Abstract
The Fcγ receptor type IIIb (CD16) is highly expressed on human neutrophils and is found in a soluble form in plasma and in other body fluids. Upon activation of neutrophils in vitro, FcγRIIIb is shed from the cell surface by proteolytic cleavage. We have now investigated the effect of metalloproteinase inhibitors and a serine proteinase inhibitor on the shedding of FcγRIIIb induced by phorbol 12-myristate 13-acetate (PMA) or cytochalasin B (cyto B) + N-formyl-methionyl-leucyl-phenylalanine (fMLP). Metalloproteinase inhibitors blocked to a large extent PMA-induced, but not cyto B + fMLP-induced shedding of FcγRIIIb. Inhibition of members of the ADAM (a disintegrin and metalloproteinase) family appeared most efficient. In contrast, the serine protease inhibitor N-methoxysuccinyl-alanine-alanine-proline-valine-chloromethylketone (MeOsuc-AAPV-CMK) largely blocked cyto B + fMLP-induced, but not PMA-induced shedding of FcγRIIIb. Metalloproteinase inhibitors in combination with the serine proteinase inhibitor resulted in full inhibition of FcγRIIIb shedding induced by either PMA or cyto B + fMLP. The shedding of FcγRIIIb that accompanied apoptosis was inhibited by 60% in the presence of inhibitors of metalloproteinases but was insensitive to inhibition of serine proteinases. These results show that distinct types of proteolytic enzyme are involved in the stimulus-induced shedding of FcγRIIIb from human neutrophils and suggest that these proteinases may become differentially activated under various physiological or pathological conditions.
Keywords: cellular activation, Fc receptor, human neutrophil
Introduction
Fcγ receptor type IIIb (FcγRIIIb, CD16), a receptor for the Fc part of IgG and highly expressed on human neutrophils, is found in a soluble form in plasma and in other body fluids [1–3]. It has been shown in vitro that, upon activation of neutrophils or during apoptosis, FcγRIIIb is shed from the cell surface by proteolytic cleavage [4–6]. However, the enzymes responsible for this process are still unknown. Earlier studies have indicated involvement of both metalloproteinases and serine proteinases in phorbol 12-myristate 13-acetate (PMA)-induced shedding of FcγRIIIb [7]. It is also unknown whether the proteinases are membrane-bound molecules or proteinases released from cytoplasmic granules. Studies from our group have suggested that the major proteinase responsible for PMA-induced FcγRIIIb shedding is membrane-bound [1,8]. However, release of proteinases from granules followed by reassociation with the cell surface cannot be excluded [9]. Recently, soluble FcγRIIIb was purified from human plasma, and C-terminal sequencing revealed a cleavage site between valine196 and serine197 [10]. Both metallo- and serine proteinases could be responsible for such cleavage [11,12].
Many proteinases are stored either within granules or located in the membrane of neutrophils. Matrix metalloproteinases, such as gelatinase B and collagenase, are stored mainly in specific and gelatinase granules, whereas serine proteinases, such as elastase, proteinase 3 and cathepsin G, are mainly found in azurophilic granules [13]. Several membrane-bound metalloproteinases, such as ADAM 8, ADAM17 and MT4-MMP, are thought to be expressed on the surface of the human neutrophil, based on the presence of RNA of these proteinases in leucocytes and the surface localization of these proteinases in other cell types [14–16]. This knowledge prompted us to investigate in more detail the FcγRIIIb shedding induced by various stimuli, resulting in different granule release patterns. We used PMA, a protein kinase C (PKC) activator, to induce release from secretory vesicles and specific granule contents. Stimulation with a combination of cytochalasin B (cyto B), an actin-disrupting agent, and N-formyl-Met-Leu-Phe (fMLP) was used to activate the neutrophil via a receptor and to release the contents of all granules (secretory vesicles, specific and azurophilic granules) [17]. To gain more information about the metalloproteinase involved in FcγRIIIb shedding we used a set of hydroxamic acid-based inhibitors, with selective inhibitory potency against gelatinase B, collagenase and ADAM-family members [18–20]. To inhibit serine proteinases, we used MeOsuc-AAPV-CMK, a well-known elastase inhibitor that also shows some inhibitory activity against cathepsin G and proteinase 3 [21], two other serine proteinases present in azurophil granules [13]. The results show that distinct types of proteolytic enzyme are involved in the stimulus-induced shedding of FcγRIIIb from human neutrophils.
Materials and methods
Materials
Phorbol 12-myristate 13-acetate (PMA), cytochalasin B (cyto B), N-formyl-Met-Leu-Phe (fMLP), N-methoxysuccinyl-Ala-Ala-Pro-Val chloromethylketone (MeOsuc-AAPV-CMK) and purified human elastase were obtained from Sigma Chemical Co., St Louis, MO, USA. Ro31–8220 and TIMP-1 were purchased from Calbiochem-Novabiochem Co., San Diego, CA, USA. Ro32–1541, Ro32–3580 and Ro32–7066 were kind gifts from Roche Discovery, Welwyn Garden City, UK. The following monoclonal antibodies (MoAbs) were obtained from our own institute: CLB-Fcgran1 (FcγRIII, CD16), DFT1 (CD43), NKI-P2 (CD44), CLB-B13·9 (CD66b) and irrelevant murine control IgG1 as well as fluoresceine-isothiocyanate (FITC)-labelled goat-antimouse-Ig. Leu-8 (CD62L) was obtained from Becton and Dickinson, San Jose, CA, USA and HP2/19 (CD50) was obtained from Immunotech, Marseille, France.
