Abstract
The effects of chronic administration of phenytoin, a common anticonvulsive drug, on immune responses were studied in mice. Anti-keyhole limpet haemocyanin (KLH) IgE antibody response after KLH-immunization was enhanced in phenytoin-treated mice. Proliferative responses of spleen cells induced with KLH, concanavalin A (ConA), lipopolysaccharide and anti-CD3 antibody were reduced in phenytoin-treated mice. Accessory function of spleen adherent cells on ConA-induced T cell proliferative response was reduced in phenytoin-treated mice. KLH-induced IL-4 production of spleen cells was enhanced, while IFN-γ production was reduced in phenytoin-treated mice. In addition, production of IL-1α, but not IL-6 and IL-12 by spleen adherent cells from phenytoin-treated mice was reduced. Natural killer cell activity was reduced in phenytoin-treated mice. These results suggest that phenytoin treatment preferentially induces a Th2 type response. We also observed that plasma ACTH and corticosterone levels were increased in phenytoin-treated mice, and speculated that phenytoin might act directly and indirectly, through HPA axis activation, on the immune system to modulate Th1/Th2 balance.
Keywords: phenytoin, Th1 / Th2 response, IgE, ACTH, corticosterone
INTRODUCTION
Phenytoin is one of the common anticonvulsive drugs used for the prevention of seizure [1]. However, phenytoin has diverse adverse effects such as gingival hypertrophy, lupus-like phenomenon, bone marrow suppression and idiosyncratic hypersensitivity reactions in which immunological mechanisms participate [2–4]. Moreover, immune functions affected by chronic administration of phenytoin also have been elucidated in humans and rodents both in vivo and in vitro [5–9]. Some animal studies have suggested that the chronic administration of phenytoin reduced the immune response against infectious and malignant diseases [10,11], but the mechanism of phenytoin-induced immune suppression is not clear. Recent studies have clarified the existence of Th1/Th2 CD4+ T cell subsets which were functionally heterogeneous populations with specific profiles of cytokine production. Th1 cells produce interferon (IFN)-γ and tumour necrosis factor (TNF)-β and participate in cell-mediated immunity, while Th2 cells produce interleukin (IL)-4, IL-5 and IL-10 and participate in humoral immunity [12]. Since Th1 type cytokines augment natural killer (NK) cell activity and delayed-type hypersensitivity (DTH), reduction of innate immune function in phenytoin-treated mice may be associated with an altered Th1/Th2 balance that shifts to Th2 dominant immune response. On the other hand, some studies have suggested that the hypothalamic-pituitary-adrenal (HPA) axis modulates immune functions [13,14]. Phenytoin has also been demonstrated to modulate the HPA axis to increase plasma corticosterone levels in mice in vivo [15,16]. Previous investigators demonstrated that corticosteroid promotes the Th2 cytokine response [17–20]. Therefore, in order to elucidate the mechanism of phenytoin-induced immune modulation, we studied the Th1/Th2 balance and plasma level of adreno-corticotrophic hormone (ACTH) and corticosterone in mice chronically administered with phenytoin in this study. Here we show evidence that chronic administration of phenytoin promotes Th2 type responses, which is accompanied by the increased plasma levels of ACTH and coticosterone.
MATERIALS AND METHODS
Animals
Male C3H/HeN mice (25–30 g body weight, 8–10 weeks of age) were used in all studies. Animals were housed in a constant temperature room (22 °C) animal facility (12 h of light, 12 h of darkness; lights on at 0700 h) of the University of Occupational and Environmental Health, Japan (UOEH). They had continuous access to water and laboratory chow. All animal experiments were performed according to the guidelines for the care and use of animals approved by UOEH.
Treatments
Mice received an intraperitoneal (i.p.) injection of phenytoin (130–140 mg/m2 of body surface, Dainippon Pharmaceutical Co., Osaka, Japan), dissolved in saline at pH 11 at a concentration of 10 mg/ml for 4 weeks. The control mice received the same volume of saline for the same length of time. The phenytoin dosage administered here was decided according to the report of Okamoto et al. [10] in which the dose used in this study could achieve a serum concentration of phenytoin (ranged from 10 to 20 µg/ml) equivalent to that of human epilepsy patients treated with phenytoin. To evaluate the toxicity of the chronic administration of phenytoin, we investigated the body weight and total cell counts of splenocytes of the phenytoin-adminstered mice and compared them to the control mice. The phenytoin-adminstered mice showed normal behaviour and no body weight reduction compared to the control mice (phenytoin-adminstered mice 32·2 ± 0·15 g, n = 10; control mice 33·4 ± 0·12 g, n = 10). There was no difference between total cell counts of splenocytes of phenytoin-adminstered mice (8·8 ± 0·29 × 107 cells, n = 10) and control mice (8·9 ± 0·16 × 107 cells, n = 10). To evaluate inflammatory responses in phenytoin administered mice, the mice were decapitated and truncal blood samples were harvested to prepare sera 12 h after single i.p. injections of lipopolysaccharide (LPS: 50 µg/mouse, Sigma Chemical Co., St Louis, MO, USA). To study antigen specific immune responses, mice were also immunized with kyehole lympet haemocianin (KLH: 100 µg/mouse, Sigma Chemical Co.) emulsified in Freund's complete adjuvant (FCA, Difco laboratories, Detroit, MI) by i.p. administration twice, on the 14th and 21st day during phenytoin treatment and sera were harvested on the 28th day. Treatments were performed between 0900 and 1000 h.
