Abstract
In vitro studies were conducted in the non-obese diabetic (NOD) mouse, prone to Type 1 autoimmune diabetes, to investigate the mechanisms involved in cell-mediated rejection of pig islet xenografts. Our previous work concerning the mechanisms of proliferation of xenogeneic lymphocytes to pig islet cells (PIC) was not indicative of PIC impairment. Consequently, a test was developed based on perifusion analysis of the alteration of basal and stimulated insulin release from adult PIC incubated with mouse splenocytes or subsets. Compared with PIC incubation alone or with syngeneic pig splenocytes, co-incubation with mouse whole spleen cells resulted in a decrease of basal and stimulated insulin release (P < 0·001). Two components of this alteration were detected separately: PIC impairment was decreased (P < 0·01) after removal of plastic-adherent cells from spleen cells, but maintained (P < 0·01) when plastic-adherent cells alone were co-incubated with PIC. The increase of murine interleukin-1β when mouse plastic-adherent spleen cells were cultured with PIC (P < 0·04) was indicative of macrophage activation. Soluble factors produced during co-incubation of mouse splenocytes or plastic-adherent cells with PIC were involved in the impairment process, since supernatant fluids collected during previous PIC–mouse cell co-incubations directly altered (P < 0·01) insulin release from PIC. Moreover, impairment of PIC by mouse spleen cells was abolished (P < 0·01) by gadolinium chloride (which inhibits macrophages), but not by cyclosporin A. Another mechanism was apparent, since co-incubation of PIC with purified mouse T cells or CD4+ T cells, re-mixed with antigen-presenting cells, led to a decrease (P < 0·01) of insulin release. This model, based on the alteration of dynamic basal and stimulated insulin release, is indicative of in vitro cell-mediated alteration of PIC in the NOD mouse. The effect of whole spleen cells was rapid, and a crucial role was played by plastic-adherent cells. Two mechanisms were responsible for the behaviour of these cells: an early direct effect (at least in part via soluble products); and the indirect presentation of PIC xenoantigens (leading to impairment by CD4+ T lymphocytes).
Keywords: islets, pig insulin release, xenograft, mouse, lymphocytes, antigen-presenting cells, primary non-function, T-cell rejection, gadolinium, cyclosporin A
Introduction
Type 1 diabetes may be treatable by pig islet grafts [1]. However, cell-mediated rejection of the xenograft is a critical factor, especially since islets may be less susceptible to hyperacute humoral rejection. Most investigations of the cellular recognition of pig cells have used pig lymphocytes or endothelial cells that are not intended to be grafted in diabetic recipients, whereas pig islets have been studied infrequently [2–4]. In this respect, our previous work concerning the intensity and mechanisms of proliferation of mouse and human immune cells to adult pig islet cells (PIC) determined that a dominant CD4 class II-restricted response was involved through an indirect recognition pathway [2,3]. As lymphocyte proliferation is not indicative of PIC impairment, the present in vitro study was undertaken to evaluate the impact of splenocytes from non-obese diabetic (NOD) mice on PIC, i.e. in a suitable model of Type 1 diabetes treated by a pig islet or foetal islet graft [4]. A test was developed based on analysis during perifusion of the alteration of basal and stimulated insulin release from PIC incubated with different mouse spleen cells. A model was first designed in which PIC were confronted with mouse whole spleen cells, i.e. a complex mixture of cells that are involved in different mechanisms. Two components were then analysed separately. The first related to alteration mediated by non-T cells, which are potentially involved in vivo in the early phenomenon known as islet primary non-function (PNF). Early graft failure due to PNF, which has also been demonstrated in the NOD mouse [5], is dependent on donor–recipient combinations [6]. The high rate of PNF in nude mice [7] or rats [8] grafted with xenogeneic islets indicates that PNF is independent of T cell-mediated events. This phenomenon appears to be associated mainly with macrophages and their secretion products (cytokines, nitric oxide, superoxide anion, hydrogen peroxide etc.) [9,10]. The second component was impairment by the T lymphocytes and subsets potentially involved in vivo in specific cellular rejection of pig islets [11,12]. It is important to control this mechanism as pig islets can survive indefinitely in diabetic nude mice [11].