Neutrophil isolation
Peripheral blood was obtained from healthy volunteers. Granulocytes were purified from the buffy coats of 500 ml of blood anticoagulated with 0·4% (w/v) trisodium citrate, as described before [22]. In short, mononuclear cells and platelets were removed by density centrifugation over isotonic Percoll (Pharmacia, Uppsala, Sweden) with a specific gravity of 1·076 g/ml. Erythrocytes were removed by a 10-min treatment with ice-cold lysis buffer (155 mm NH4Cl, 10 mm KHCO3 and 0·1 mm EDTA). The remaining granulocytes were washed twice in phospate-buffered saline (PBS) and were resuspended in incubation medium [132 mm NaCl, 6 mm KCl, 1 mm CaCl2, 1 mm MgSO4, 1·2 mm K2HPO4, 20 mm HEPES, 5·5 mm glucose and 0·5% (w/v) human serum albumin (pH 7·4)] at a concentration of 107 cells/ml. The purity and viability of the neutrophils was over 95%.
Cell treatment
Neutrophils (107/ml) in incubation medium were preincubated in a shaking waterbath for 5 min at 37°C. After 10 min of incubation with the inhibitors, as indicated in the figures, neutrophils were activated at 37°C for 10 min with PMA (200 ng/ml) or for 5 min with cytochalasin B (0·5 µg/ml), followed by fMLP (1 µm) for 10 min at 37°C. Cell-free supernatants were collected, and cell pellets were resuspended in ice-cold PBS containing bovine serum albumine (0·1% v/v). Cell expression of FcγRIIIb, l-selectin and CD66b was determined by FACScan analysis. Neutrophils were incubated with MoAb for 45 min at 4°C in PBS containing bovine serum albumin (0·1% v/v). The cells were subsequently washed and stained with (FITC)-labelled goat-antimouse-Ig for 30 min at 4°C. The cells were again washed, and the fluorescence was measured by flow cytometry (FACScan, Becton and Dickinson, San Jose, CA, USA).
Soluble FcγRIIIb measurement
Soluble FcγRIIIb (sFcγRIIIb) in neutrophil supernatant samples was measured by ELISA as described before [23]. In short, 96-well ELISA plates were coated with the FcγRIII-specific MoAb CLB-Fcgran1, and sFcγRIIIb in the samples was detected with a biotinylated polyclonal rabbit-antihuman-FcγRIIIb antibody. After addition of streptavidin poly-horseradish peroxidase and substrate buffer, the colour reaction was allowed to proceed for 15 min and was stopped by addition of 2 m H2SO4. The absorbance at 450 nm was measured in a Titertek multiscan ELISA reader (Flow Laboratory, Rockville, MD, USA). The concentration of sFcγRIIIb in each sample was calculated from a standard curve obtained with serial dilutions of a human plasma pool containing 5 nm sFcγRIIIb [1].
Elastase measurement
Elastase in neutrophil supernatant samples was measured by ELISA as described before [24]. In short, 96-well ELISA plates were coated with an elastase-specific rabbit-antihuman polyclonal antibody, and elastase in the samples was detected with a biotinylated polyclonal rabbit-antihuman-elastase supplemented with 0·1% (v/v) bovine/rabbit (9:1, v/v) serum. After addition of streptavidin poly-horseradish peroxidase and substrate buffer, the colour reaction was allowed to proceed for 15 min and was stopped by addition of 2 m H2SO4. The absorbance at 450 nm was measured in a Titertek multiscan ELISA reader (Flow Laboratory, Rockville, MD, USA). The concentration of elastase in each sample was calculated from a standard curve obtained by serial dilutions of purified elastase from sputum [24].
Immunoprecipitation from neutrophils
Neutrophils were labelled with 125iodide according to the manufacturer's instructions (Pierce Chemical Co., Rockford, IL, USA). After labelling, the cells were resuspended in incubation medium and were activated for 10 min with PMA (200 ng/ml) or for 5 min with cytochalasin B (0·5 µg/ml), followed by fMLP (1 µm) for 10 min at 37°C. Proteins were immunoprecipitated from the cell-free medium with CLBFcRgran1 covalently coupled to CNBr-activated Sepharose 4B as described before [1]. Immunoprecipitated proteins were treated with N-glycanase to remove N-linked sugars according to the manufacturer's instructions (Genzyme, Boston, MA, USA). Immunoprecipitated, deglycosylated proteins were subjected to SDS-PAGE and autoradiography.