Splenocytes and blood sample preparation
On the 28th day of phenytoin treatment, mice were sacrificed by cervical dislocation and exsanguinated between 0900 and 1000 h to avoid circadian variation, and the serum and plasma samples obtained were stored at − 80 °C. At the same time, spleen cell suspensions were prepared by teasing spleens in ice-cold phosphate buffered saline pH 7·4 (PBS). For proliferative responses, splenocytes (2 × 106/ml) were resuspended in Eagle Hanks Amino Acid (EHAA) medium [21] supplemented with 10% heat-inactivated fetal calf serum (FCS, Bio Whittaker, Walkersville, MD, USA), 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Dainippon Pharmaceutical Co.). For NK cell assay and cytokine production studies, splenocytes (2 × 107/ml) were resuspended in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% heat-inactivated FCS, 2 mm l-glutamine and penicillin-streptomycin (RPMI-10% FCS). To prepare accessory cells and T cells, splenocytes from control and phenytoin-treated mice were incubated in culture dishes (Falcon #3002) at 37 °C for 2 h. T cells were purified from nonadherent cells passed through nylon-wool columns (Wako Co., Tokyo, Japan) [22]. Purified T cells were composed of more than 95% Thy-1 (+) cells detected by flow cytometry (data not shown). After 4 rinses, the adherent cells were scraped off from culture dishes using a rubber policeman and used as accessory cells. Adherent cells were composed of more than 80% MHC class II antigens (+) cells, less than 10% Ig (+) cells and 10% Thy-1 (+) cells detected by flow cytometry, respectively. Cell viability was estimated by the trypan blue dye exclusion test.
Splenocyte proliferation assay
Splenocytes (2 × 105) were cultured with KLH (50 µg/ml, 100 µg/ml), concanavalin A (ConA; 10 µg/ml, EY Laboratories, San Mateo, CA, USA), LPS (10 µg/ml) or anti‐mouse CD3 monoclonal antibody (1 µg/ml, Biosource, Camarillo, CA) in 0·2 ml of EHAA-10% FCS medium in wells of flat-bottomed microtitre culture plates (Falcon #3072, Becton Dickinson Co., Lincoln Park, NJ, USA) at 37 °C for 72 h in 5% CO2 and 95% humidified air. To evaluate accessory cell function, spleen adherent cells (5 × 104), which were treated with 100 µg/ml mitomycin C (Kyowahakko. Kogyo Co., Tokyo, Japan) at 37 °C for 30 min and then washed 4 times with PBS, and T cells (2 × 105) from control and phenytoin-treated mice were cocultured with or without ConA (5 µg/ml). The cells were labelled with 0·5 µCi of [3H]-thymidine (Amersham plc, Buckinghamshire, UK) for the last 18 h and were harvested by a semiautomated cell harvester (Abekagaku Co., Chiba, Japan). [3H]-thymidine incorporated into the cells was counted by a liquid scintillation counter (Aloka Co., Tokyo, Japan) [23]. The results were expressed as the mean ± SE of the stimulation index of 3–5 independent experiments in which the [3H]-thymidine count of the stimulated group was divided by the [3H]-thymidine count of the control group.
Cytokine production in vitro
Splenocytes (5 × 106/ml) from KLH-immunized mice were cultured with KLH (50 µg/ml, 100 µg/ml) in RPMI-10% FCS for 18 h at 37 °C in 5% CO2 and 95% humidified air in 24-well tissue culture plates (Falcon #3047). To study cytokine production of the accessory cells, spleen adherent cells (1 × 106/ml) were cultured with 0·005% Staphylococcus aureus Cowan I (SAC) (Calbiochem-Novabiochem Co., La Jolla, CA, USA) in RPMI-10% FCS at 37°C for 18 h in 24-well tissue culture plates (Falcon #3047). The supernatants were collected and stored at − 80 °C until being used for cytokine assay.
Enzyme linked immunosorbent assay (ELISA)
The concentration of IL-1α, IL-4, IL-6, IL-12 and IFN-γ in the supernatants of splenocyte and adherent cell culture, and the serum level of IFN-γ were measured by sandwich ELISA. As capture antibodies, rat anti‐mouse IL-1α, IL-4, IL-6, IL-12 and IFN-γ monoclonal antibodies (PharMingen, San Diego, CA, USA) were used. As detection antibodies, biotinylated rat anti‐mouse IL-1α, IL-4, IL-6, IL-12 and IFN-γ monoclonal antibodies (PharMingen) were used, respectively. Briefly, 96-well flat-bottomed microtitre plates (Maxisorp, Nunc, Denmark) were coated with capture antibodies for 18 h, washed with PBS, and blocked with 1% bovine serum albumin (Sigma Chemical Co.) solution in PBS (PBS/1% BSA). The culture supernatants and standards were added to each well, and plates were incubated at 37°C for 1 h. The plates were washed with PBS, the detection antibodies were added to each well, and incubated 37°C for 1 h. A 1/1000 dilution of streptoavidin-conjugated alkaline phosphatase (Sigma Chemical Co.) was added to each well. After incubation at 37 °C, plates were washed and a developing solution consisting of 1 mg/ml p-nitrophenylphosphate (Zymed Laboratory Inc., San Francisco, CA, USA) as substrate in 10 mm diethanolamine-0·5 mm MgCl2 buffer, pH 9·4, was added. Colour development was determined on a Immunoreader NJ-2000 (Japan Intermed Co., Tokyo, Japan) at a wavelength of 405 nm [24]. Serum level of KLH-specific IgG and IgE antibody was also measured by ELISA. Briefly, 96-well flat-bottomed microtitre plates (Maxisorp) were coated with 10 µg/ml KLH at 37 °C for 2 h and 4 °C for 18 h. After blocking with PBS/1% BSA, serially diluted serum samples were added to the plates and incubated at 37 °C for 2 h. After the plates were washed with PBS, 1/2000 dilution of horseradish peroxidase-conjugated goat anti‐mouse IgG antibody (Binding Site Co., Birmingham, U.K.) or rat anti‐mouse IgE antibody (Serotec Inc., Oxford, U.K.) was added to the wells. The plates were then incubated at 37 °C for 2 h. After washing with PBS, o-phenylenediamine dihydrochloride (5·5 × 10−4 M, Wako Junyaku Co., Kyoto, Japan) in 0·01% H2O2 solution was added as substrate, and incubated at 37 °C for 15 min. The reaction was stopped with 8N H2SO4 and the optical density (OD) at 490 nm was measured using an Immunoreader NJ-2000 [24]. The results were expressed as antibody titre.
