Abstract
Haemodialysis is a widespread option for end-stage renal disease (ESRD). Long-term success of dialysis is, however, limited by a high rate of serious bacterial and viral infections. We compared T cell functions in ESRD patients undergoing haemodialysis (n = 20), or were not dialysed and received conventional medical treatment (n = 20). Healthy volunteers (n = 15) served as controls. The T cell phenotype was examined by immunofluorescence using fluorochrome-labelled monoclonal antibodies and FACS analysis. The concentration of soluble CD95/Fas and of tumour necrosis factor-α receptor type 1 (sTNFR1) in the sera was quantified by ELISA. Activation-induced programmed T cell death was triggered by anti-CD3/CD28 antibodies and measured by 7-AAD staining. All immunological tests were performed at least 1 month after dialysis initiation. T cell proliferation in response to phytohaemagglutinin or anti-CD3 monoclonal antibodies was moderately diminished in non-dialysed patients and markedly reduced in haemodialysis patients compared to healthy controls (P < 0·01 and P < 0·001, respectively). In a mixed lymphocyte culture the proliferative response of T cells from dialysed patients was significantly diminished (P < 0·001). T cells of both non-dialysed and dialysed patients have augmented CD95/Fas and CD45RO expression, increased sCD95/Fas and sTNFR1 release and spontaneously undergo apoptosis. Culture of T cells from haemodialysis patients with anti-CD3/CD28 antibodies increased the proportion of CD4+ T cells committing activation-induced cell death by a mean 7·5-fold compared to T-helper cells from non-dialysed patients (P < 0·001). Renal failure and initiation of haemodialysis results in a reduced proliferative T cell response, an aberrant state of T cell activation and heightened susceptibility of CD4+ T cells to activation-induced cell death.
Keywords: apoptosis, end-stage renal failure, haemodialysis, T cell activation
Introduction
Renal failure is a major public-health issue due to its permanent increase among the general population, and the escalation of the cost of the renal function replacement therapies such as dialysis and kidney transplantation. The rate of success has, however, been reduced by various complications including thromboembolic events, haemostasis disorders and arterial occlusive accidents [1–3]. Despite continuous improvement of dialysis techniques, infections have remained one of the major causes of morbidity of patients in end-stage renal disease (ESRD). Epidemiological studies within the United States indicate that in chronic haemodialysis patients, bacterial and viral infection rank second place in mortality and morbidity, behind cardiovascular disease. Septicaemia accounts for more than 75% of mortality rate in haemodialysis ESRD patients, and an average of 7·6 bacteraemia episodes per 100 patient-years [4–6]. A European prospective cross-sectional study revealed that bacterial infections have a monthly incidence of approximately 1% [7].
The high risks of bacterial and viral infections in ESRD patients are related to an acquired immunodeficiency [5]. Uremic toxins may cause defects in cell-mediated immunity. Clinical evidence of impaired lymphocyte [8,9], granunlocyte [10,11] and monocyte [5,12] functions, which progress during the development of uremic retention, has been reported. Immune defects may also occur as a direct consequence of therapy, mainly in cell-mediated immunity [13]. Although some of the infections can be attributed to catheter and dialysis device installations, the high incidence of viral and bacterial infections in particular suggests that long-term haemodialysis treatment may be associated with the development of defects in host immunity [7,14,15]. This has been also reported after implantation of left-ventricular assist devices (LVAD) [16,17].
The objective of the present study was to investigate T cell proliferative responses, changes in T cell phenotype, the presence of soluble death-inducing receptors and activation-induced T cell death in ESRD patients undergoing haemodialysis or conventional medical treatment (non-dialysed) in comparison to healthy humans.
