cells. RPMI 1640 with 10% human AB serum was used as negative control. One sample of non-stimulated cells was immediately harvested for fixation. Stimulated cells and unstimulated cultured cells were incubated in 37°C, 5% CO2, for 2, 6 or 24 h, respectively.
Fixation and cytokine staining of the human renal proximal tubular cells
Cultured cells were harvested after indicated periods of time and washed with Earls buffer salt solution (EBSS, Gibco Ltd, Paisley, UK) containing Ca2+ and Mg2+ supplemented with 0·01 m HEPES buffer and transferred to adhesion slides (Bio-Rad, GMBH, Munich, Germany). The method used for cytokine staining at the single-cell level has been described previously [12]. In short, viable cells were allowed to bind to the slide surface for 15 min at room temperature, and fixed in 2% formaldehyde in PBS, pH 7·4 (Sigma) for 15 min and stored in − 20°C until assayed. Slides were rehydrated in EBSS and the endogenous peroxidase activity was blocked by 30 min incubation of 1% H2O2 in EBSS supplemented with 0·1% saponin. Saponin permeabilizes the cell membrane and the Golgi complex. Unspecific Fc receptor interactions were blocked with the addition of 5% human AB-serum in EBSS for 5 min at 37°C. Presence of endogenous biotin was blocked with avidin/biotin (Vector Laboratories Burlingame, CA, USA) supplemented with 0·1% saponin and incubated for 15 min each. Thereafter the cells were incubated for 30 min with a panel of cytokine-specific monoclonal antibodies (2–5 µg/ml) dissolved in EBSS and 0·1% saponin (Table 1). An isotype-specific control antibody was used to confirm specificity of the staining. To prevent non-specific hydrophobic interactions, the cells were incubated with normal goat serum for 30 min. Biotinylated goat antimouse immunoglobulin G1 (IgG1) and biotinylated goat antirat IgG were used as secondary antibodies and incubated for 30 min. Finally the cells were incubated with Vectastain avidin-biotin horseradish peroxidase complex (Vector Laboratories) for 30 min and a colour reaction was developed during 8–10 min by 3′-diaminobenzidine tetrahydrochloride (DAB) (Vector Laboratories) for peroxidase staining. The nucleated cells were counterstained blue with Mayer's haematoxylin for 3 s. The slides were then mounted with phosphate-buffered glycerol. We have shown previously that cytokine positive cells had a specific juxtanuclear staining pattern which was in total congruence with the mRNA expression as measured by reversed transcriptase-polymerase chain reaction (RT-PCR) and in situ hybridization [13]. In addition two-colour staining combined with mRNA detection by in situ hybridization and cytokine protein expression with the saponin paraformaldehyde-based immunostaining induced a 98% colocalization of the cytokine both at the messenger and protein levels [14].
Table 1.
Description of cytokine-specific antibodies used in the human epithelial cells
| Cytokine | Antibody | Isotype | Manufacturer |
|---|---|---|---|
| IL-1α | 1277–89–7 | Mouse IgG1 | Immunokontakt, Switzerland |
| 1277–82–29 | |||
| 1277–143–4 | |||
| IL-1β | 2-D-8 | Mouse IgG1 | Immunokontakt, Switzerland |
| IL-1ra (mix) | 1384–92–17–19 | Mouse IgG1 | Biomedicals, Switzerland |
| IL-6 | MQ2–6A3 | Rat IgG2a | PharMingen, CA, USA |
| IL-8 | NAP-1 | Mouse IgG1 | M. Ceska, Sandoz, Vienna, Austria |
| IL-10 | JES3–19F1 | Rat IgG2A | PharMingen, CA, USA |
| TNF-α | MoAb 1 and 11 | Mouse IgG1 | PharMingen, CA, USA |
| G-CSF | BVD13–3A5 + | Rat IgG1 | PharMingen, CA, USA |
| BVD11–37G1 | Rat IgG2a | ||
| GM-CSF | BVD2–21C11 | Rat IgG2A | PharMingen, CA, USA |
Statistical method
Wilcoxon signed-rank test was used for comparison of cytokine expressing cells in unstimulated versus stimulated cultured epithelial cells.
