Abstract
Colonic administration of a hapten, 2,4,6-trinitrobenzene sulphonic acid (TNBS) has been shown to induce colitis in rats. We are using this model to investigate the role of colonic antigens in the immunopathology. In this study, we show that colitis can be suppressed by oral administration of haptenized colonic antigens prior to the TNBS enema. Moreover, our data suggest that haptenization of the colonic antigens is not essential because oral feeding of non haptenized colonic antigens too protects rats from TNBS-induced colitis. Thus, unmodified colonic antigens may be involved in the induction of oral tolerance, and possibly in the pathogenesis in this model of colitis. Further, we show that the protective immunity or oral tolerance induced by non haptenized colonic antigens can be passively transferred to naïve rats by mesenteric T lymphocytes. Interestingly, oral feeding of small intestinal antigens, haptenized and non haptenized, does not protect rats from colitis, suggesting a specific role for colonic antigens. These data underscore the usefulness of this rat model in the identification of pathogenic antigens in colitis and in the development of therapeutic strategies based on oral tolerance.
Keywords: animal model, TNBS, passive transfer, tolerance
Introduction
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC) and Crohn's disease (CD), the major chronic inflammatory diseases of the gastrointestinal (GI) tract in humans [1]. While the cause of these disorders remains unknown, various features strongly suggest the involvement of immune response, particularly autoimmune reactions, in the pathogenesis of IBD [2,3]. Although, antigen-driven responses in IBD have not been fully characterized, several studies have indicated the important role of intestinal epithelial antigens in IBD [4–6]. The possible sources of pathogenic antigens in the intestine are food-derived antigens, normal colonic antigens (autoantigens), and antigens of commensal microorganisms [7]. The latter two have received much attention because there is no substantial evidence for the involvement of food-derived antigens in IBD [8].
Animal models of IBD, including genetically engineered rodent models, have helped us to understand some aspects of the intricacy of immune responses in IBD [9]. Of interest is the hapten-induced rodent model of colitis [10,11]. A contact-sensitizing agent, 2,4,6-trinitrobenzene sulphonic acid (TNBS), haptenizes self proteins to invoke delayed-type hypersensitivity (DTH) response to modified self antigen [12]. A single dose of TNBS, instilled rectally in 50% ethanol, produces chronic colitis in rats and mice [10,11]. Among several experimental animal models of colitis, TNBS-induced colitis is unique in that the disease can be passively transferred by lymphocytes and can be suppressed by oral administration of haptenized colonic antigens [11,13]. Thus, TNBS-induced colitis appears to be mediated by colonic antigens. Oral tolerance to colonic antigens has been thought to provide protection, via suppression of the peripheral immune response, against the disease [11,13,14]. However, it has been shown that TNBS alone can induce oral tolerance and protect the animals from TNBS-induced colitis [14]. This raises a question about the role of normal colonic autoantigens in the pathogenesis and protection. We considered three scenarios. First, if normal colonic antigens are involved in the pathogenesis, which is more likely in the human disease, one would expect that oral tolerance to the unmodified colonic proteins should also provide protection against the disease. Secondly, if the haptenization of proteins or the hapten itself is absolutely essential for the induction of oral tolerance, oral administration of unmodified colonic proteins should fail to provide protection. Finally, oral tolerance may be induced by either unmodified or TNBS-modified proteins or TNBS alone. We reasoned that resolutions of these issues would provide us clues to search for the colon antigen(s) involved in the pathogenesis and protective immunity in colitis.
Most of the reports describing oral tolerance in TNBS-induced colitis have used only colonic extracts for oral administration [11,13]. This raises an interesting question as to whether the tolerizing antigen is present exclusively in the colon or is expressed elsewhere in the GI tract. Organ specificity, if any, of the tolerizing antigen would be of great significance in the elucidation of the pathogenetic, and protective mechanisms and in the development of novel therapeutic strategies. In this study, we demonstrate that orally fed unmodified colonic autoantigens can induce protective immune response. We further show that small intestinal antigens do not induce protective oral tolerance in the TNBS-induced colitis.
Materials and methods
Animals
Virgin female Sprague-Dawley rats (250–300 g) obtained from Harlan Inc., USA were used in this study. They were maintained in controlled temperature (25°C) and light/dark (14:10 h) cycle. The animals were housed with one rat per cage. Standard chow pellets and drinking water were provided ad lib. Internal Animal Care and Use Committee (IACUC) approved the protocol.