Results
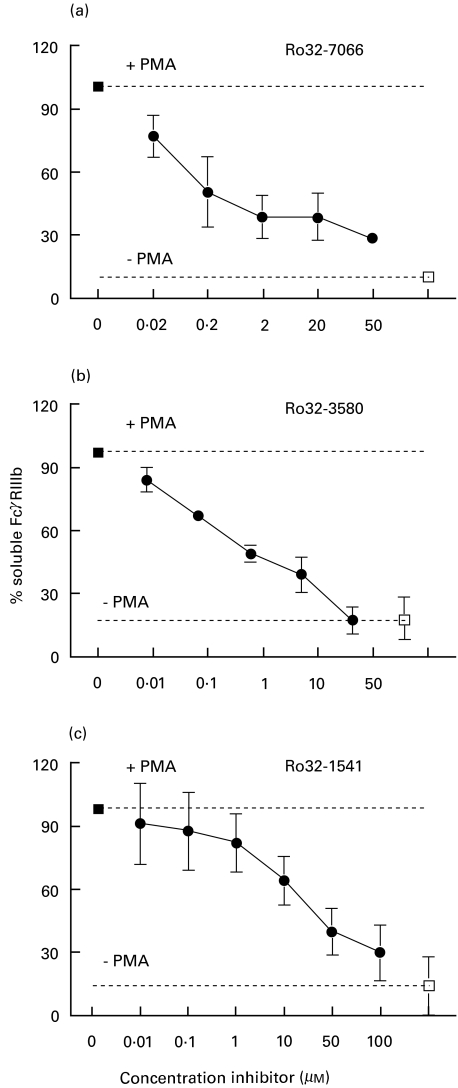
Earlier studies with hydroxamic-acid based inhibitors have already indicated that a metalloproteinase is involved in PMA-induced FcγRIIIb shedding [7,8,10]. In an attempt to identify this metalloproteinase, we used a new set of hydroxamic-acid based inhibitors with selectivity against collagenase (Ro32–1541), gelatinase B (Ro32–3580) and the ‘a disintegrin and metalloproteinase’ (ADAM) family (Ro32–7066) [18–20]. The dose-dependent effect of these inhibitors on PMA-induced FcγRIIIb shedding from human neutrophils was measured by ELISA (Fig. 1). We measured soluble FcγRIIIb instead of FcγRIIIb expression, because surface expression is a result of both shedding and up-regulation from intracellular stores of this receptor [25]. Because the surface expression varies greatly between individuals [26] due to differences in expression of NA1 and NA2 FcγRIIIb [23] and the occurrence of FcγRIIIb deletions [27], we normalized the amount of shed FcγRIIIb. From these results, the 50% inhibitory concentration (IC50) for each inhibitor was determined (Table 1). Ro32–7066, directed against ADAM-family proteinases, was the most potent inhibitor for the PMA-induced FcγRIIIb shedding, with an IC50 of 0·5 µm, although this inhibitor used at 50 µm still blocked FcγRIIIb shedding by only 70% (Fig. 2). The metalloproteinase inhibitors did not block CD66b up-regulation during neutrophil activation, indicating that fusion of the secondary granules with the cell membrane was normal, as shown before [8]. In the presence of PMA plus tissue inhibitor of metalloproteinase type 1 (TIMP-1), the physiological inhibitor of matrix metalloproteinases [28], we found 127% ± 7% (n = 3) of the FcγRIIIb shedding induced by PMA in the absence of TIMP-1 (P > 0·05), suggesting that this kind of metalloproteinase is not involved in FcγRIIIb shedding under physiological circumstances.
Fig. 1.
Inhibition of PMA-induced FcγRIIIb shedding by metalloprotease inhibitors measured by ELISA. Human neutrophils were preincubated with various concentrations of Ro32–7066 (a), Ro32–3580 (b) or Ro32–1541 (c) for 10 min at 37°C. After 10 min of PMA (200 ng/ml) stimulation, supernatants were collected and soluble FcγRIIIb was measured by ELISA. The concentration of soluble FcγRIIIb ±SD in the absence of inhibitors (closed squares) was taken as 100%. This concentration amounted to 0·9 ± 0·4 pmol/ml (n = 4) in (a), 1·2 ± 0·5 pmol/ml (n = 4) in (b) and 2·0 ± 0·9 pmol/ml (n = 5) in (c). The percentage of soluble FcγRIIIb ±SD in the absence of PMA and without inhibitors (open squares) is also indicated. The results shown were obtained in (n) independent experiments.
Table 1.
Mean IC50 of hydroxamic-acid based metalloproteinase inhibitors on FcγRIIIb shedding in human neutrophils
| Stimulus | ||
|---|---|---|
| PMA | cyto B + fMLP | |
| Ro32–7066 (ADAM inhibitor) | 0·5 µm | No inhibition |
| Ro32–3580 (gelatinase B inhibitor) | 3·9 µm | No inhibition |
| Ro32–1541 (collagenase inhibitor) | 35·3 µm | No inhibition |
Results represent the mean IC50 (µm) of the stimulus-induced Fc γ RIIIb shedding of four independent experiments.