RESULTS
Cytokine-producing human renal proximal tubular cells
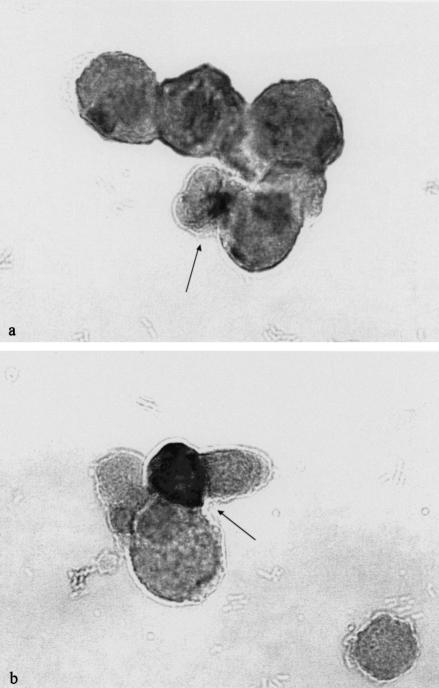
Cytokine-producing renal tubular epithelial cells generated different staining morphology depending on the type of cytokine studied. Two different patterns of cytokine staining were observed. TNF-α, IL-6, IL-8, IL-10, G-CSF and GM-CSF generated a restricted staining to the juxtanuclear area of the Golgi stacks (Fig. 1a). Exogenous addition of recombinant or natural cytokines including TNF, IL-6 and G-CSF to these epithelial cells never generated this specific perinuclear staining pattern. In addition, two-colour staining confirmed that the staining signal was localized to the Golgi endoplasmatic reticulum [15]. All cytokines listed above have a leader sequence, which directs their secretion through the Golgi-endoplasmatic pathway. The identification of these types of cytokine-producing cells was therefore facilitated by the localized reactivity to the Golgi complex. In contrast, staining for IL-1α, IL-1β and IL-1ra generated a diffuse cytoplasmic staining including the perinuclear site (Fig. 1b). The identification of the IL-1 expressing cells thus required both a diffuse cytoplasmic and a nuclear staining signal. This may be explained by the absence of a leading sequence in the IL-1 family [16]. Preincubation with recombinant or natural cytokine with the cytokine-specific monoclonal antibody caused total abolition of the staining signal. Renal epithelial cells were also allowed to adhere to culture slides before E. coli stimulation. Compared to stimulation of renal cells in suspension, no significant differences regarding cytokine production was observed (data not shown). Viability, judged as CFDA-positive cells, was > 90% in cell cultures harvested at initiation of the cell culture period, and 70–90% in cell cultures harvested after 24 h. These figures were confirmed by trypan blue exclusion. Both non-stimulated cells and cells stimulated with E. coli revealed the same values.
Fig. 1.
(a) Photomicrograph illustrating morphological characteristics of renal proximal tubular cell expression for IL-6, IL-8, IL-10, G-CSF, GM-CSF and TNF-α following stimulation with heat-inactivated E. coli. The picture shows the staining morphology of the juxtanuclear focal staining signal with anti-IL-6 monoclonal antibodies. Original magnification × 693. (b) Comparative pattern of renal proximal tubular cell staining for the IL-1 family following stimulation with heat-inactivated E. coli. The figure shows the diffuse staining morphology that occurs throughout the cytoplasm as well as generating a perinuclear site with staining for IL-1 isotypes. Original magnification × 693.