Materials and methods
Specific pathogen-free (SPF) pigs: islet isolation
Large-White SPF pigs (80–120 kg, 20–30 weeks-old) were used as previously described [13]. Pancreata were removed, soaked in betadine solution, inflated with ice-cold sterile modified University of Wisconsin (mUW) solution and transported in a sterile receptacle containing mUW. Pancreata were then re-inflated with mUW solution containing 2 mg/ml Liberase (Roche Diagnostics, Meylan, France) and placed in a digestion chamber filled with mUW solution kept at 36°C. Crude islets were pelleted by centrifugation, suspended in mUW and purified on a continuous Optiprep gradient (Life International Technology, Cergy-Pontoise, France) with a COBE 2991 processor. Islet purity, as assessed by dithizone staining, exceeded 90%. Insulin release from islets in response to different stimuli was highly reproducible. Islets were then treated with EDTA and dispase to obtain islet cell suspensions, before the cells were enumerated and suspended in Ham's F10 medium (5·5 mmol/l glucose; Biomédia, Boussens, France) supplemented with 2% Ultroser (BioSepra SA, Villeneuve la Garenne, France), penicillin, streptomycin and Fungizone (Seromed, Berlin, Germany) at 37°C in a CO2 incubator.
Mice and splenocyte preparations
Three-month-old, non-diabetic, female NOD mice (Clea, Tokyo, Japan), bred in our laboratory since 1986, were used for all experiments. After spleens were removed in Hank's balanced salt solution (HBSS; Seromed, Berlin, Germany), using 2% foetal calf serum (FCS), splenocytes were flushed out and erythrocytes were lysed by ammonium chloride. The cell suspension was resuspended in RPMI (Eurobio, Les Ulis, France)−10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin. In some experiments, spleen cells were depleted of plastic-adherent cells after two successive incubations on plastic Petri dishes (Nunc, Rostkilde, Denmark) for 3 h each at 37°C. Enriched antigen-presenting cell (APC) preparations were recovered after adherence of spleen cells by incubation on plastic Petri dishes for 3 h at 37°C. After depletion of plastic-adherent cells from spleen cells, T cells or CD4+ T-cell fractions were purified by positive selection using microspheres (Dynabeads™, Dynal, Oslo, Norway) coated with MoAbs against Thy 1.2. or CD4, respectively. Mouse CD8+ T-cell fractions were enriched by negative selections (R & D Systems, Abington, UK). The efficiency of depletions or enrichments was controlled by flow cytometry analysis.
Co-incubation of PIC with mouse spleen cells and subsets: perifusion analysis of basal and stimulated insulin release
Islet β-cell function was assessed after co-incubation of 105 PIC with different types of mouse cells in flat-bottomed, 24-well, microtitre plates (Nunc) incubated at 37°C in 5% CO2 in a final volume of 1·5 ml for 1, 2, 3 or 7 days. The effect of whole mouse spleen cells (1·5 × 106) was first studied. In some experiments, spleen cells (1·5 × 106) were introduced into the assay after removal of plastic-adherent cells, whereas in other experiments, 300 000 plastic-adherent cells were directly incubated with PIC. Co-cultures of PIC with spleen cells were also performed in the presence of different doses of gadolinium chloride (GdCl3, Prolabo, Paris, France) or cyclosporin A (Sandoz, Rueil-Malmaison, France). Supernatant fluids from 4-day co-cultures were harvested to test whether the soluble factors produced during co-incubation of mouse whole spleen cells or plastic-adherent cells with PIC could be involved in impairment of insulin release. These supernatant fluids (1 ml) were added three times (days 1, 2 and 3) to fresh PIC, and then perifused at day 4. In some of these co-incubations of PIC with supernatant fluids, blocking antibodies to murine IL-1, IFNγ and TNFα (BD Pharmingen, Heidelberg, Germany) were added (10 µg/ml) during the 4 days in order to evaluate the role of IL-1, IFNγ and TNFα in impairment of insulin release. Insulin release was also measured after co-incubation of PIC for 7 days with 1·5 × 106 mouse T cells, and CD4+ or CD8+ cell subsets, re-mixed or not with 150 000 plastic-adherent APC, in the absence or presence of 6 U/ml mouse IL-2 (R & D Systems).