Induction of chronic inflammation
Four to six rats were used in each experimental group, as shown in Table 1. Rats were lightly anaesthetized with 3:1 ketamine-xylazine mixture, 0·1 ml/100 g body weight were given intraperitoneally. A number 20 metal feeding tube (Popper & Sons, New York) was inserted rectally into the colon such that the tip was 8 cm proximal to the anus, approximately at splenic flexure area. TNBS dissolved in 50% ethanol was instilled into the colon at a dose of 30 mg per rat. Rats were sacrificed on 15th day for assessment of damage and for collection of tissue, and blood samples.
Table 1.
Gross morphological disease scores in different treatment groups
| Gross morphology | |||||
|---|---|---|---|---|---|
| Groups | No. of Rats | None | Mild | Moderate | Severe |
| Control | 4 | 4 | |||
| RE-TNBS | 7 | 3 | 4 | ||
| RE-ETOH | 6 | 2 | 4 | ||
| OR-TNBS | 4 | 4 | |||
| HCE-TNBS | 4 | 2 | 2 | ||
| NHCE-TNBS | 5 | 1 | 3 | 1 | |
| HSE-TNBS | 5 | 1 | 4 | ||
| NHSE-TNBS | 5 | 5 | |||
| TL-TNBS | 5 | 1 | 3 | 1 | |
| TS-TNBS | 6 | 1 | 4 | 1 | |
| NTL-TNBS | 4 | 1 | 3 | ||
| NTS-TNBS | 4 | 1 | 3 | ||
Control, untreated; RE-TNBS, TNBS (30 mg) in ethanol (50%) given as enema; RE-ETOH, ethanol (50%) alone given as enema; OR-TNBS, TNBS (10 mg) was given orally (single dose) prior to rectal TNBS in ethanol; HCE-TNBS, haptenized colon extract fed rats followed by TNBS in ethanol; NHCE-TNBS, nonhaptenized colon extract fed rats followed by rectal TNBS in ethanol; HSE-TNBS & NHSE-TNBS, haptenized and nonhaptenized small intestinal extracts fed rats followed by rectal TNBS in ethanol; TL-TNBS and TS-TNBS, rats that received through tail vein T lymphocytes from mesenteric lymph nodes (TL) or from spleen (TS) from rats that were fed with nonhaptenized colon extract for 5 times on alternate days. Seven days after lymphocyte transfer, rats were challenged with rectal TNBS in ethanol and then were sacrificed on the 15th day as in other groups. As controls, T lymphocytes from mesenteric lymph nodes and spleen from normal naïve rats were given via tail vein used as controls followed by rectal TNBS in ethanol and then were sacrificed on the 15th day (NTL-TNBS and NTS-TNBS groups, respectively).
Assessment of colonic inflammation and damage
Colon was removed and divided into two parts: 0–8 cm, that is from anus to about splenic flexure area (distal colon) and >8 cm to caecum (proximal colon) and weighed. The tissue samples from distal and proximal colon were longitudinally divided and the tissue specimens were immediately examined using a stereo microscope and any visible damage was scored on 0–5 scale, as described by Morris et al. [10]. Half of the specimens were frozen in dry ice immediately and stored at −80°C, till use. The other halves were fixed in 10% formalin by jelly role technique, processed for paraffin block, and sections were stained with haematoxylene and eosin stain. The small intestine was also similarly processed. Histological assessment by light microscopy was performed in blinded fashion on coded slides by a pathologist among us. (P.S.A). The thickness of the distal colon wall was determined on the H&E stained sections by measuring the distance from serosal surface to luminal surface of the mucosal layer by light microscopy fitted with a measuring scale.
Myeloperoxidase (MPO) activity of distal colon, proximal colon, and small intestine were measured [15–17] using 4-aminoantipyrine as substrate. MPO activity was expressed as unit per mg of protein. A unit of MPO activity was defined as that converting 1 µmol of hydrogen peroxide to water in 1 min at 22°C.
Statistical methods
Data are expressed as mean ±SEM. Parametric data were analysed using the Student's two-tailed t-test for unpaired observations, using Microsoft Excel. With all statistical analyses, P ≤ 0·05 was considered significant.