Constitutive cytokine expression in cultured unstimulated tubular epithelial cells
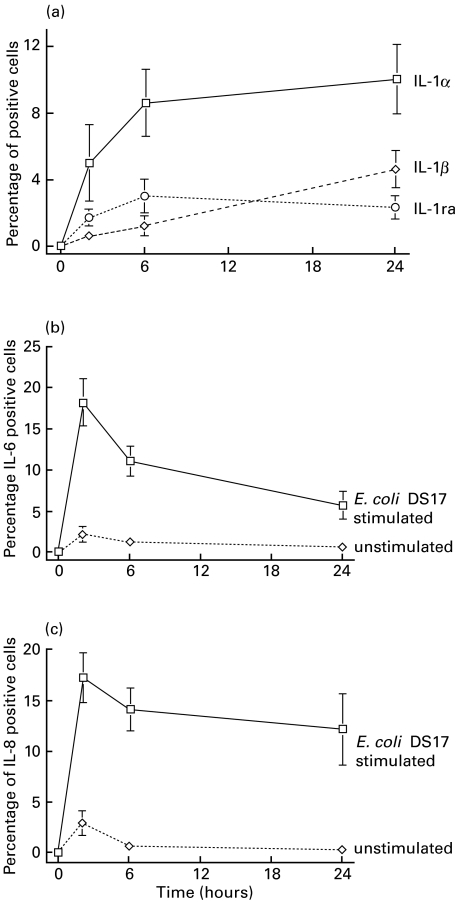
In non-stimulated cultured cells < 3% of the cells showed a constitutive protein expression of IL-8 and even less for IL-6. The IL-1 group of cytokines were not expressed at any time-point (Fig. 2a,b,c). Nor could we detect any constitutive expression of TNF-α, IL-10, G-CSF or GM-CSF.
Fig. 2.
(a) Kinetic response curve showing the incidence of IL-1α, IL-1β and IL-1 receptor antagonist-expressing cells (percentage of positive cells, mean values and s.e.m.) after challenge with E. coli DS-17. The incidence of IL-1α, IL-1β and IL-1ra-expressing cells in the unstimulated cultured epithelial cells were always below 1/1000 cells. The data are means of experiments performed in the epithelial cells in nine donors. (b) The graph illustrates the incidence of IL-6 expressing cells (percentage of positive cells, mean values and s.e.m.) after challenge with E. coli DS-17 and a low constitutive expression in non-stimulated cells. (c) The graph illustrates the rapid induction of IL-8 expressing cells (percentage of positive cells, mean values and s.e.m.) after challenge with E. coli DS-17 and a low constitutive expression in non-stimulated cells.
E. coli-induced cytokine production in human renal proximal tubular cells
After E. coli exposure we found that the frequency of IL-1α producing epithelial cells gradually increased over time. A peak incidence at 24 h was induced when 10% of all cultured cells expressed IL-1α (P < 0·008 compared to non-stimulated cells) (Fig. 2a). IL-1α was the most predominant cytokine in the IL-1 family. Four donor samples out of 9 expressed IL-1β at 2 and 6 hours, respectively, while eight samples had detectable IL-1β-producing cells at 24 h (Fig. 2a). The mean frequency of IL-1β-expressing cells increased to a maximum of 5% at 24 h (P < 0·02 at 24 h versus non-stimulated cells). A similar kinetic response was observed for IL-1ra-synthesizing cells with a peak level of 3%, P < 0·02 compared to non-stimulated cells (Fig. 2a).
The number of IL-6 and IL-8-expressing cells after E. coli exposure were more prominent and present in all tested samples (Fig. 2b,c). Peak levels of expression were seen at 2 h with 18% and 17% of the epithelial cells synthesizing each of these cytokines (P < 0·008 and P < 0·02 versus non-stimulated cells, respectively). A gradual decrease was thereafter observed at 6 and 24 h.
Low levels of TNF-α and GM-CSF-expressing cells were detected in 3/9 donated epithelial samples each. A peak was seen after 2 h, followed by a rapid decline and loss of expression at 24 h. In addition, in 1 out of 9, IL-10 and G-CSF-producing cells were found at 6 h.
Similar results of cytokine production were achieved when another E. coli strain was used to stimulate the renal epithelial cells. Thus, the results found were not restricted to the strain E. coli DS-17 (data not shown).