In all cases, insulin release was determined at the end of the co-incubation period. PIC were maintained by a filter in a perifusion chamber (Swinnex 13, Millipore Corp, Bedford, MA), i.e. a small enclosed area continuously exposed to liquid propelled by a peristaltic pump (flow rate: 1 ml/min) through tubing from tanks containing Krebs Ringer bicarbonate buffer [0·5% bovine serum albumin, 5 mmol/l pyruvate, fumarate and glutamate (Sigma), but different glucose concentrations]. A multi-way stopcock on the tubing allowed the liquid to be changed instantly, so that PIC were alternately perifused with media, stimulating or not stimulating insulin release. PIC were first perifused for 30 min in 2·8 mmol/l glucose, then for 20 min in 20 mmol/l glucose and finally, in 2·8 mmol/l glucose. Samples of the liquid at the outlet of the chamber (same flow rate as at the entry) were collected every minute, allowing continuous radioimmunoassay (CIS Bio International, Gif-sur-Yvette, France) of the insulin released. Chambers and perifusion media were kept at 37°C in a water-bath, and perfusates were gassed with a 95% O2−5% CO2 mixture. In some experiments, insulin release was calculated as the area under the curve obtained throughout the perifusion period. Modifications of this area between experimental and control conditions were then expressed as percentages of variation.
Interleukin 1 secretion
Under conditions similar to those described above, 100 µl supernatant fluids were removed after 2 days of co-incubation for interleukin-1 (IL-1) assays. Murine IL-1 concentrations were determined by ELISA (Biotrak ELISA system, Amersham Life Science), using a solid phase involving a capture antibody bound to the wells of a microtitre plate and a horseradish peroxidase-conjugated antibody for detection. Standard curves were generated using recombinant mouse IL-1.
Statistical analysis
Data are presented as mean values ±s.e.m. The statistical significance of differences was evaluated using Student's t-test or Mann–Whitney test, when n < 5; P < 0·05 was considered as significant.
Results
Decrease of insulin release from perifused PIC incubated with mouse whole spleen cells
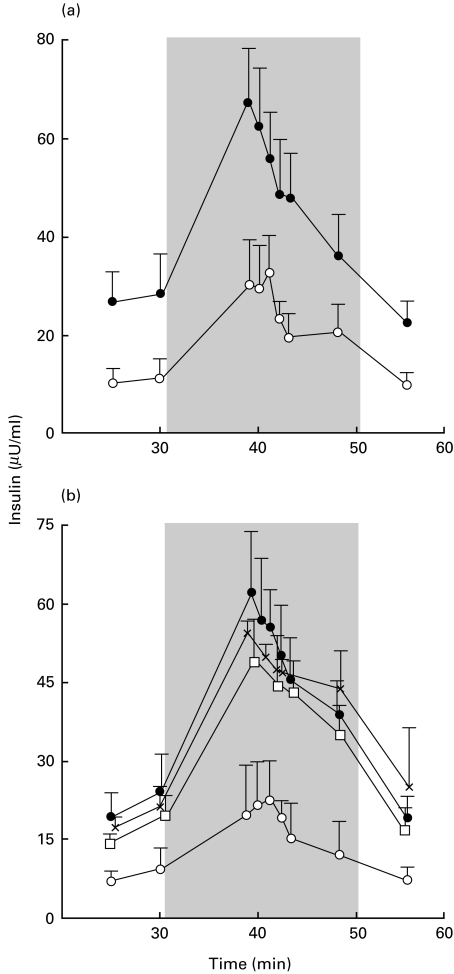
Compared with PIC incubated alone or with syngeneic pig splenocytes, co-incubation with NOD mouse splenocytes led to a decrease in basal and stimulated insulin release (P < 0·001 for both, Fig. 1). The decrease was detected after 24 (data not shown), 48 or 7 days of co-incubation (Fig. 1). The magnitude of this alteration was dependent on the number of mouse splenocytes (data not shown). No alteration in insulin release was detected when PIC were co-incubated with irradiated NOD splenocytes (Fig. 1). A decrease in basal and stimulated insulin release from PIC was similarly detected after co-culture with non-diabetes-prone Balb/c or C57BL6 splenocytes (P < 0·001) and splenocytes from NOD mice (data not shown).