Preparation of colonic and small intestinal tissue extracts from rats
Colon and small intestinal specimens of normal homologous rats were removed and cut into small pieces. Tissue was extensively washed in normal saline, homogenized in phosphate buffered saline pH 7·4 (PBS) with Polytron, then sonicated. Crude extract was centrifuged for 10 min at 1000 × g to remove large particles. The supernatant was used. Protein concentration was estimated by Bio-Rad protein assay. Haptenization of tissue extracts was performed by incubating the extracts with 0·1% TNBS per mg of total protein for 4 h at room temperature. Unbound TNBS was removed by dialysis against PBS overnight.
Oral administration of antigens
Rats were fed with 500 µg of haptenized or nonhaptenized tissue extract in PBS every other day over a period of 9 days (total 5 times) using a number 20 gastric feeding needle (Popper and Sons). For oral TNBS group, 10 mg of TNBS per rat was fed once only. After 7 days of rest, following these oral feedings, rats were challenged with (30 mg/rat) intra rectal TNBS in 50% ethanol, as mentioned above. Rats were sacrificed after 15 days, serum collected, and intestinal tissue specimens were processed as described above.
Passive transfer of lymphocytes
Mesenteric lymph nodes and spleens were dissected from the rats fed with nonhaptenized colonic proteins. T cells were isolated using a rat T-cell isolation column (Pierce, Rockford, IL, USA) following manufacturer’s instructions. One million T cells from Mesenteric lymph nodes or spleen were injected into each rat via tail vein in 200 µl of PBS. After 7 days of rest, rats were challenged with TNBS as described above.
As control, following the same method, T-cells were harvested from spleen and mesenteric lymph nodes from two naïve Sprague-Dawley Rats. Normal T-cells, either from spleen or mesenteric lymph nodes were injected into the tail vein of 8 rats (4 for splenic T-cells and 4 for mesenteric lymph node derived T-cells) followed by TNBS enema. Rats were sacrificed after 15 days.
Results
Induction and suppression of colitis in rats
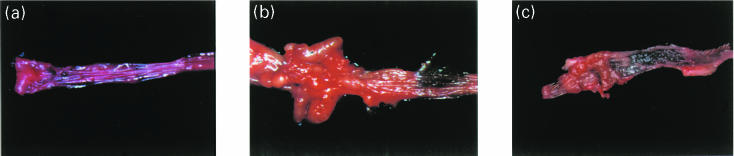
Rectal administration of TNBS in ethanol (RE-TNBS) clearly produced colitis in rats, whereas ethanol alone (RE-ETOH) did not produce colitis. The disease progression was monitored by visual examination of stool consistency, appearance of blood in the stool, and loss of body weight (data not shown). Macroscopically, animals that received 30 mg TNBS in 50% ethanol developed grossly visible thickening of colon wall, inflammation, and ulcers. There were often multiple separate sites of inflammation in the distal 8 cm of colon. Additionally segmental pericolic accumulation of mesenteric fat and fibrinous adhesion to small bowel, and uterine horn were frequently observed. The ulcers appeared as necrotic areas with white slough at the base or linear and was surrounded by thickened inflamed mucosa (Fig. 1b). The proximal colon and small intestine looked normal.
Fig. 1.
Gross appearance of colonic mucosa in the distal colon. a, Normal colonic mucosa b, Significant inflammation in the distal 8 cm of colon with oedema, ulceration, nodularity, and thickening of the wall in a representative rat that received rectal TNBS in ethanol. c, The colonic mucosa from a representative rat that was fed with nonhaptenized colon extract prior to rectal TNBS in ethanol. There is only mild oedema and erythema without any ulceration or nodularity.
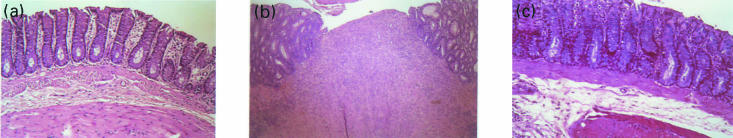
Histological examination of distal colon revealed inflammation extending through the mucosa, submucosa, and often transmural. Ulceration of the mucosa and extensive infiltration by polymorphonuclear leucocytes, lymphocytes, eosinophils, and macrophages were apparent (Fig. 2b). There were also thickening of the wall with fibro-muscular hypertrophy. Proximal colon and small intestine were unaffected.