DISCUSSION
In this study we present evidence that human proximal epithelial cells can synthesize IL-1α, IL-1β, IL-1ra, IL-6 and IL-8 at the protein level following stimulation with the heat-inactivated E. coli strain DS-17. Our study also shows that in cells from some of the tested patients there was a low-level expression of TNF-α and GM-CSF and also in a single patient of IL-10 and G-CSF. This is, to our knowledge, the first time that E. coli-induced production of the IL-1 cytokines has been reported in normal cells of renal tubular origin.
Acute pyelonephritis is associated with increased secretion of cytokines in the urine, which are most probably locally produced in the kidneys. We have demonstrated previously mRNA for pro-inflammatory and regulatory cytokines in the mouse kidney after experimental acute pyelonephritis [17,18]. Furthermore, women suffering of acute pyelonephritis have higher IL-8 in the urine than in serum [19]. These findings support the hypothesis of a local renal cytokine production during acute pyelonephritis, but do not explain the cellular source. In the present study the renal cortical epithelial cells responded in a time-dependent manner to the E. coli strain DS-17. IL-1α, IL-1β and IL-1 ra demonstrated an initial and comparatively slower kinetic response within the first 6 h and thereafter either levelled out or continued to increase. The range of epithelial cells expressing the IL-1 family cytokines after E. coli exposure was between 3% and 10%. It has been shown previously that little of IL-1α is secreted but instead generates intracellular signalling either in combination with an intracellular receptor or directly [4,20,21]. In the current study we found a similar kinetic response for IL-1β, known to be secreted by cell-surface–ICE interactions [22–24] or via the alternative non-ICE-dependent processing [22,25]. Over time we demonstrate here an increased incidence of IL-1α, IL-1β and IL-1ra-expressing cells reflecting an accumulation of the protein. A different kinetic response was found for IL-6 and IL-8, where peak levels were already reached at 2 h, whereafter they decreased or remained stationary (Fig. 2b,c). Our results with regard to IL-6 and IL-8 are in concert with previous findings using transformed cell lines, where a similar time-dependent response curve was observed [26]. The observation of IL-1 production is especially important since it can induce NO through iNOs and de novo expression of endothelial cell–leucocyte adhesion molecules, such as ICAM-1, which facilitates the adhesion and attachment of polymorphonuclear leucocytes [27] recruited through the production of IL-8. The activation of polymorphonuclear leucocytes by IL-1, TNF-α and G-CSF play a central role in acute pyelonephritis by activating their phagocytic function as well as cytokine-producing capacity, thereby enhancing the bacterial clearance. IL-1 and IL-6 may also be involved in the regulation of endogenous antimicrobial peptides [28,29] of importance for bacterial clearing in pyelonephritis. Endogenous antimicrobial peptides are under NFκB-regulation [30]. Similarly, IL-1 and IL-6 may up-regulate the constitutive production through this route.
The current E. coli strain used expresses P-fimbriae, which has been shown to enhance the IL-6 and IL-8 response in epithelial cells independently of the endotoxic activity of its LPS [31], but induction of the IL-1 family has not been demonstrated. Another possible candidate that induces cytokine production is LPS–CD14 interactions. We could, however, not demonstrate cell-surface CD14 expression (data not shown). In addition, culture of these cells in the absence of serum, excluding soluble CD14 interactions resulted in maintained IL-1 induction, indicating that other pathways dominate in E. coli activation of epithelial cells. It has been suggested that a signal transduction molecule in the LPS receptor complex may belong to the IL-1 receptor/toll-like receptor (TLR) superfamily, and that the LPS signalling cascade uses an analogous molecular framework for IL-1 signalling in mononuclear phagocytes and endothelial cells [32]. It has been shown recently that although a coexpression of CD14 synergistically enhances the LPS signal transmission through TLR2, responsiveness to LPS at high concentrations bypasses the requirement for LBP and CD14 [33]. However, the relevant pathway for renal epithelial cells remains to be elucidated.