Fig. 1.
Marked decrease of dynamic insulin release from perifused pig islet cells (PIC) incubated with mouse whole spleen cells. (a and b) Insulin release of 100 000 PIC cultured (•) alone (n = 15), (×) with 1·5 × 106 syngeneic pig splenocytes (n = 2), or with 1·5 × 106 NOD mouse whole spleen cells (□) irradiated (n = 2) or (○) not (n = 15). Perifusion was performed after 48 h (a) or 7 days (b) of co-incubation. The grey area corresponds to the glucose stimulation period. Each point indicates the mean value ±s.e.m. P < 0·001 between insulin release from PIC cultured alone or with mouse spleen cells.
Insulin release from PIC decreased rapidly after impairment by mouse plastic-adherent cells
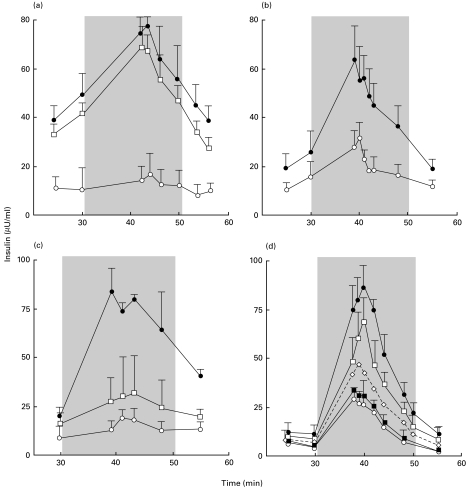
Impairment of PIC by NOD mouse splenocytes was abolished (P < 0·01) after removal of plastic-adherent cells (Fig. 2). The decrease in basal and stimulated insulin release was recovered during co-incubation of PIC with plastic-adherent cells alone (P < 0·01) (Fig. 2). The magnitude of this alteration was dependent on the number of plastic-adherent cells (the effect disappeared when PIC were co-incubated with fewer than 150 000 cells) (Fig. 2). Co-incubation of PIC with plastic-adherent cells from Balb/c mice decreased basal and stimulated insulin release similarly to PIC incubation with NOD splenocytes (data not shown).
Fig. 2.
Involvement of plastic-adherent cells in early impairment of pig islet cell (PIC) function by spleen cells from NOD mice. (a) Insulin release from 100 000 PIC cultured for 48 h alone (•), with 1·5 × 106 whole mouse spleen cells (○), or with 1·5 × 106 mouse spleen cells depleted of plastic-adherent cells (□). Each point indicates the mean value ±s.e.m. of 10 experiments. P < 0·01 between PIC cultured with whole spleen cells or spleen cells depleted of plastic-adherent cells. (b) Insulin release from PIC cultured for 48 h alone (•) or with 300 000 mouse plastic-adherent cells (○). Each point indicates the mean value ±s.e.m. of 10 experiments. P < 0·01 between PIC cultured alone or with plastic-adherent spleen cells. (c) Insulin release from PIC cultured for 48 h alone (•), with supernatant fluids from previous co-cultures of PIC and mouse spleen cells (○), or with plastic-adherent spleen cells (□). Each point indicates the mean value ±s.e.m. of three experiments. P < 0·01 between PIC cultured alone or with supernatant fluids. (d) Effects of 0·01 µg/ml (▪), 1 µg/ml (◊), or 100 µg/ml (□) of gadolinium chloride on insulin release from PIC co-cultured with mouse spleen cells for 48 h. Comparisons were performed with co-cultures of PIC and spleen cells without gadolinium chloride (○) or with PIC alone (•). Each point indicates the mean value ±s.e.m. of three experiments. P < 0·01 between PIC cultured with mouse spleen cells in the absence and presence of Gad. The grey area corresponds to the glucose stimulation period.
Early impairment of insulin release by plastic-adherent cells may have resulted mainly from functional alteration of PIC, as the number of PIC counted in light microscopy after 48 h co-incubations with NOD plastic-adherent cells (n = 2) was not decreased in comparison with PIC alone incubated for the same time period (10% versus 8% and 15% versus 12%, respectively).