Fig. 2.
Histology of the distal colon a, Normal colonic mucosa. b, Severe transmural inflammation with ulceration on the luminal surface (top) is clearly evident in a rat that received TNBS in ethanol by enema. c, A rat that was fed with nonhaptenized colon extract prior to rectal TNBS showing minimal amount of inflammatory cells without any ulceration and thickening
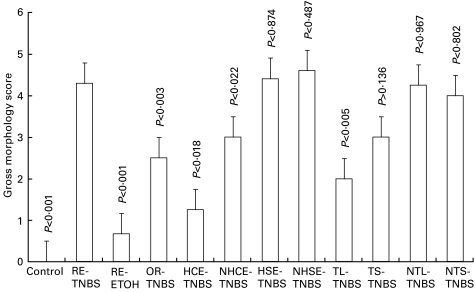
The degree of gross inflammation was scored on a scale of 0–5, as described by other investigators [10,14] and graded as: 0 meant no inflammation, mild (score 1), moderate (scores 2, and 3), and severe (scores 4, and 5) (Table 1). Figure 3 demonstrates statistical analysis of gross morphology scores in various groups. RE-TNBS group showed significant (P < 0·001) inflammatory changes when compared with normal controls and RE-ETOH groups.
Fig. 3.
Gross morphology scores of the distal colon (0–8 cm) in different groups and their statistical analysis. RE-TNBS showed significant (P < 0·001) inflammation when compared to normal control and the RE-ETOH group. The inflammatory scores were significantly (P < 0·005 to P < 0·02) less in the HCE-TNBS, NHCE-TNBS, TL-TNBS and OR-TNBS groups when compared with RE-TNBS group showing protection. However, the inflammation scores in HSE-TNBS, NHSE-TNBS, NTL-TNBS, NTS-TNBS and TS-TNBS groups were not significantly different than the RE-TNBS group. Abbreviations as given in Table 1.
Histopathological changes were scored as no inflammation (score 0), mild (score 1), moderate (score2) or severe (score 3) by the single pathologist (PSA) who was unaware of the groups, and the results are shown in Table 2. Statistical analysis of the histological data was not used because of the inherent difficulty of objective morphometric parameters and, in several specimens, the pathological changes, although severe but localized with patchy distributions. Hence, an overall assessment was made by the pathologist, and the individual values are shown in Table 2. Both gross and microscopic examinations convincingly showed significant inflammation induced by TNBS (Tables 1 and 2, Figs 1 and 2).
Table 2.
Shows the histological score of each of the animals in various treatment groups. The histological assessment is described in detail in the text
| Group | No. in group | Inflammation average score | Individual scores |
|---|---|---|---|
| Control | (4) | 0 | (0,0,0,0) |
| RE-TNBS | (7) | 2·43 | (2,3,2,3,2,2,3) |
| RE-ETOH | (6) | 0·17 | (1,0,0,0,0,0) |
| OR-TNBS | (4) | 0·5 | (0,1,1,0) |
| HCE-TNBS | (4) | 0·75 | (2,0,1,0) |
| NHCE-TNBS | (5) | 1·2 | (0,2,2,1,1) |
| HSE-TNBS | (5) | 2·3 | (2·5,0,3,3,3) |
| NHSE-TNBS | (5) | 2·8 | (2,3,3,3,3) |
| TL-TNBS | (5) | 2·2 | (2,2,3,2,2) |
| TS-TNBS | (6) | 1·67 | (2,1,3,0,2,2) |
| NTL-TNBS | (4) | 2·75 | (2, 3, 3, 3) |
| NTS-TNBS | (4) | 2·5 | (2·5, 1·5, 3, 3) |
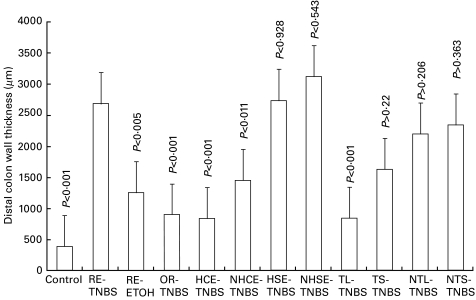
As additional objective and quantitative measure of the inflammatory changes, we have used thickness of the distal colon wall measured in H&E stained tissue sections and MPO activity in crude tissue extracts. The thickness of the distal colon walls in different groups of rats, and its statistical analysis are shown in Fig. 4. The mean ±SEM thickness in the RE-TNBS group was 2684 ± 345µm when compared to normal control (385 ±5µm) (P < 0·001) and RE-ETOH control (1251 ± 131µm) (P < 0·005). The choice of MPO as an index was based on previous studies [15–17], and the specificity of MPO as a marker for neutrophil infiltration. This study confirms the previously reported correlation between severity of colitis and MPO activity. The MPO activity in the distal colon affected with colitis was significantly higher than in the untreated control group (P = 0·007) (Fig. 5). Rectal administration of 50% ethanol alone did not affect the MPO activity (P = 0·44 versus untreated control group), indicating that inflammatory response was induced by TNBS (Fig. 5). Histology and MPO activities in the proximal colon and small intestine were in the normal range.