We found a low incidence of G-CSF, GM-CSF and IL-10-producing cells. These results are in line with our previous analyses of urine samples from adult females with acute pyelonephritis, where we found that only a few patients secreted IL-10 or G-CSF in their urine [34]. The lack of cytokine response in the current study is therefore not surprising, although the reasons can not be explained.
In conclusion, we demonstrate E. coli-induced production of IL-1α, IL-1β, IL-1ra, IL-6 and IL-8 and also occasionally TNF-α and GM-CSF in human proximal tubular cells. This is of importance for the understanding of local immunity to bacterial infections in the urinary tract. Our results indicate that the innate cytokine response occurs at the renal tubular epithelial site.
Acknowledgments
This study was supported by the Swedish Medical Research Council grant 10850 and Funds from the Karolinska Institute and Magn. Bergvall Foundation. We thank Lena Radler for excellent technical help and Dr Ed Morgan, Pharmingen, San Diego, CA, USA, for providing the cytokine specific monoclonal antibodies.
REFERENCES
- 1.Dentener MA, Bazil V, von Asmuth EJ, Ceska M, Buurman WA. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–291. [PubMed] [Google Scholar]
- 2.Trachtman H, Futterweit S, Singhal PC, Sankaran R, Franki N. Renal tubular cell–E. coli interaction products stimulate nitric oxide production in cultured rat renal medullary interstitial and mesangial cells. Res Commun Mol Path Pharmacol. 1996;94:227–38. [PubMed] [Google Scholar]
- 3.Graves BJ, Hatada MH, Hendrickson WA, Miller JK, Madison VS, Santow Y. Structure of interleukin 1α at 2·7-Å resolution. Biochemistry. 1990;29:2679–84. doi: 10.1021/bi00463a009. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Muzio M, Ghezzi P, Colotta F, Introna M. Negative regulators of the interleukin-1 system; receptor antagonists and a decoy receptor. Int J Clin Lab Res. 1999;26:7–14. doi: 10.1007/BF02644768. [DOI] [PubMed] [Google Scholar]
- 5.Aderka D, Le J, Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989;143:3517–23. [PubMed] [Google Scholar]
- 6.Kaboré AF, Simard M, Bergeron MG. Local production of inflammatory mediators in an experimental model of acute obstructive pyelonephritis. J Infect Dis. 1999;179:1162–72. doi: 10.1086/314700. [DOI] [PubMed] [Google Scholar]
- 7.Hoch RC, Schraufstätter IU, Cochrane CG. In vivo, in vitro, and molecular aspects of interleukin-8 and the interleukin-8 receptors. J Lab Clin Med. 1996;128:134–45. doi: 10.1016/s0022-2143(96)90005-0. [DOI] [PubMed] [Google Scholar]
- 8.Agace W, Hedges S, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal Gram-negative infection. J Clin Invest. 1993;92:780–5. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedges S, Svenson M, Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992;60:1295–301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Söderhäll M, Bergerheim USR, Jacobson SH, et al. Molecular evidence for pap-G specific adhesion of Escherichia coli to human renal cells. J Urol. 1997;157:346–50. [PubMed] [Google Scholar]
- 11.Dive C, Cox H, Watson JV, Workman P. Polar fluorescein derivatives as improved substrate probes for flow cytoenzymological assay of cellular esterases. Mol Cell Probes. 1988;2:131–45. doi: 10.1016/0890-8508(88)90035-7. [DOI] [PubMed] [Google Scholar]
- 12.Sanders B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde–saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 13.Dolhain RJEM, Andersson U, Ter Har NT, et al. Detection of intracellular interferon-γ by light microscopy using an immunoperoxidase technique: correlation with the corresponding mRNA and protein product. J Leukoc Biol. 1993;54:545–51. doi: 10.1002/jlb.54.6.545. [DOI] [PubMed] [Google Scholar]
- 14.Sparrelid E, Emanuel D, Fehniger T, Andersson U, Andersson J. Interstitial pneumonitis in bone marrow transplant recipients is associated with local production of TH2-type cytokines and lack of T cell-mediated cytotoxicity. Transplantation. 1997;64:1207–8. doi: 10.1097/00007890-199706270-00013. [DOI] [PubMed] [Google Scholar]