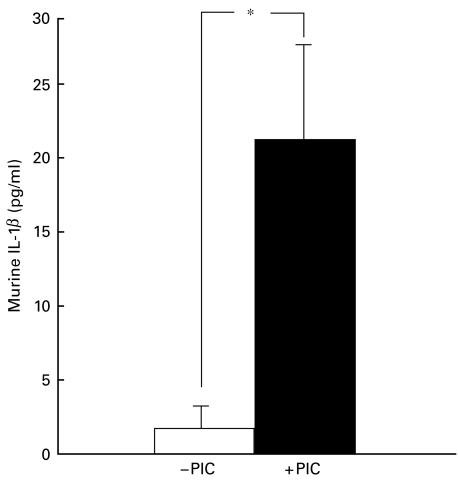
To determine whether soluble factors could be involved in the alteration of insulin release, supernatant fluids from previous co-cultures of PIC and mouse spleen cells were added to fresh PIC. PIC cultured with those supernatant fluids showed decreased insulin release (P < 0·01) compared with PIC cultured alone (Fig. 2). Similarly, addition of supernatant fluids from previous co-cultures of PIC and plastic-adherent cells inhibited (P < 0·01) insulin release (Fig. 2). This effect of supernatant fluids from previous co-cultures of PIC and plastic-adherent cells could have been due, at least in part, to the direct effect of murine IL-1, IFNγ and TNFα on PIC. Two experiments suggested that blocking antibodies to IL-1, IFNγ or TNFα reduced the inhibition of insulin release (20 and 21% reduction for IL-1, 25 and 19% for IFNγ, and 30 and 28% for TNFα). The production of murine IL-1 increased significantly (P < 0·04) when plastic-adherent spleen cells were cultured with PIC (Fig. 3).
Fig. 3.
In vitro production of interleukin-1β (IL-1β) by 300 000 plastic-adherent spleen cells from NOD mice cultured for 48 h alone (□) or with 100 000 pig islet cells (PIC, ▪). Columns indicate the mean values ±s.e.m. of three experiments. *P < 0·04 between IL-1β production from mouse cells cultured alone or with PIC.
GdCl3, when added in vitro during co-cultures, abolished the alteration of insulin release from PIC cultured with NOD mouse splenocytes in a dose-dependent manner (P < 0·01) (Fig. 2d), whereas the alteration of insulin release from PIC co-incubated with mouse splenocytes was unchanged by cyclosporin A (data not shown). The highest cyclosporin A dose used (100 µg/ml) did not alter insulin release from PIC, but completely blocked proliferation of the same mouse splenocytes co-incubated for the same time with the same PIC preparations (data not shown).
Decrease of insulin release from PIC co-incubated with T cells, including CD4+ cells, following presentation by mouse APC
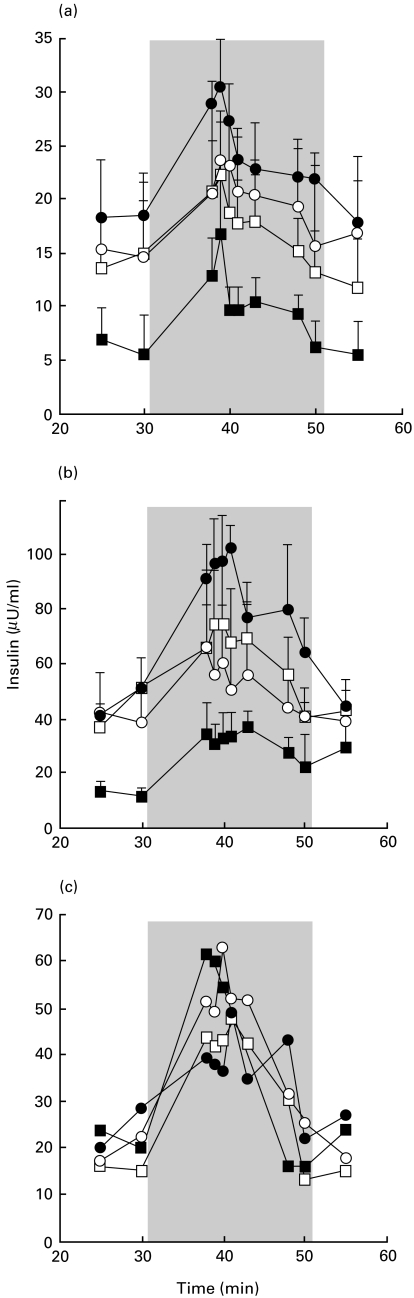
Seven-day co-incubation of PIC with purified mouse T cells re-mixed with 150 000 plastic-adherent APC led to a decrease (P < 0·01) in insulin release (Fig. 4). However, this effect was not detected after co-incubation with mouse T cells or APC alone. This decrease in insulin release could have resulted mainly from lysis of PIC, since PIC counted in light microscopy after 7 days of co-incubation with T cells (n = 2) decreased by 67 and 71% in comparison with PIC alone.