Fig. 4.
The thickness of the distal colon wall in different groups is shown here. There was a significant (P < 0·005 to P < 0·001) increase in the thickness in the RE-TNBS group when compared to normal controls and RE-ETOH groups. However, the rats in the groups of OR-TNBS, HCE-TNBS, NHCE-TNBS, TL-TNBS and TS-TNBS showed protection when compared with RE-TNBS (P < 0·02 to P < 0·001). The protection in the TS-TNBS group was much less than TL-TNBS group. However, no protection was evident in HSE-TNBS, NHSE-TNBS, NTL-TNBS and NTS-TNBS groups. Abbreviations as given in Table 1.
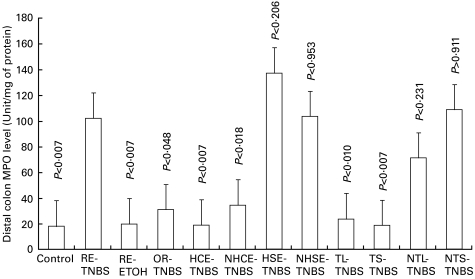
Fig. 5.
MPO levels in the distal colon of rats in various groups. The legends are as described in Table-1. When compared with the RE-TNBS group, the MPO levels were significantly (P-values ranged from <0·003 to <0·01) lower in the HCE-TNBS, NHCE-TNBS, TL-TNBS and TS-TNBS groups, but not with HSE-TNBS, NHSE-TNBS, NTL-TNBS and NTS groups showing protection in the former groups. The MPO values in the protected group were not significantly different than the control and RE-ETOH groups.
The effect of oral feeding of haptenized or nonhaptenized colonic antigens prior to the TNBS enema was examined using gross and microscopic features (Tables 1 and 2, Figs 1–4), and MPO activity (Fig. 5). After oral administration of haptenized and nonhaptenized colonic proteins (HCE-TNBS and NHCE-TNBS groups, respectively), there was considerable reduction of colonic inflammation, as evidenced by decrease in diarrhea, oedema, ulcerations, adhesions, intestinal wall thickness, and histological changes (Figs 1c and 2c). In several animals, mucosa in the distal colon appeared almost normal morphology as determined by both gross and histological examination (Tables 1 and 2). Clearly, the oral feeding of colon-derived antigens showed significant suppression of colitis. The gross morphology score was significantly (P < 0·02) reduced in both HCE-TNBS and NHCE groups when compared with RE-TNBS group (Fig. 3). Similarly, the thickness of the distal colon was also reduced in HCE-TNBS (P < 0·001) and NHCE-TNBS group (P < 0·01) (Fig. 4). This was further confirmed by the measurement of MPO activities (Fig. 5). When compared to the untreated control group, the MPO activities in the distal colon of the haptenized and non haptenized colon extract fed groups were not significantly different (P = 0·56 and 0·1, respectively, Fig. 5). On the contrary, compared to RE-TNBS group, the MPO activity in both HCE-and NHCE-fed groups was significantly lower (P < 0·007 & P < 0·02, respectively, Fig. 5). Rats fed with haptenized and nonhaptenized small intestinal proteins (HSE-TNBS and NHSE-TNBS) did not show any protection as shown in Table 1 and Figs 3–5. Oral administration of TNBS followed by rectal TNBS (OR-TNBS group) also showed protection (Figs 3–5).