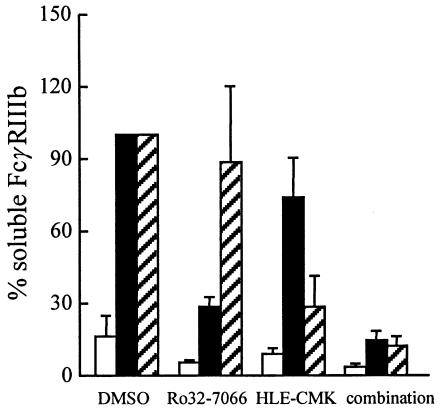
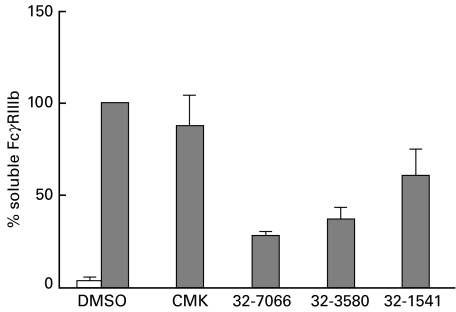
Fig. 2.
PMA-induced and cyto B + fMLP-induced FcγRIIIb release from neutrophils. Human neutrophils (107/ml) were preincubated with vehicle (DMSO), Ro32–7066 (50 µm), MeOSuc-AAPV-CMK (CMK; 200 µm) or the combination of Ro32–7066 and CMK for 10 min at 37°C. After stimulation of the neutrophils with PMA (200 ng/ml) for 10 min at 37°C (filled bars) or with a combination of cyto B (0·5 µg/ml) for 5 min + fMLP (1 µm) for 10 min at 37°C (hatched bars), supernatants were collected and soluble FcγRIII was measured by ELISA. As a negative control, unstimulated neutrophils are shown (open bars). The concentration ±SD of FcγRIIIb in the supernatants of the PMA incubations [2·0 ± 0·9 pmol/ml (n = 4)] was taken as 100% and compared with the concentration of FcγRIIIb when protease inhibitors were also present. The concentration ±SD of FcγRIIIb in the cyto B + fMLP incubations [3·4 ± 1·4 pmol/ml (n = 4)] was taken as 100% and compared with the concentration of FcγRIIIb when protease inhibitors were also present. The results shown were obtained in four independent experiments.
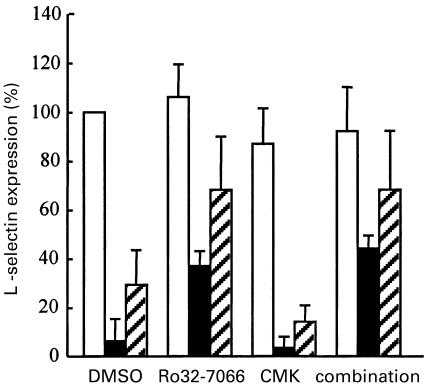
We also investigated other molecules that are known to be shed from the neutrophil surface after activation [29–32]. l-selectin down-regulation after PMA stimulation was inhibited by metalloproteinase inhibitors, again with Ro32–7066 being the most potent inhibitor (31% ± 14% inhibition, P < 0·001; Fig. 3). Earlier studies have already indicated that l-selectin shedding is susceptible to hydroxamic acid-based metalloproteinase inhibitors [33–35]. Down-regulation of CD43, CD44, CD50 and CD53 was not inhibited by metalloproteinase inhibitors (data not shown).
Fig. 3.
PMA-induced and cyto B + fMLP-induced l-selectin down-regulation from neutrophils. Human neutrophils (107/ml) were preincubated with vehicle (DMSO), Ro32–7066 (50 µm), MeOSuc-AAPV-CMK (CMK; 200 µm), or a combination of inhibitors for 10 min at 37°C. After stimulation with PMA (200 ng/ml) for 10 min at 37°C (filled bars), or with a combination of cyto B (0·5 µg/ml) for 5 min + fMLP (1 µm) for 10 min at 37°C (hatched bars), l-selectin expression was determined by FACS analysis. Unstimulated neutrophils are shown as open bars. The expression of l-selectin ±SD on DMSO-incubated cells without inhibitors [MFI 346 ± 59, n = 5] was taken as 100% and compared with the expression of l-selectin on cells incubated with activators ± inhibitors.