Fig. 4.
Alteration of insulin release from pig islet cells (PIC) by mouse T cells or subsets. Insulin release of 100 000 PIC cultured alone (•) or with mouse spleen cell subsets for 7 days. The grey area corresponds to the glucose stimulation period during perifusion. (a) PIC were co-incubated with 150 000 mouse plastic-adherent, antigen-presenting cells (APC) (n = 4; ○), 1·5 × 106 mouse purified T cells, or T cells mixed with APC (n = 5, □, and n = 5, ▪, respectively). P < 0·01 between insulin release from PIC alone and PIC cultured with T cells mixed with APC. (b) PIC were co-incubated with APC (n = 4; ○), 1·5 × 106 mouse CD4+ cells, or CD4+ cells mixed with APC (□ and ▪, respectively, n = 4 each). P < 0·01 between insulin release from PIC alone and PIC cultured with CD4+ cells mixed with APC. (c) Representative experiment in which PIC were co-incubated with APC (n = 1; ○), 1·5 × 106 mouse CD8+ cells, or CD8+ cells mixed with APC (n = 1, □, and n = 1, ▪, respectively).
Similarly, co-incubation of PIC with purified mouse CD4+ T cells mixed with 150 000 APC also led to a decrease (P < 0·01) in insulin release (Fig. 4). However, this effect was not detected after co-incubation with mouse CD4+ cells or APC alone. No decrease in insulin release was detected when PIC were co-incubated with purified CD8+ cells mixed with APC (Fig. 4), even in the presence of IL-2.
Discussion
Although the results presented here are preliminary, the in vitro study concerning the NOD mouse prone to Type 1 autoimmune diabetes provides information about mechanisms likely to be involved in cell-mediated impairment of adult pig islets. Our previous study concerning the intensity and mechanisms of mouse spleen cell proliferation in the presence of PIC showed a dominant CD4 class II-restricted response involving an indirect recognition pathway [2,3]. However, the fact that T lymphocyte proliferation is not indicative of the mechanisms involved in impairment of target cells led us to develop a test based on dynamic analysis during perifusion of the alteration of insulin release from PIC incubated with mouse cells. To our knowledge, this is the first functional study investigating cellular xenogeneic effects on adult pig islets.
This test showed that the decrease in basal and stimulated insulin release from PIC was very rapid with whole mouse spleen cells. The number of PIC used in these experiments was based on preliminary experiments (data not shown) and optimal proliferation conditions. Although the number of mouse splenocytes was relatively high, overcrowding was unlikely. For example, co-incubation of PIC with the same number of mouse splenocytes depleted of plastic-adherent cells did not result in decreased insulin release, nor did co-incubation of PIC with the same number of autologous pig splenocytes alter insulin release. This suggests that the decreased insulin release observed with whole mouse splenocytes was actually due to xenogeneic impairment. This cellular effect was apparent from the first days of PIC–spleen cell confrontation and thus, well before lymphocyte proliferation which is detectable no earlier than the fourth day and peaks after 7 days [2,3]. This early effect in vitro suggests that deleterious effects on β-cell function during in vivo islet xenografts occur rapidly once immune cells are attracted in situ. The mechanisms involved in PIC impairment resulting from the mixture of cells contained in mouse spleen cells are complex. A combination of depletion, enrichment and pharmacological inhibition experiments allowed us to distinguish two mechanisms, both dependent on plastic-adherent cells.