Passive transfer of protective immunity
To examine whether the protection is mediated by immuno-regulatory cells, we transferred mesenteric lymphnode-derived T lymphocytes from NHCE-tolerized rats to naïve rats intravenously. After 8 days, the rats were given TNBS rectally (TL-TNBS group, Tables 1 and Figs 3–5). Similarly, splenic T cells from the same animals were also transferred to naïve animals followed by rectal TNBS (TS-TNBS). Colitis was significantly suppressed in rats that received lymphocytes from mesenteric lymph nodes from tolerized rats (P < 0·01 to P < 0·001, Figs 3–5). Passive transfer of splenic lymphocytes also provided somewhat less but significant protection (Figs 3–5). Passive transfer of normal mesenteric and splenic T-lymphocytes, however, did not show any protection (Figs 3–5).
Discussion
Oral administration of TNBS alone is known to induce oral tolerance and confer protection against TNBS-induced colitis in mice [14]. We have confirmed that orally fed TNBS alone (OR-TNBS group) can partially protect the animals in our rat model. One recent study has described suppression of TNBS-induced colitis following oral administration of colonic antigens from colitis affected colon extract after induction of colitis with TNBS [13]. In this protocol the colonic proteins are almost certainly haptenized as they used colon extract after TNBS-induced colitis developed [13]. Our data show that protection against TNBS-induced colitis can be achieved even by oral administration of unmodified colonic proteins (autoantigens). Most importantly, our data suggest that haptenization of antigens with TNBS or the oral feeding of TNBS is not an absolute requirement for the induction of oral tolerance. These data further support the hypothesis that oral tolerance to normal colonic autoantigens is involved in the suppression of TNBS-induced colitis. We believe that immune response to haptenized colonic antigens is required for the induction of colitis in this model because the disease is induced only after TNBS enema. However, the protective mechanism can be due to a bystander process in which the regulatoy T cells suppress responses to a tissue specific antigen(s) which is currently unknown.
Organ-specificity of the tolerizing antigens
One important aspect of our data is that small intestinal antigens, in contrast to colonic antigens, failed to induce tolerance (Table 1, Figs 3–5). Rats fed with haptenized small intestinal extract (HSE) or nonhaptenized small intestinal extracts (NHSE) produced severe colitis following rectal administration of TNBS. The MPO activity in the distal colon of HSE-or NHSE-fed rats was similar to RE-TNBS group and was significantly higher than the MPO activity in the untreated control group (P < 0·002, Fig. 5). In all the groups used in this study, there were no gross morphological changes in the small intestine following rectal administration of TNBS and the MPO activity in small intestine of TNBS-treated rats was not significantly different than in the untreated control group (data not shown). These data suggest that the tolerizing antigen is present only in colon but not in small intestine.
Passive transfer of protective immunity
In mice, oral administration of haptenized colonic antigens induces TGF-beta and Th2-type cytokines that suppresses TNBS-induced colitis [11,12]. Colitis in this model is associated with Th1 T cell response and can be passively transferred to naïve mice by lymphocytes [18]. Thus, induction and suppression of TNBS-induced colitis are mediated by distinct sets of T cells [11–13,18]. In our rat model, tolerance was induced by nonhaptenized colonic antigens. Passive transfer of mesenteric and splenic T lymphocytes from normal rats did not show any tolerance. These data clearly underscore the role of lymphocytes, most likely regulatory T cells, in the tolerization process. At present, we do not know the mechanism of tolerance induction in TNBS-induced colitis in rats. However, taken together with the observation that only colonic but not small intestinal antigen(s) induced T-cell mediated tolerance, we conclude that regulatory T-cells are generated to colon-specific antigens following the oral administration. However, the specific protein(s) involved in this process is unknown.