NK cell assay
Splenic NK cell activity was measured in a standard 4-h chromium release assay [25]. YAC-1 cells as targets were radiolabeled by incubating 2 × 106 cells/ml in RPMI 1640–10% FCS and 100 µCi of sodium [51Cr] chromate (Amersham Plc, Buckinghamshire, UK) in a culture tube (Falcon #2057) at 37°C for 60 min. After washing four times with PBS, the concentration was adjusted to 1 × 105 cells/ml in RPMI-10% FCS. Mixtures of 100 µl of spleen cell suspensions (100 × 104, 50 × 104 and 25 × 104) and 100 µl of labelled target cells (1 × 104) were cocultured in 96-well round bottomed microtitre culture plates (Falcon #3077) at 100 : 1, 50 : 1 and 25 : 1 effecter-to-target (E:T) ratios. Plates were incubated at 37 °C for 4 h in 5% CO2 and 95% humidified air. Aliquots of 150 µl of supernatants were recovered from each well, and the amount of radioactivity was determined using a γ-counter (Aloka Co.). Percent specific lysis was calculated using the following formula:
 |
where maximum release was obtained by incubating target cells in 0·1% Triton X 100 (Sigma Chemical Co.) and spontaneous release was obtained by incubating target cells alone.
Assay of ACTH and corticosterone
The plasma level of ACTH and corticosterone was determined by a commercial RIA kit following the manufacturer's protocol (ACTH: Mitsubishikasei Co., Tokyo, Japan. corticosterone: ICN Biomedicals Inc., Costa Mesa, CA.).
Statistical analysis
The Mann–Whitney U-test was used for comparison of the mean values. Pairs of means were considered statistically different at the confidence interval of P < 0·05.
RESULTS
Effects of phenytoin treatment on antibody response
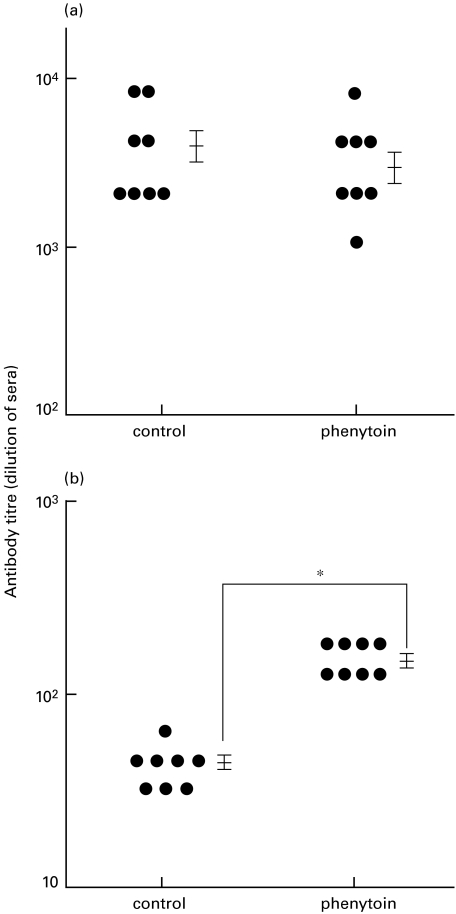
Mice were administered phenytoin i.p. every day for 28 days and immunized with KLH in FCA twice on day 14 and 21. Anti-KLH IgG and IgE antibody responses in the sera were determined on day 28. As shown in Fig. 1, the KLH-specific IgE response was significantly enhanced in phenytoin-treated mice, while the IgG response was not changed. KLH-specific antibodies were not detected in either control or phenytoin-treated mice, without KLH-immunization.
Fig. 1.
KLH-specific IgG (a) and IgE (b) antibody responses in phenytoin-treated mice. Phenytoin-treated and control mice were immunized with KLH twice. The sera were individually collected and their anti-KLH antibodies were assessed by ELISA. Each dot represents an individual mouse. Results are expressed by dilutional number of sera as antibody titre. The vertical bars represent mean ± SE. *significantly different.