A first mechanism, i.e. early direct impairment by plastic-adherent cells, was responsible for a rapid decrease in insulin release. Depletion reduced impairment, while enrichment reproduced the alteration in insulin release. The early decrease in insulin release from PIC induced by mouse plastic-adherent cells could have been due to lysis or functional inhibition of PIC. However, the absence of a decrease in the number of PIC in the presence of mouse plastic-adherent cells (as revealed by microscopy) is concordant with the notion of functional impairment of PIC, at least during the early stages. This in vitro effect seems consistent with in vivo studies in nude rodents, suggesting that early xenogeneic islet primary non-function (PNF) is a non-T-cell response mainly mediated by macrophages [7,8]. The role of macrophages in the PNF of islets grafted into xenogeneic rodents has also been suggested because of the improvement of early graft function following gadolinium chloride-induced inhibition of macrophages [9,14,15]. In this respect, our study found that the use of gadolinium in the in vitro assay caused inhibition of early impairment by mouse spleen cells without toxicity to PIC. Cyclosporin A was unable to abolish this alteration, confirming the involvement of a non-T-cell phenomenon. The alteration ininsulin release from PIC incubated with supernatant fluids from previous co-cultures of PIC and mouse whole spleen cells or plastic-adherent cells suggested that impairment by these mouse cells was at least partly mediated by soluble factors. The fact that murine IL-1β production markedly increased during co-incubation with PIC is indicative of macrophage activation. Some mouse cytokines appeared to be at least partially involved in the alteration of insulin release from PIC incubated with supernatant fluids from previous co-cultures of PIC and mouse spleen cells. This was suggested in the present study by blocking experiments with MoAbs directed against IL-1, IFNγ or TNFα. However, since species disparity between the pig and the mouse reduces the probability of cytokine cross-reaction, murine IL-1β, IFNγ or TNFα may not have been the main toxic products for PIC, as they could be in other species combinations. Other non-species-specific soluble factors, such as nitric oxide or oxygen metabolites, may have been involved. The deleterious effect of such factors during PNF, or of in vitro macrophage toxicity to islets, has been suggested [9,10,14]. Nevertheless, the test described here could provide a model for in vivo PNF of pig islets. The intensity of the impairment observed in this in vitro model confirms that in vivo PNF of pig islet xenografts would be a serious obstacle. Thus, a therapeutic approach is needed that can counteract PNF while inhibiting plastic-adherent cells. This approach is particularly necessary as our study suggests that these same cells play an additional major role as APC in T-cell aggression.
The in vitro test of the alteration of insulin release from perifused PIC enabled us to demonstrate a second mechanism, i.e. impairment by T lymphocytes. T-cell-mediated impairment of PIC in vitro required the presence of mouse APC. The decrease in the number of PIC at the end of co-incubation with mouse T cells is consistent with the notion that the drop in insulin release was mainly due to lysis of PIC. This T-cell mediated alteration was reproduced by co-incubation of PIC with CD4+ cells mixed with APC, whereas CD8+ cells showed no detectable effect. This is consistent with studies indicating that CD4+ cells constitute a major lymphocyte subset involved in xenograft rejection [12,16]. As mouse T-cell-mediated aggression of PIC in vitro involves T lymphocytes and an indirect pathway for recognition of PIC xenoantigens, the present data are also consistent with our previous report of a dominant CD4 class II-restricted response, with an indirect recognition pathway, for mouse or human T-cell proliferation against these same PIC [2,3]. Thus, therapeutic methods targeting APC are needed to help combat this indirect recognition and aggression.
In addition to studies of aggression mechanisms, the in vitro test described here seems suitable for modelling what might happen in vivo during pig islet xenografts. This test is based on dynamic analysis of the loss of insulin release, which corresponds to the judgement criterion for the failure of an islet graft. Analysis of the alteration of insulin release during perifusion is more precise than with static incubation. The test allows the different chronological steps of the rejection process to be studied and their impact to be compared. Finally, the test is suitable for determining whether drugs intended to control these two stages of cellular xenorejection alter basal and stimulated insulin release.
Acknowledgments
The authors are grateful to Mrs M. Ouary, Mr C. Chevallier, Mrs M. Allard and Mrs S. Pogu for their excellent collaboration. This work was supported by grants from the Pays de la Loire and Brittany regions. Pig islets were isolated from SPF pigs bred by a collaboration with AFSSA (Ploufragan, France, Dr Ph. Vannier).