The tolerance induced by nonhaptenized colonic protein is currently unknown. This could be attributed to either colonic autoantigens or antigens of commensal colonic microorganisms [19] or both. We speculate that colonic autoantigen(s) is likely to be involved in the tolerization process because oral feeding of crude extracts of LS-180 colon cancer cell line also conferred protection to 4 of 6 rats used in one experiment (A. Dasgupta, unpublished observations). It is reasonable to presume that most intestinal antigens are shared by colon and small intestine and therefore, the number of colon specific tolerizing antigens is likely to be small or even a single colon-specific antigen may be involved. In this scenario, it should be possible to isolate the target antigen(s) by biochemical techniques using this rat model as an assay system. Clearly, such an approach will help us design a novel therapeutic strategy for IBD based on oral tolerance. Haptenization of colonic proteins is not a known mechanism in human IBD, although genetic and chemical alterations cannot be ruled out. With the availability of a known colonic antigen in future, the study of induction and suppression of colitis in this model will facilitate understanding of at least some antigen-specific immunological pathways in IBD.
Acknowledgments
This work was presented, in part, at the 100th annual meeting of the American Gastroenterological Association, Orlando, FL, May 1999. This work is supported, in part, by a research grant, NIADDK RO1 DK47673–05S1 from the National Institutes of Health, Bethesda, MD.
References
- 1.Podolsky D. Inflammatory bowel disease. N Engl J Med. 1991;325:928–35. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 2.Das KM. Relationships of extracolonic involvement in inflammatory bowel disease: New insights into autoimmune pathogenesis. Dig Dis Sci. 1999;44:1–13. doi: 10.1023/a:1026629528233. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Autoimmunity and ulcerative colitis: can two enigmas make sense together? Gastroenterology. 1995;109:307–12. doi: 10.1016/0016-5085(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 4.Hibi T, Ohara M, Toda K, et al. In vitro anticolon antibody production by mucosal or peripheral blood lymphocytes from patients with ulcerative colitis. Gut. 1990;31:1371–6. doi: 10.1136/gut.31.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta A, Mandal A, Das KM. Circulating Immunoglobulin G1 in patients with ulcerative colitis against the colonic epithelial proteins detected by a novel monoclonal antibody. Gut. 1993;35:1712–7. doi: 10.1136/gut.35.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das KM, Dasgupta A, Mandal A, et al. Autoimmunity to cytoskeletal protein Tropomyosin, A clue to the pathogenetic mechanism of ulcerative colitis. J Immunol. 1993;150:2487–93. [PubMed] [Google Scholar]
- 7.Sartor RB. The role of luminal bacteria in colitis: More than an antigenic drive (comment) Eur J Clin Invest. 1998;28:1027–9. doi: 10.1046/j.1365-2362.1998.00404.x. [DOI] [PubMed] [Google Scholar]
- 8.Chao LP, Steele J, Rodrigues C, et al. Specificities of antibodies secreted by hybridomas generated from activated B cells in the mesenteric lymph nodes of patients with inflammatory bowel disease. Gut. 1998;29:35–40. doi: 10.1136/gut.29.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson CO, Sartor RB, Tennyson GS, et al. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 10.Morris GP, Beck PL, Herridge MS, et al. Hapten induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–03. [PubMed] [Google Scholar]
- 11.Neurath MF, Fuss I, Kelsall BL, et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asherson GL, Zembala M, Perera MACC, et al. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the Peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977;33:145–55. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- 13.Ilan Y, Weksler-Zangen S, Ben-Horin S, et al. Treatment of experimental colitis by oral tolerance induction: a central role for suppressor lymphocytes. Am J Gastroenterol. 2000;95:966–73. doi: 10.1111/j.1572-0241.2000.01935.x. [DOI] [PubMed] [Google Scholar]
- 14.Elson CO, Beagley KW, Sharmanov AT, et al. Hapten-induced model of murine inflammatory bowel disease. Mucosal immune responses and protection by tolerance. J Immunol. 1996;157:2174–85. [PubMed] [Google Scholar]
- 15.Bradley PP, Prievat DA, Christensen RD, et al. Measurement of cutaneous inflammation; estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1992;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 16.Worthington V, Zacka J. A comprehensive manual on enzymes and related biochemicals. Am Biotechnol Lab. 1994;12:72. [PubMed] [Google Scholar]
- 17.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase assay. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 18.Neurath MF, Fuss I, Kelsall BL, et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β mediated oral tolerance. J Exp Med. 1996;183:2605–16. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdu EF, Bercik P, Cukrowska B, et al. Oral administration of antigens from intestinal flora anaerobic bacteria reduces the severity of experimental acute colitis in BALB/c mice. Clin Exp Immunol. 2000;120:46–50. doi: 10.1046/j.1365-2249.2000.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]