Effects of phenytoin treatment on lymphocyte proliferative response
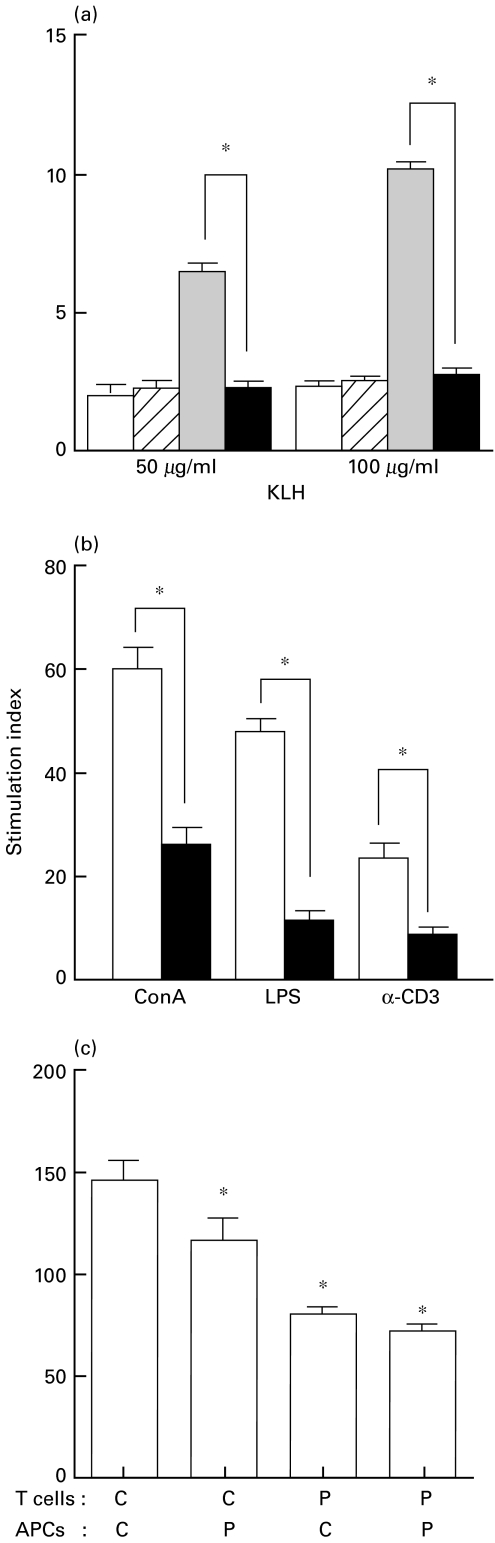
Since antigen-specific IgE and IgG antibody responses are dependent on T cell responses, KLH-specific T cell responses were next studied in vitro. As shown in Fig. 2a, in vitro culture of spleen cells from control mice immunized with KLH showed potent proliferative responses to stimulation with KLH. However, spleen cells from phenytoin-treated mice immunized with KLH did not respond to KLH. Spleen cells from KLH-nonimmunized mice did not respond to KLH in both control and phenytoin-treated mice. Nonspecific mitogenic responses induced by Con A and LPS were also impaired in spleen cells from phenytoin-treated mice (Fig. 2b). This is consistent with a previous report [10]. Furthermore, T cell responses induced by a cross-linking of CD3 was also impaired in spleen cells from phenytoin-treated mice. These results suggest that T cell functions are impaired by phenytoin-treatment.
Fig. 2.
Effect of phenytoin treatment on splenocyte proliferative response. a, KLH-specific proliferative response of splenocytes from phenytoin-treated mice. Splenocytes (2 × 105) were cultured with 50 or 100 µg/ml KLH for 3 days. Control mice (n = 5, □), phenytoin-treated mice (n = 5, □/), control mice with KLH-immunization (n = 5,  ), phenytoin-treated mice with KLH-immunization (n = 5, ▪). b, Effect of phenytoin treatment on splenocyte proliferative response to mitogens. Splenocytes from control (□) or phenytoin-treated (▪) mice were cultured with ConA, LPS or anti-CD3 antibody for 3 days and their response was evaluated by [3H]-thymidine incorporation. c, Effect of phenytoin treatment on accessory cell funciton. Purified T cells (2 × 105) and spleen adherent cells (5 × 104) from control (C) (n = 3) and phenytoin-treated mice (P) (n = 3) were cultured with ConA for 3 days. The cells were labelled with 0·5 µCi [3H]-thymidine for the last 18 h, harvested and [3H]-thymidine incorporated by splenocytes was counted. Results are expressed as mean ± SE of stimulation index. *significantly decreased from the control group.
), phenytoin-treated mice with KLH-immunization (n = 5, ▪). b, Effect of phenytoin treatment on splenocyte proliferative response to mitogens. Splenocytes from control (□) or phenytoin-treated (▪) mice were cultured with ConA, LPS or anti-CD3 antibody for 3 days and their response was evaluated by [3H]-thymidine incorporation. c, Effect of phenytoin treatment on accessory cell funciton. Purified T cells (2 × 105) and spleen adherent cells (5 × 104) from control (C) (n = 3) and phenytoin-treated mice (P) (n = 3) were cultured with ConA for 3 days. The cells were labelled with 0·5 µCi [3H]-thymidine for the last 18 h, harvested and [3H]-thymidine incorporated by splenocytes was counted. Results are expressed as mean ± SE of stimulation index. *significantly decreased from the control group.
Effect of phenytoin treatment on accessory cell function
Since T cell proliferative responses to antigens and mitogens require accessory cells such as macrophages, the accessory cell function of spleen adherent cells from the control and phenytoin-treated mice in ConA responses was studied. It was seen that the accessory function of spleen adherent cells from phenytoin-treated mice was significantly reduced as compared to the control mice (Fig. 2c). However, spleen adherent cells from control mice did not restore the T cell response from phenytoin-treated mice to control level. These results suggest that phenytoin affects both T cell and accessory cell function.