References
- 1.Groth CG, Korsgren O, Tibell A, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–4. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 2.Rivereau AS, You S, Lalain S, Gouin E, Saï P. In vitro xenorecognition of adult pig pancreatic islet cells by splenocytes from non-obese diabetic or non-diabetes prone mice. Transplantation. 1998;66:633–8. doi: 10.1097/00007890-199809150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Lalain S, Chaillou L, Gouin E, Saï P. Intensity and mechanisms of in vitro xenorecognition of adult pig pancreatic islet cells by CD4+ and CD8+ lymphocytes from type I diabetic or healthy subjects. Diabetologia. 1999;42:330–5. doi: 10.1007/s001250051159. [DOI] [PubMed] [Google Scholar]
- 4.Mandel TE, Kovarik J, Koulmanda M. A comparison of organ cultured fetal pancreas allo-, iso-, and xeno-(pig) in non-immunosuppressed non-obese diabetic mice. Am J Pathol. 1995;147:834–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Faust A, Rothe H, Schade U, Lampoter E, Kolb H. Primary nonfunction of islet grafts in autoimmune diabetic mice is prevented by treatment with interleukin-4 and interleukin-10. Transplantation. 1996;62:648–52. doi: 10.1097/00007890-199609150-00019. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med. 1990;172:291–302. doi: 10.1084/jem.172.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuch BE, Casamento FM. Comparison of fetal pig pancreatic development in scid and nude mice. Xenotransplant. 1997;41:150. [Google Scholar]
- 8.Brandhorst D, Brandhorst H, Zwolinski A, Nahidi F, Jahr H, Bretzel RG. Primary non-function of isolated pig proislet after intraportal transplantation in athymic nude rats. Hormone Metabolism Research. 1998;30:A9. [Google Scholar]
- 9.Marquet RL, Bonthuis F, Duval SY, et al. Macrophages and nitric oxide are involved in primary nonfunction of islet xenografts. Transplant Proc. 1995;27:252–3. [PubMed] [Google Scholar]
- 10.Jahr H, Bretzel RG, Wacker T, et al. Toxic effects of superoxide, hydrogen peroxide, and nitric oxide on human and pig islets. Transplant Proc. 1995;27:3220–1. [PubMed] [Google Scholar]
- 11.Maki T, O'Neil JJ, Porter J, Mullon CJ, Solomon BA, Monaco AP. Porcine islets for xenotransplantation. Transplantation. 1996;62:136–8. doi: 10.1097/00007890-199607150-00028. [DOI] [PubMed] [Google Scholar]
- 12.Simeonovic CJ, Ceredig R, Wilson JD. Effect of GK1.5 monoclonal antibody dosage on survival of pig proislet xenografts in CD4+ T cell-depleted mice. Transplantation. 1990;49:849–56. doi: 10.1097/00007890-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gouin E, Rivereau AS, Duvivier V, et al. Perifusion analysis of insulin secretion from specific pathogen-free large white pig islets shows satisfactory functional characteristics for xenografts in humans. Diabete Metab. 1998;23:208–14. [PubMed] [Google Scholar]
- 14.Deng S, Ketchum RJ, Kucher T, Weber M, Naji A, Brayman KL. Primary nonfunction of islet xenografts in rat recipients results from non-T-cell mediated immune response. Transplant Proc. 1997;29:1726–7. doi: 10.1016/s0041-1345(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 15.Lazar G, Farkas G, Csanadi J, Lazar G. Gadolinium chloride-induced macrophage blockade prevents rejection of human insulinoma cell xenograft in rats. Transplantation. 1997;63:729–32. doi: 10.1097/00007890-199703150-00020. [DOI] [PubMed] [Google Scholar]
- 16.Chitilian HV, Laufer TM, Stenger K, Shea S, Auchincloss HJ. The strength of cell-mediated xenograft rejection in the mouse is due to the CD4+ indirect response. Xenotransplant. 1998;5:93–8. doi: 10.1111/j.1399-3089.1998.tb00014.x. [DOI] [PubMed] [Google Scholar]