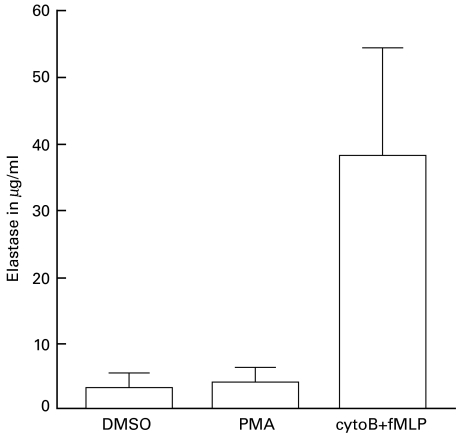
No inhibition of FcγRIIIb shedding was measured with hydroxamic-acid-based metalloproteinase inhibitors when neutrophils were stimulated with the combination of cyto B + fMLP (Table 1). These results suggested a role for serine proteinases, especially elastase, because exogenously added elastase is capable of cleaving FcγRIIIb from the cell surface [36,37]. No FcγRIIIb shedding was measured when neutrophils were incubated with cyto B alone (data not shown). As a control for release of the azurophilic granule contents, we measured elastase by ELISA. Cyto B + fMLP clearly induced more release of elastase from the azurophilic granules than did PMA (Fig. 4). To test whether endogenous elastase is involved in FcγRIIIb shedding, a known elastase inhibitor (MeOsuc-AAPV-CMK) was used, at an effective inhibiting concentration of 200 µm against endogenous elastase activity (data not shown). No inhibition of PMA-induced FcγRIIIb shedding was found, but MeOsuc-AAPV-CMK inhibited the cyto B + fMLP-induced FcγRIIIb shedding by 70% (Fig. 2). The combination of Ro32–7066 with MeOsuc-AAPV-CMK completely blocked both PMA-induced and cyto B + fMLP-induced FcγRIIIb shedding (Fig. 2). In contrast to FcγRIIIb shedding, no inhibiting effect was found with MeOsuc-AAPV-CMK on either PMA-induced or cyto B + fMLP-induced down-regulation of l-selectin (Fig. 3), CD43 or CD44 (data not shown). However, l-selectin down-regulation induced by cyto B + fMLP was inhibited by Ro32–7066 (39% ± 32% inhibition, P < 0·01; Fig. 3). The combination of Ro32–7066 and MeOsuc-AAPV-CMK had a similar effect on the shedding induced by either PMA or cyto B + fMLP, as had Ro32–7066 alone (Fig. 3).
Fig. 4.
Elastase release upon neutrophil activation with different stimuli. Human neutrophils (107/ml) were stimulated with PMA (200 ng/ml) for 10 min at 37°C, or with a combination of cyto B (0·5 µg/ml) for 5 min + fMLP (1 µm) for 10 min at 37°C. Supernatants were collected and elastase was measured by ELISA. The results shown represent the concentration of elastase ±SD of six independent experiments.
To investigate whether oxygen radicals are involved in the activation of metalloproteinases responsible for PMA-induced FcγRIIIb shedding, we studied neutrophils from three patients with chronic granulomatous disease (CGD). Neutrophils from these patients are incapable of generating oxygen radicals after activation [38]. However, PMA-induced FcγRIIIb shedding was only slightly inhibited in these cells (78% ± 9% of the shedding induced in normal cells; P < 0·05). Addition to normal neutrophils of diphenylene iodonium (DPI), an inhibitor of oxygen radical formation, did not affect FcγRIIIb shedding (P > 0·05; n = 3).
FcγRIIIb is shed from activated neutrophils, but is also released from the surface during apoptosis [5,6]. All metalloproteinase inhibitors tested were capable of inhibiting FcγRIIIb shedding during apoptosis, but the serine proteinase inhibitor MeOsuc-AAPV-CMK was not (Fig. 5). This observation is similar to the situation of PMA-induced FcγRIIIb shedding. Annexin-V binding, a feature of apoptotic cells [6], was not affected by these inhibitors (data not shown).
Fig. 5.
Inhibition of FcγRIIIb release by serine and metalloprotease inhibitors during apoptosis. Human neutrophils were incubated with vehicle (DMSO), MeOSuc-AAPV-CMK (CMK; 200 µm), Ro32–7066 (10 µm), Ro32–3580 (20 µm) or Ro32–1541 (50 µm) for 24 h at 37°C ( ). The amount of soluble FcγRIIIb was determined by ELISA. The concentration ±SD of soluble FcγRIIIb in the 24-h supernatants without inhibitors [1·9 ± 0·6 pmol/ml (n = 4)] was taken as 100%. The percentage of soluble FcγRIIIb at t = 0 is also shown (□). The results shown were obtained in four independent experiments.
). The amount of soluble FcγRIIIb was determined by ELISA. The concentration ±SD of soluble FcγRIIIb in the 24-h supernatants without inhibitors [1·9 ± 0·6 pmol/ml (n = 4)] was taken as 100%. The percentage of soluble FcγRIIIb at t = 0 is also shown (□). The results shown were obtained in four independent experiments.
To investigate whether the PMA-induced and the cyto B + fMLP-induced FcγRIIIb shedding result in similarly cleaved soluble FcγRIIIb, we precipitated soluble FcRIIIb from radiolabelled, activated neutrophils. After deglycosylation, the cleavage products were subjected to SDS-PAGE and autoradiography. All soluble fragments of FcγRIIIb migrated with a similar apparent molecular mass (data not shown), suggesting that a similar cleavage site is used by different proteinases, although small differences in molecular weight will not be detected.