Patients and methods
Patients and clinical features
Immunological investigations were carried out in 20 patients undergoing haemodialysis three times per week (chronically uremic patients; mean creatinine 8·26 ± 0·48), in 20 non-dialysed patients (predialysis), non-catabolic renal failure patients (ESRD-chronically azotemic patients; with a mean serum creatinine value of 6·33 ± 0·41 mg/dl), and in 15 apparently healthy volunteers. The study groups were age- and sex-matched and all immunological tests were performed in the dialysis group at least 1 month after initiation of dialysis. Poplysulphone and cellulose acetate filters were utilized for haemofiltration. None of the renal failure patients had concurrent opportunistic or viral infections. Blood samples were acquired before scheduled dialysis treatment. Some demographic and clinical features are shown in the Table 1.
Table 1.
Demographic and clinical features
| Uremic patients (n = 20) | Azotemic patients (n = 20) | |
|---|---|---|
| Age years, median (range) | 58 (31–85) | 53 (33–78) |
| Gender; male:female | 11: 9 | 13: 7 |
| Serum-creatinine; mg/100 ml | 8·26 ± 0·48* | 6·33 ± 0·41* |
| Kt/V | 1·46 ± 0·06 | – |
| Creatinine-clearance; ml/min | – | 11·6 ± 1·03 |
| WBC; G/l | 6·17 ± 0·42 | 6·85 ± 0·4 |
| C-reactive protein; mg/100 ml | 0·71 ± 0·17 | 0·43 ± 0·13 |
Data except age and gender are expressed as mean ±s.e.m. Statistical significance:
P < 0·01 when compared to each other.
Immunophenotype of circulating T cells
Cell counts were measured by Coulter counter analysis. Fluorochrome-labelled monoclonal antibodies (MoAbs) to CD3, CD4, CD8, CD45RO and CD95 (Becton Dickinson, McKinley, MN, USA) were utilized in two-colour immunoflourescence analyses as described previously [16]. Immunofluorescence was measured by a FACScan 500 flow cytometer (Becton Dickinson, San Jose, CA, USA).
T cell proliferation
Whole blood samples had been anticoagulated with edetic acid, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient centrifugation [17]. PBMC were resuspended in culture medium (Roswell Park Memorial Institute Medium), and 200 μl medium containing 105 cells was added per well of 96-well flat-bottomed tissue culture plates. For blastogenesis assays cells were stimulated with phytohaemagglutinin (PHA; 7 μg/ml; Sigma, St Louis, MO, USA) or MoAb to CD3 (10 μg/ml) for 48 h at 37°C. For mixed lymphocyte culture (MLC) assays, 5 × 105 responder cells (isolated from either healthy humans, predialysis and dialysis patients) were added to irradiated (25 Gy) stimulator cells (5 × 105 cells) (obtained from controls) or to the medium only, and cultured for 5 days at 37°C. Cells were pulsed for 16 h with [3H]thymidine (3·7 × 104 Bq/well) after 48 h in the blastogenesis assays, and after 5 days in the MLC. Cells were harvested and [3H]thymidine uptake was measured in a liquid scintillation counter.
Quantification of soluble CD95 (sCD95)
Serum samples were obtained from study populations and kept frozen. Circulating serum levels of sCD95 were measured in a commercial enzyme-linked immunosorbent assay (ELISA) using polyclonal antibodies against human CD95 (Cytoscreen, BioSource International, Inc., Camarillo, CA, USA), as described previously [18]. The sensitivity limit of the ELISA was 20 pg/ml. The amount of protein in each serum sample was calculated according to a standard curve of optical density values constructed for known levels of sCD95 protein.
Quantification of soluble tumour necrosis factor-α receptor type 1 (sTNFR1)
Serum samples were obtained from study populations and kept frozen. Circulating serum levels of sTNFR1 (TNFR p55, CD120a) were measured by ELISA [19], using polyclonal antibodies against human TNFR1 (R&D Systems Inc, McKinley Place, MN, USA). The sensitivity limit of the ELISA was 3 pg/ml. The amount of protein in each sample was calculated according to a standard curve of optical density values constructed for known levels of sTNFR1 protein.