Effect of phenytoin treatment on cytokine production
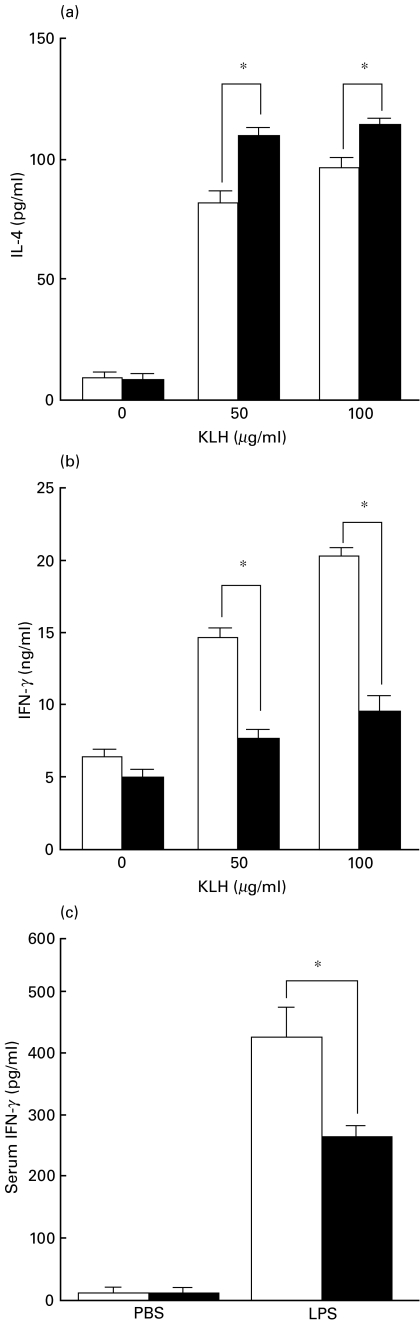
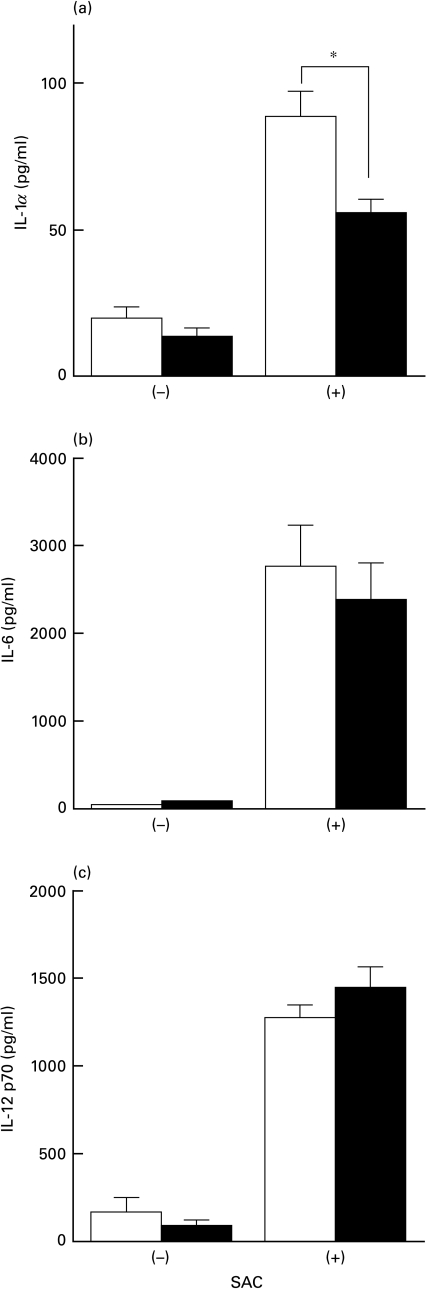
Next we studied cytokine production of spleen cells from phenytoin-treated mice. Spleen cells from control or phenytoin-treated mice immunized with KLH were cultured in vitro with KLH. The cytokines in the supernatant were then studied. As shown in Fig. 3, IL-4 (Fig. 3a) was significantly enhanced, but IFN-γ (Fig. 3b) was significantly reduced in spleen cells from phenytoin-treated mice. IL-2 was also reduced in spleen cells from phenytoin-administered mice (data not shown). Spleen cells from nonimmunized mice did not produce any cytokines. This result suggests that T cells are polarized to the Th2 type in phenytoin-treated mice and is consistent with the results that the IgE antibody response is enhanced in phenytoin-treated mice, as shown in Fig. 1. The reduced IFN-γ production was also observed in vivo after LPS-administration in phenytoin-treated mice (Fig. 3c). IL-4 could not be detected in vivo. IL-1α production by spleen adherent cells after SAC-stimulation was also reduced in phenytoin-treated mice as compared to the control mice (Fig. 4). However, there was no significant difference in IL-6 and IL-12 production between control and phenytoin-treated mice.
Fig. 3.
Effects of phenytoin treatment on cytokine production. KLH-specific IL-4 (a) and IFN-γ (b) production by splenoctyes from phenytoin-treated mice. Splenocytes (5 × 106/ml) from control (□) or phenytoin-treated (▪) mice immunized with KLH were stimulated with 50 or 100 µg/ml KLH for 18 h and culture supernatants were harvested. Cytokines were detected by ELISA. Results are expressed as mean ± SE of cytokines (pg or ng/ml) produced by 5 × 106 splenocytes (n = 5). *significantly different. c, Serum level of IFN-γ in phenytoin-treated mice. Control (□) or phenytoin-treated (▪) mice were injected i.p. with LPS or PBS and their sera were harvested 18 h later. IFN-γ levels in their sera were detected by ELISA. Results are expressed as mean ± SE (n = 5). * significantly different.