Discussion
In this study we reveal two different proteolytic processes for FcγRIIIb shedding from the surface of the human neutrophil, namely a metalloproteinase-mediated pathway, mainly active after PMA activation, and a serine proteinase-mediated pathway, which is mainly active after cyto B + fMLP activation. The metalloproteinase is probably an ‘a disintegrin and metalloproteinase’ (ADAM)-family member, because a selective inhibitor of ADAM-family proteinases appeared to be the most potent in inhibiting FcγRIIIb shedding. Unfortunately, neutrophils cannot be manipulated by transfection with antisense oligonucleotides or retroviral transduction, because these cells have a low level of protein synthesis, do not divide any more and go into apoptosis within 24 h after isolation. Thus, the only way to analyse the importance of certain enzymes in the FcγRIIIb shedding process is by pharmacological means.
Although ‘selective’ inhibitors of gelatinase B and collagenase [18,19] partly inhibited PMA-induced FcγRIIIb shedding, it is unlikely that these granule matrix metalloproteinases are the proteinases involved in the physiological process of FcγRIIIb shedding. Earlier studies had already cast doubt on the role of gelatinase B in the FcγRIIIb shedding process, because an inhibitory MoAb against gelatinase B did not prevent FcγRIIIb shedding after neutrophil activation [8]. Involvement of collagenase is also highly unlikely because TIMP-1, a known collagenase inhibitor [39], did not block the FcγRIIIb shedding. It is more likely that a metalloproteinase in the ADAM family [possibly TNF-α-converting enzyme (TACE, ADAM17) or a related proteinase], is the proteinase responsible for FcγRIIIb shedding. TACE has been detected on the surface of neutrophils [15], and the presence of mRNA of another ADAM family member, CD156 or ADAM8, has been described in granulocytes [14]. Apparently, TACE is capable of cleaving ectodomains of numerous proteins from cells [40,41], but the activation mechanism of this metalloproteinase is still unknown. In PMA-induced FcγRIIIb shedding oxygen radicals do not play an important role, a conclusion based on our experiments with neutrophils from CGD patients and with neutrophils in which the NADPH oxidase activity was inhibited by diphenylene iodonium (DPI). Activation with a serine proteinase is also unlikely, because the elastase inhibitor MeOsuc-AAPV-CMK did not inhibit FcγRIIIb shedding when neutrophils were stimulated with PMA. However, serine proteinase-mediated FcγRIIIb shedding does exist. When human neutrophils were activated with cyto B + fMLP, FcγRIIIb shedding was blocked by MeOsuc-AAPV-CMK. Because this is a potent elastase inhibitor and earlier studies have shown that exogenously added elastase cleaves FcγRIIIb from the cell surface [36,37], we presume that elastase is the major enzyme involved in this pathway. This idea is consistent with the fact that cyto B + fMLP induced considerable release of elastase from human neutrophils, whereas PMA did not. Thus, elastase released from cyto B + fMLP-activated neutrophils probably binds to the cell surface and subsequently causes FcγRIIIb shedding. A previous study has shown that fMLP alone did not release FcγRIIIb from human neutrophils [42]. In this study only the surface expression was taken as a measure for FcγRIIIb shedding. However, this surface expression is the result of both shedding and up-regulation of FcγRIIIb, and therefore not a good measure for shedding [25]. FcγRIIIb shedding induced by cyto B + fMLP is not inhibited by the ADAM inhibitor; this might be due to the presence of cyto B, which inhibits actin polymerization. Previous studies have shown that actin polymerization is involved in FcγRIIIb shedding [43].
l-selectin down-regulation was also sensitive to metalloproteinase inhibitors, with Ro32–7066 as the most potent one. This suggests that l-selectin shedding from the human neutrophil surface, together with FcγRIIIb shedding, can be mediated by a TACE-like enzyme. However, in contrast to FcγRIIIb shedding, TACE inhibition had effect both on the PMA-induced and on the cyto B + fMLP-induced l-selectin down-regulation. Apparently, actin polymerization is not involved in l-selectin down-regulation. Inhibition of serine proteinases had no effect on l-selectin down-regulation at all. CD43, CD44, CD50 and CD53 down-regulation is probably not mediated via metalloproteinases, but more likely via serine proteinases [7,31,32].
Cleavage of FcγRIIIb by a metalloproteinase or by a serine proteinase resulted in similar FcγRIIIb fragments, as judged by autoradiography after SDS-PAGE. This suggests that cleavage mediated by a metalloproteinase or a serine proteinase utilizes the same cleavage site. However, C-terminal sequencing is required to obtain a definitive answer.
Based on the findings presented here and the single cleavage site found in plasma-derived soluble FcγRIIIb [10], we have as yet no clue as to which proteolytic process will prove to be dominant in vivo. However, FcγRIIIb release from apoptotic neutrophils could only be blocked with metalloproteinase inhibitors in vitro. Earlier studies in our laboratory have already indicated that soluble FcγRIIIb in plasma is solely derived from neutrophils and is a measure for the turn-over of neutrophils in the human body [1,44]. This indicates that in a healthy individual the metalloproteinase-mediated pathway is probably the most important one in FcγRIIIb shedding. The serine proteinase-mediated pathway will be more important when neutrophils are releasing their granule contents, for instance in inflammatory processes. This could be the reason for the elevated levels of soluble FcγRIIIb found at inflammatory sites [3]. Further studies are required to identify the physiological role of these two different FcγRIIIb-shedding pathways.