T cell apoptosis in vivo
A flow cytometry apoptosis detection kit (Becton Dickinson Systems, McKinley, MN, USA) was used to identify programmed cell death. PBMC (3 × 105) were triple-stained with phycoerythrin-conjugated MoAb to CD3, 10 μl flourescein isothiocyanate-labelled annexin V (R&D Systems, Minneapolis, MN, USA) to detect phosphatidylserine expression on cells during early apoptotic phases and 7-aminoactinomycin (7-AAD) to exclude dead cells [20,21]. The samples were analysed on a FACStar 500.
Activation-induced cell death
The amount of 3 × 106 PBMC from dialysis, predialysis and healthy humans (each group, n = 6) were placed on 48-well plates and cultured with MoAb to CD3/CD28 (10 μg/ml) or isotype control antibodies for 18 h at 37°C. Resting or stimulated T cells were stained simultaneously with fluorochrome-conjugated anti-CD4 MoAb or antiannexin V/7-AAD [21], and subjected to flow cytometry analysis.
Statistical analysis
Continuous variables such as proliferative responses to various stimuli, expression of CD95 and CD45RO, serum level of sCD95 and sTNFR, or annexin V binding were analysed by Student's t-test. A value of P < 0·05 was considered to be statistically significant.
Results
Immunophenotypic analysis of T cells
As shown in Table 2, the percentage of circulating T lymphocytes was significantly lower in dialysis as well as in preadialysis patients (P < 0·01) compared with healthy control volunteers (29·4 ± 1·7; 21·3 ± 1·2; 19·7 ± 1·4, respectively). Among T cell subsets the mean number of circulating CD4+ T cells was significantly lower in dialysis (521 ± 46) than in the predialysis group (702 ± 63) of patients (P < 0·001), or healthy controls (727 ± 87). In both groups of dialysed and non-dialysed ESRD patients the mean counts of CD8+ T cells was significantly reduced.
Table 2.
Changes in the number and the phenotype of T lymphocytes
| Lymphocytes | Controls (n = 15) | Predialysis (n = 20) | Dialysis (n = 20) |
|---|---|---|---|
| T cells (%) | 29·4 ± 1·7 | 21·3 ± 1·2* | 19·7 ± 1·4* |
| CD3+/μl | 1259 ± 118 | 1079 ± 95* | 917 ± 85** |
| CD4+/μl | 727 ± 87 | 702 ± 63 | 521 ± 46** |