Fig. 4.
Cytokine production of spleen adherent cells stimulated with SAC. a, IL-1α; b, IL-6; c, IL-12 p70. Spleen adherent cells (1 × 106/ml) from control and phenytoin-treated mice were cultured with 0·005% SAC for 18 h and culture supernatants were harvested. Cytokines were detected by ELISA. Results are expressed as mean ± SE of cytokines (pg/ml) produced by 106 spleen adherent cells (n = 6). * significantly different.
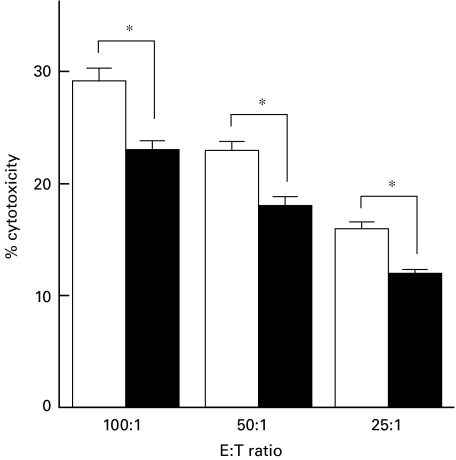
Effect of phenytoin-treatment on NK cell activity
NK cell activity was also studied in phenytoin-treated mice and we found that splenic NK cell activity was significantly reduced in phenytoin-treated mice as compared to the control mice (Fig. 5). This is consistent with a previous report [10].
Fig. 5.
NK cell activity of splenocytes from phenytoin-treated mice. Splenocytes from control (□) or phenytoin-treated (▪) mice were cultured with 51Cr-labelled YAC-1 cells at effector to target cell ratio of 100 : 1, 50 : 1 and 25 : 1 for 4 h and released 51Cr was counted. Results are expressed as mean ± SE of specific cytotoxicity (n = 5). * significantly different.
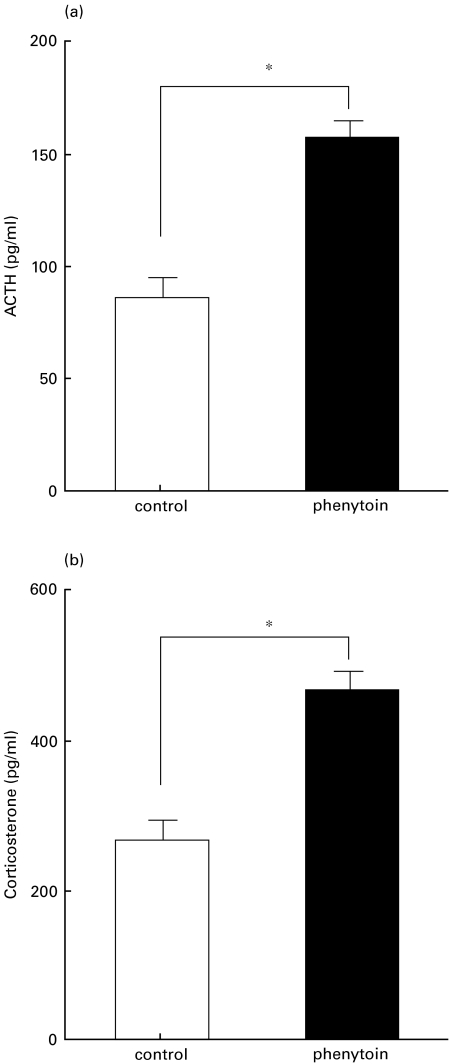
Effect of phenytoin-treatment on plasma ACTH and corticosterone level
It is known that cellular immune function is affected by the HPA axis. In particular, the cellular immune function was previously reported to be suppressed by corticosterone and corticotropin-releasing factor [26,27]. On the other hand, phenytoin is known to act on the central nervous system to modulate neural activity. To study the effects of phenytoin-treatment on the HPA axis, plasma levels of ACTH and corticosterone were determined. Plasma levels of ACTH and corticosterone were significantly higher in phenytoin-treated mice than in the control mice (Fig. 6).
Fig. 6.
Serum level of (a) ACTH and (b) corticosterone in control (□) or phenytoin-treated (▪) mice. Results are expressed as mean ± SE of 5 mice. * significantly different.
DISCUSSION
In this report, the effects of phenytoin on immune functions were studied in mice. Chronic administration of phenytoin caused an enhanced IgE antibody response and reduced T cell proliferative responses. The reduced T cell proliferative response depended on the impaired function of both T cells and accessory cells. In addition, the cytokine profile was also altered in phenytoin-treated mice in which the Th1 type cytokine response (IFN-γ) was reduced and the Th2 type cytokine response (IL-4) was promoted. Furthermore, NK cell activity in the spleen was reduced. Hence, reduced NK cell activity by administration of phenytoin may partly account for the reduced production of IFN-γ, as IFN-γ is one of the important soluble factors which enhances NK cell activity [28,29]. These results in this study suggest that chronic administration of phenytoin inhibits Th1 responses and promotes Th2 responses which results in a suppressed cellular immunity and in an augmented IgE response in phenytoin-treated mice.