Acknowledgments
The authors thank Dr David Bradshaw and Dr John Nixon (Roche Research Centre, Welwyn Garden City, Herts, UK) for their kind gift of inhibitors used in this study and Marion Kleijer for her excellent technical assistance. This study was supported by grant 900–512–092 from the Netherlands Organization for Scientific Research (NWO).
References
- 1.Huizinga TWJ, de Haas M, Kleijer M, Nuijens JH, von Roos D, dem Borne AEG Kr. Soluble Fcγ receptor III, in human plasma originates from release by neutrophils. J Clin Invest. 1990;86:416–23. doi: 10.1172/JCI114727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sautès C, Teillaud C, Mazières N, et al. Soluble Fcγ receptor (sFcγR): detection in biological fluids and production of a murine recombinant sFcγR biologically active in vitro and in vivo. Immunobiology. 1992;185:207–21. doi: 10.1016/s0171-2985(11)80642-x. [DOI] [PubMed] [Google Scholar]
- 3.Fleit HB, Kobasiuk CD, Daly C, Furie R, Levy PC, von Webster RO. A soluble form of FcγRIII is present in human serum and other body fluids and is elevated at sites of inflammation. Blood. 1992;79:2721–8. [PubMed] [Google Scholar]
- 4.Huizinga TWJ, van der Schoot CE, Jost C, et al. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988;333:667–9. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- 5.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, von Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (FcγRIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 6.Homburg CH, de Haas M, dem Borne AEG, et al. Human neutrophils lose their surface FcγRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1994;85:532–40. [PubMed] [Google Scholar]
- 7.Bazil V, Strominger JL. Metalloprotease and serine protease are involved in cleavage of CD43, CD44, and CD16 from stimulated human granulocytes. Induction of cleavage of L-Selectin via CD16. J Immunol. 1994;142:1314–22. [PubMed] [Google Scholar]
- 8.Middelhoven PJ, Ager A, Roos D, Verhoeven AJ. Involvement of a metalloprotease in the shedding of human neutrophil FcγRIIIb. FEBS Lett. 1997;414:14–8. doi: 10.1016/s0014-5793(97)00959-9. [DOI] [PubMed] [Google Scholar]
- 9.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteases. J Cell Biol. 1995;131:775–89. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J, Moldovan I, Galinha A, et al. Identification of the cleavage site involved in production of plasma soluble Fc gamma receptor type III (CD16) Eur J Immunol. 1998;28:2101–7. doi: 10.1002/(SICI)1521-4141(199807)28:07<2101::AID-IMMU2101>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 11.Renesto P, Si-Tahar M, Moniatte M, Balloy V, van Dorsselaer A, Pidard D, Chignard M. Specific inhibition of thrombin-induced cell activation by the neutrophil proteinase elastase, cathepsin G, and proteinase 3: evidence for distinct cleavage sites within the aminoterminal domain of the thrombin receptor. Blood. 1997;89:1944–53. [PubMed] [Google Scholar]
- 12.Murphy G, Crabbe T. Gelatinases A and B. Meth Enzymol. 1995;248:470–84. doi: 10.1016/0076-6879(95)48030-7. [DOI] [PubMed] [Google Scholar]
- 13.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 14.Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S. CD156 (human ADAM8): expression, primary amino acid sequence, and gene location. Genomics. 1997;41:56–62. doi: 10.1006/geno.1997.4607. [DOI] [PubMed] [Google Scholar]
- 15.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 16.Puente XS, Pendás AM, Llano E, Velasco G, López-Otín C. Molecular cloning of a novel membrane-type matrix metalloproteinase from a human breast carcinoma. Cancer Res. 1996;56:944–9. [PubMed] [Google Scholar]
- 17.Kuijpers TW, Tool ATJ van der Schoot et al. Membrane surface antigen expression on neutrophils: a reappraisel of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–11. [PubMed] [Google Scholar]
- 18.Bottomley KM, Borkakoti N, Bradshaw D, et al. Inhibition of bovine nasal cartilage degradation by selective matrix metalloproteinase inhibitors. Biochem J. 1997;323:483–8. doi: 10.1042/bj3230483. (compound xiii is Ro32-1541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottemley KM, Johnson WH, Walter DS. Matrix metalloproteinase inhibitors in arthritis. J Enzy Inhib. 1998;13:79–101. doi: 10.3109/14756369809035829. (compound 25 is Ro32-3580) [DOI] [PubMed] [Google Scholar]
- 20. Ro32-7066; range of IC50 against ADAM family members: 5–20 nM (Roche, Welwyn Garden City, Herts, UK)
- 21.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR. Characterization of proteinase-3 (Pr-3), a neutrophil serine protease. Structural and functional properties. J Biol Chem. 1991;266:9540–8. [PubMed] [Google Scholar]