- 15.Henter J-I, Söder M, Andersson U. Identification of individual tumor necrosis factor/cachectin-producing cells after lipopolysaccharide induction. Eur J Immunol. 1988;18:983–8. doi: 10.1002/eji.1830180703. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J, Björk L, Dinarello CA, Towbin H, Andersson U. Lipopolysaccharide induces human interleukin 1 receptor antagonist- and interleukin 1-production in the same cell. Eur J Immunol. 1992;22:2617–23. doi: 10.1002/eji.1830221022. [DOI] [PubMed] [Google Scholar]
- 17.Khalil A, Brauner A, Bakhiet M, et al. Cytokine expression during experimental Escherichia coli pyelonephritis in mice. J Urol. 1997;158:1576–80. [PubMed] [Google Scholar]
- 18.Tullus K, Wang J, Lu Y, Burman LG, Brauner A. Interleukin-1α and interleukin-6 in the urine, kidney, and bladder of mice inoculated with Escherichia coli. Pediatr Nephrol. 1996;10:453–7. doi: 10.1007/s004670050138. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson SH, Hylander B, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in serum and urine in patients with acute pyelonephritis in relation to bacterial virulence associated traits and renal function. Nephron. 1994;67:172–9. doi: 10.1159/000187923. [DOI] [PubMed] [Google Scholar]
- 20.Maier JA, Statuto M, Ragnotti G. Endogenous interleukin-1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol Cell Biol. 1994;14:1845–51. doi: 10.1128/mcb.14.3.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin MU, Falk W. The interleukin-1 receptor complex and interleukin-1 signal transduction. Eur Cytokine Netw. 1997;8:5–17. [PubMed] [Google Scholar]
- 22.Gjörloff-Wingren A, Björkdahl O, Labuda T, et al. Fusion of a signal sequence to the interleukin-1β gene directs the protein from cytoplasmic accumulation to extracellular release. Cell Immunol. 1996;169:226–37. doi: 10.1006/cimm.1996.0113. [DOI] [PubMed] [Google Scholar]
- 23.Kuida K, Lippke JA, Ku G, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–3. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Allen H, Banerjee S, et al. Mice deficient in interleukin-1 converting enzyme (ICE) are defective in production of mature interleukin-1β and resistant to endotoxic shock. Cell. 1995;80:401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Ku G, Harding MW, et al. Response to local inflammation of IL-1β converting enzyme-deficient mice. J Immunol. 1997;158:1818–24. [PubMed] [Google Scholar]
- 26.Agace W, Hedges S, Andersson U, Andersson J, Ceska M, Svanborg C. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infect Immun. 1993;61:602–9. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuthrich RP, Jevnikar AM, Takei F, Glimcher LH, Kelley VE. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmun lupus nephritis. Am J Pathol. 1990;136:441–50. [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–51. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 29.Frohm-Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmaco M, Boman A, Mangoni ML, et al. Effect of glucocorticoids on the synthesis of antimicrobial peptides in amphibian skin. FEBS Lett. 1997;416:273–5. doi: 10.1016/s0014-5793(97)01216-7. [DOI] [PubMed] [Google Scholar]
- 31.Hedlund M, Wachtler C, Johansson E, et al. P-fimbriae-dependent, lipopolysaccharide-independent activation of epithelial cytokine response. Mol Microbiol. 1999;33:693–703. doi: 10.1046/j.1365-2958.1999.01513.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang FX, Kirschning CJ, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–4. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 33.Kirschning CJ, Wesche H, Ayres TM, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–7. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson SH, Lu Y, Brauner A. Soluble interleukin 6 receptor, interleukin 10 and granulocyte-colony stimulating factor in acute pyelonephtitis—relationship to markers of bacterial virulence and renal function. Nephron. 1998;80:401–7. doi: 10.1159/000045211. [DOI] [PubMed] [Google Scholar]