Although there are variable studies concerning the effect of phenytoin on immunoglobulin production in vivo [6,9,30,31], phenytoin administration did not reduce the serum levels of total IgG and IgE in this study (data not shown). This may be due to the species and strains of animals used for experiments, administered dose of phenytoin, and the antigen used for immunization. However, this is the first report showing that phenyotin has a different effect on antigen-specific IgG and IgE response and that this difference is caused by the different effect of phenytoin on Th1 and Th2 responses.
Concerning the molecular mechanism of the action of phenytoin, it is reported that phenytoin acts on extracellular sites of voltage-dependent sodium channels to inhibit Na+ currents, and lymphocytes and macrophages also have been shown to express voltage-dependent sodium channels [32–34]. Accordingly, phenytoin may directly affect lymphocytes and macrophages. Therefore, we considered the direct effect of phenytoin on immune cells by in vitro culture. In vitro culture of spleen cells with phenytoin (10−8 to 10−6 M) for 72 h did not have any significant effect on mitogen-induced proliferation and cytokine and immunoglobulin production (data not shown). These results suggest that the effects of phenytoin on the immune system may be an indirect action, although this acute in vitro model may be not equivalent to the chronic administration of phenytoin in vivo.
In this study increased levels of plasma ACTH and corticosterone were also demonstrated in phenytoin-treated mice. This is consistent with previous studies confirming that the administration of phenytoin to mice causes an increase in plasma corticosterone levels [15,16]. Accordingly, we speculated that chronically upregulated plasma levels of corticosterone played an important role in shifting the immune system to a Th2 dominant response in phenytoin-treated mice, because it has been reported that corticosteroid acts on CD4+ T cells and antigen presenting cells such as macrophages to promote Th2 type responses [17–20]. Recent studies have revealed the presence of direct immunommodulation by the nervous system, independent of the HPA axis [35]. The increased levels of plasma ACTH suggest the presence of upregulation of corticotropin releasing factor that has been elucidated to activate the sympathetic nervous system in phenytoin-treated mice [36,37]. Hence, there is a possibility that phenytoin may have an effect on the immunological function through the modulation of the neural pathway.
In conclusion, chronic treatment of phenytoin has an inhibitory activity on Th1 and accessory cell function, in which ACTH and corticosterone may be involved, and results in Th2 cell predominancy. Dysregulation of the Th1 and Th2 balance by phenytoin may play a role in the adverse effects in which immunological mechanisms have been suggested. In addition, these findings have an important therapeutic implication especially for allergic patients and tumour bearing patients who have been receiving phenytoin for a long time, because the decreased Th1 cytokine response and augmented Th2 cytokine response may have unpleasant effects on disease course in these patients.
REFERENCES
- 1.Meshkibaf MH, Subhash MN, Lakshmana KM, Sridhara Rama Rao BS. Effect of chronic administration of phenytoin on regional monoamine level in rat brain. Neurochem Res. 1995;20:773–8. doi: 10.1007/BF00969688. [DOI] [PubMed] [Google Scholar]
- 2.Schweiger FJ, Kelton JG, Messner H, Klein M, Berger S, McIlroy WJ, Falk J, Keating A. Anticonvulsant-induced marrow suppression and immune thrombocytopenia. Acta Haemat. 1988;80:54–8. doi: 10.1159/000205599. [DOI] [PubMed] [Google Scholar]
- 3.Leeder JS. Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia. 1998;39:S8–16. doi: 10.1111/j.1528-1157.1998.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 4.Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. Epilepsia. 1998;39:S3–7. doi: 10.1111/j.1528-1157.1998.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 5.Grob PJ, Herold GE. Immunological abnormalities and hydantoins. Br Med J. 1972;2:561–3. doi: 10.1136/bmj.2.5813.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seager J, Coovadia HM, Soothill JF. Reduced immunoglobulin concentration and impaired macrophage function in mice due to diphenylhydantoin. Clin Exp Immunol. 1978;33:437–40. [PMC free article] [PubMed] [Google Scholar]
- 7.Bardana EJ Jr, Gabourel JD, Davies GH, Craig S. Effects of phenytoin on man's immunity. Evaluation of changes in serum immunoglobulins, complement, and antinuclear antibdy. Am J Med. 1983;74:289–96. doi: 10.1016/0002-9343(83)90630-7. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi K, McCormick CI, Neuwelt EA. Immunosuppression by phenytoin: Implication for altered immune competence in brain-tumor patients. J Neurosurg. 1984;61:1085–90. doi: 10.3171/jns.1984.61.6.1085. [DOI] [PubMed] [Google Scholar]