- 22.Roos D, de Boer M. Purification and cryopreservation of phagocytes from human blood. Meth Enzymol. 1986;132:225–43. doi: 10.1016/s0076-6879(86)32010-x. [DOI] [PubMed] [Google Scholar]
- 23.Koene HR, de Haas M, Kleijer M, von Roos D, dem Borne AEG Kr. NA-phenotype-dependent differences in neutrophil FcγRIIIb expression cause differences in plasma levels of soluble FcγRIII. Br J Haematol. 1996;93:235–41. doi: 10.1046/j.1365-2141.1996.4971038.x. [DOI] [PubMed] [Google Scholar]
- 24.Teeling JL, de Groot ER Eerenberg et al. Human intravenous immunoglobulin (IVIG) preparations degranulate human neutrophils in vitro. Clin Exp Immunol. 1998;114:264–70. doi: 10.1046/j.1365-2249.1998.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosi MF, von Zakem H. Surface expression of Fcγ receptor III (CD16) on chemoattractant-stimulated neutrophils is determined by both surface shedding and translocation from intracellular storage compartments. J Clin Invest. 1992;90:462–70. doi: 10.1172/JCI115882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huizinga TWJ, Kerst M Nuyens et al. Binding characteristics of dimeric IgG subclass complexes to human neutrophils. J Immunol. 1989;142:2359–64. [PubMed] [Google Scholar]
- 27.De Haas M, Kleijer M, van Zwieten R, von Roos D, dem Borne AEG Kr. Neutrophil FcγRIIIb deficiency, nature and clinical consequences: a study of 21 individuals from 14 families. Blood. 1995;86:2403–13. [PubMed] [Google Scholar]
- 28.Murphy G, Willenbrock F. Tissue inhibitors of matrix metalloendopeptidases. Meth Enzymol. 1995;248:496–510. doi: 10.1016/0076-6879(95)48032-3. [DOI] [PubMed] [Google Scholar]
- 29.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins are inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 30.Campanero MR, Pulido R Alosno et al. Down-regulation by tumor necrosis factor-α of neutrophil cell surface expression of the sialophorin CD43 and the hyaluronate receptor CD44 through a proteolytic mechanism. Eur J Immunol. 1991;21:3045–8. doi: 10.1002/eji.1830211222. [DOI] [PubMed] [Google Scholar]
- 31.del Pozo M, Pulido R, Muñoz C, et al. Regulation of ICAM-3 (CD50) membrane expression on human neutrophils through a proteolytic shedding mechanism. Eur J Immunol. 1994;24:2586–94. doi: 10.1002/eji.1830241104. [DOI] [PubMed] [Google Scholar]
- 32.Mollinedo F, Martín-Martín B, Gajate C, Lazo PA. Physiological activation of human neutrophils down-regulates CD53 cell surface antigen. J Leukocyt Biol. 1998;63:699–706. doi: 10.1002/jlb.63.6.699. [DOI] [PubMed] [Google Scholar]
- 33.Walcheck B, Kahn J, Fisher JM, et al. Neutrophil rolling altered by inhibition of l-selectin shedding in vitro. Nature. 1996;380:720–3. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 34.Bennett TA, Lynam EB, Sklar LA, Rogelj S. Hydroxymate-based metalloprotease inhibitor blocks shedding of l-selectin adhesion molecule from leukocytes. Functional consequences for neutrophil aggregation. J Immunol. 1996;156:3093–7. [PubMed] [Google Scholar]
- 35.Preece G, Murphy G, Ager A. Metalloproteinase-mediated regulation of l-selectin levels on leukocytes. J Biol Chem. 1996;271:11634–40. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- 36.Tosi MF, Berger M. Functional differences between the 40 kDa and 50–70 kDa IgG Fc receptors on human neutrophils revealed by elastase treatment and antireceptor antibodies. J Immunol. 1988;141:2097–103. [PubMed] [Google Scholar]
- 37.Remold-O'Donnell E, Parent D. Specific sensitivity of CD43 to neutrophil elastase. Blood. 1995;86:2395–402. [PubMed] [Google Scholar]
- 38.Roos D, de Boer M, Kuribayashi F, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–81. [PubMed] [Google Scholar]
- 39.Taylor KB, Windsor LJ, Caterina NCM, Bodden MK, Engler JA. The mechanism of inhibition of collagenase by TIMP-1. J Biol Chem. 1996;271:23938–45. doi: 10.1074/jbc.271.39.23938. [DOI] [PubMed] [Google Scholar]
- 40.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 41.Buxbaum JD, Liu KN, Luo Y, et al. Evidence that tumor necrosis factor-α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–7. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 42.Dong ZM, Murphy JW. Cryptococcal polysaccharides induce l-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J Clin Invest. 1996;97:689–98. doi: 10.1172/JCI118466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Middelhoven PJ, van Buul JD, Kleijer M, Roos D, Hordijk PL. Actin polymerization induces FcγRIIIb (CD16) shedding from human neutrophils. Biochem Biophys Res Commun. 1999;255:568–74. doi: 10.1006/bbrc.1999.0244. [DOI] [PubMed] [Google Scholar]
- 44.Huizinga TWJ, de Haas M van Oers et al. The plasma concentration of soluble FcγRIII, is related to the production of neutrophils. Br J Haematol. 1994;87:459–63. doi: 10.1111/j.1365-2141.1994.tb08298.x. [DOI] [PubMed] [Google Scholar]