- 9.Basaran N, Hincal F, Ciger A. Humoral and cellular immune parameters in untreated and phenytoin- or carbamazepine-treated epileptic patients. Int J Immunopharmacol. 1994;16:1071–7. doi: 10.1016/0192-0561(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto Y, Shimizu K, Tamura K, Miyao Y, Yamada M, Tsuda N, Matsui Y, Mogami H. Effects of phenytoin on cell-mediated immunity. Cancer Immunol Immunother. 1988;26:176–9. doi: 10.1007/BF00205612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade-Mena CE, Sardo-Olmedo JA, Ramirez-Lizardo EJ. Effects of phenytoin administration on murine immune function. J Neuroimmunol. 1994;50:3–7. doi: 10.1016/0165-5728(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 12.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- 14.Blalock JE. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–11. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 15.Hansen DK, Holson RR, Sullivan PA, Grafton TF. Alteration in maternal plasma corticosterone levels following treatment with phenytoin. Toxicol Appl Pharmacol. 1988;96:24–32. doi: 10.1016/0041-008x(88)90243-8. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan-Jones P, Hansen DK, Sheehan DM, Holson RR. The effect of teratogens on maternal corticosterone levels and cleft incidence in A/J mice. J Craniofac Genet Dev Biol. 1992;12:183–9. [PubMed] [Google Scholar]
- 17.Ramírez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406–12. [PubMed] [Google Scholar]
- 18.Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–95. [PubMed] [Google Scholar]
- 19.DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160:2231–7. [PubMed] [Google Scholar]
- 20.Espey MG, Basile AS. Glutamate augments retrovirus-induced immunodeficiency through chronic stimulation of the hypothalamic-pituitary-adrenal axis. J Immunol. 1999;162:4998–5002. [PubMed] [Google Scholar]
- 21.Corradin GH, Etlinger HM, Chiller JM. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymphnode cells from primed mice. J Immunol. 1977;119:1048–55. [PubMed] [Google Scholar]
- 22.Yamashita U, Hamaoka T. The requirement of Ia-positive accessory cells for the induction of hapten-reactive cytotoxic T lymphocytes in vitro. J Immunol. 1979;123:2637–43. [PubMed] [Google Scholar]
- 23.Harrison MR, Thurmond G, Thomas GH. A simple and versatile harvesting device for processing radioactive label incorporated into and/or released from cells in microculture. J Immunol Methods. 1974;4:11–20. doi: 10.1016/0022-1759(74)90027-1. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida Y, Wang MQ, Liu JN, Shan BE, Yamashita U. Immunomodulating activity of chinese medicinal herbs and Oldenlandia diffusa in particular. Int J Immunopharmacol. 1997;19:359–70. doi: 10.1016/s0192-0561(97)00076-3. [DOI] [PubMed] [Google Scholar]
- 25.Cerottini JC, Engers HD, MacDonald HR, Brunner KT. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte culture. J Exp Med. 1974;140:203–25. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin M, Hauger RL, Brown M, Britton KT. CRF activates autonomic nervous system and reduces natural killer cytotoxicity. Am J Physiol. 1988;255:R744–7. doi: 10.1152/ajpregu.1988.255.5.R744. [DOI] [PubMed] [Google Scholar]
- 27.Irwin M, Vale W, Rivier C. Central corticotropin-releasing factor mediates the suppressive effect of stress on natural killer cytotoxicity. Endocrinology. 1990;126:2837–44. doi: 10.1210/endo-126-6-2837. [DOI] [PubMed] [Google Scholar]
- 28.Sayers TJ, Mason LH, Wiltrout TA. Trafficking and activation of murine natural killer cells: Differing roles for IFN-γ and IL-2. Cell Immunol. 1990;127:311–26. doi: 10.1016/0008-8749(90)90135-e. [DOI] [PubMed] [Google Scholar]
- 29.Novelli F, Giovarelli M, Reber-Liske R, Virgllita G, Garotta G, Forni G. Blockade of physiologically secreted IFN-γ inhibits human T lymphocyte and natural killer cell activation. J Immunol. 1991;147:1445–52. [PubMed] [Google Scholar]
- 30.Margaretten NC, Warren RP. Effect of phenytoin on antibody production: Use of a murine model. Epilepsia. 1987;28:77–80. doi: 10.1111/j.1528-1157.1987.tb03627.x. [DOI] [PubMed] [Google Scholar]
- 31.Ishizaka A, Nakanishi M, Kasahara E, Mizutani K, Sakiyama Y, Matsumoto S. Phenytoin-induced IgG2 and IgG4 deficiencies in a patient with epilepsy. Acta Paediatr. 1992;81:646–8. doi: 10.1111/j.1651-2227.1992.tb12322.x. [DOI] [PubMed] [Google Scholar]
- 32.Pieri C, Recchioni R, Moroni F, Balkay L, Márián T, Trón L, Damjanovich S. Ligand and voltage gated sodium channels may regulate electrogenic pump activity in human, mouse and rat lymphocytes. Biochem Biophys Res Commun. 1989;160:999–1002. doi: 10.1016/s0006-291x(89)80100-7. [DOI] [PubMed] [Google Scholar]
- 33.Damjanovich S, Pieri C. Electroimmunology: Membrane potential, ion-channel activities, and stimulatory signal transduction in human T lymphocytes from young and elderly. Ann N Y Acad Sci. 1991;621:29–39. doi: 10.1111/j.1749-6632.1991.tb16966.x. [DOI] [PubMed] [Google Scholar]
- 34.Negulyaev YA, Vedernikova EA. Sodium-selective channels in membranes of rat macrophages. J Membr Biol. 1994;138:37–45. doi: 10.1007/BF00211067. [DOI] [PubMed] [Google Scholar]
- 35.Downing JEG, Miyan JA. Neuronal immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–9. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 36.Sundar SK, Cierpial MA, Kilts C, Ritchie JC, Weiss JM. Brain IL-1-induced immunosuppression occurs through activation of both pituitary-adrenal axis and sympathetic nervous system by corticotropin-releasing factor. J Neurosci. 1990;10:3701–6. doi: 10.1523/JNEUROSCI.10-11-03701.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of β2-adrenergic receptor by Th1 and Th2 clones. J Immunol. 1997;158:4200–10. [PubMed] [Google Scholar]