| CD8+/μl | 520 ± 53 | 383 ± 41* | 299 ± 29** |
| CD4:CD8 ratio, mean | 1·42 ± 0·09 | 2·02 ± 0·19* | 1·93 ± 0·19* |
Peripheral T cells and their subsets were identified by immunofluorescence and FACS analysis. Data represent the mean ±s.e. Statistically significant differences between T cells from dialysis and predialysis patients versus controls:
P < 0·01
P < 0·001. The value of CD4:CD8 ratio from each patient was used to calculated the mean.
T cell proliferation
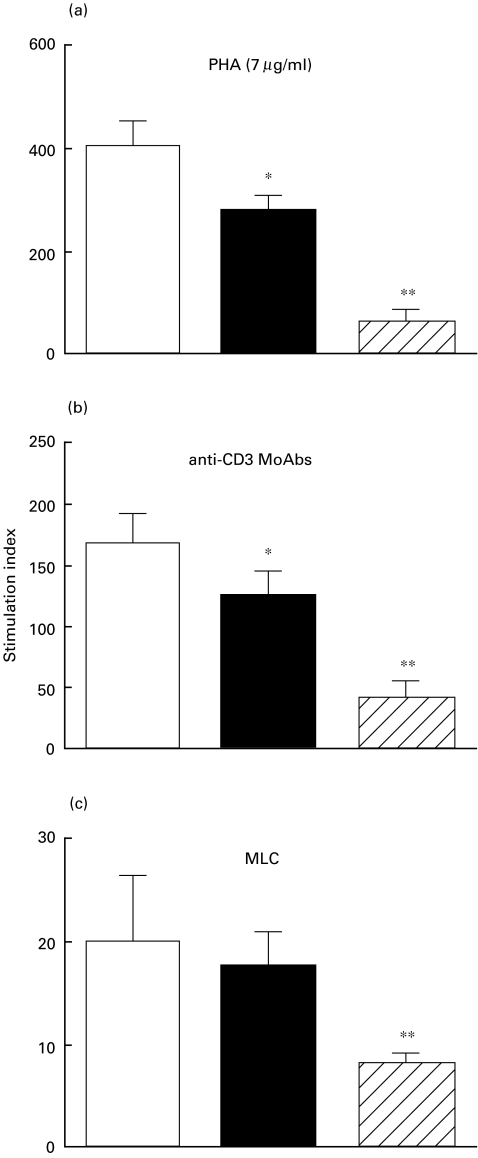
The results presented in Fig. 1a demonstrate that the proliferative capacity of T cells from ESRD-predialysis and dialysis patients in response to PHA were significantly reduced in comparison to controls (P < 0·01 and P < 0·001, respectively). As can be seen from Fig. 1b, after activation of T cells with anti-CD3 MoAb, the mean stimulation index (SI) of T cells from dialysed (40·9 ± 14·5) and non-dialysed (125·9 ± 19·7) patients were markedly reduced (P < 0·01 and P < 0·001, respectively) versus healthy controls (168·9 ± 23·9). After allogeneic MLC, the SI of T cells only from dialysed patients was significantly diminished (P < 0·001) as compared to the control group (Fig. 1c).
Fig. 1.
T cell proliferative responses. PBMC from 15 healthy volunteers, 20 non-dialysed ESRD patients and 20 dialysed patients were stimulated with phytohaemagglutinin (PHA; 7 μg/ml), anti-CD3 mAbs (10 μg/ml) or in a mixed lymphocyte culture (MLC). [3H]thymidine incorporation was measured, and the results are presented as mean of stimulation index ±s.e. Statistical significance: *P < 0·01; **P < 0·001. □, Healthy controls; ▪, ESRD-predialysis;  , dialysis.
, dialysis.
Expression of CD95 and CD45RO on peripheral T cells
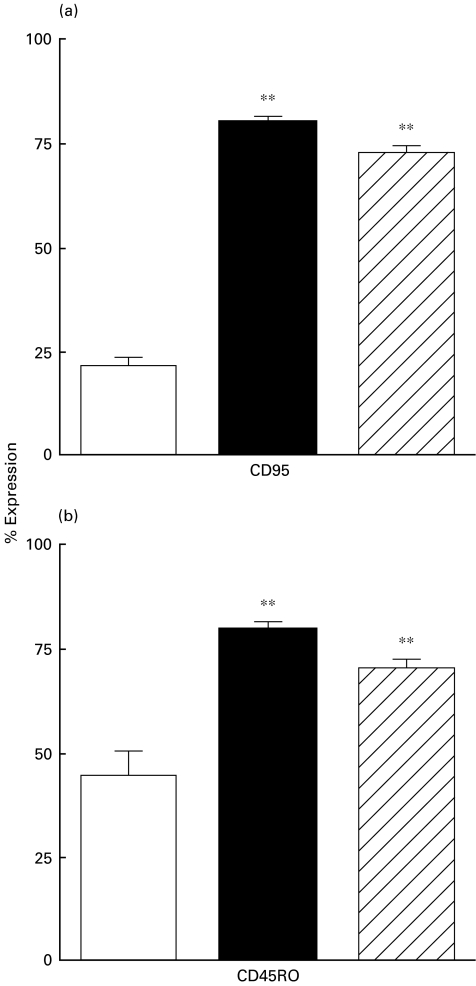
As shown in Fig. 2a, the expression of CD95 (Fas, Apo1), a molecule associated with a pathway of cellular apoptosis, was increased to a similar degree in CD3+ T cells from the non-dialysed (77·4 ± 1·6) and dialysed (73·3 ± 1·2) patients compared with healthy control volunteers (21·8 ± 1·9). Correspondingly, as can be seen from Fig. 2b, the percentage of cells expressing CD45RO, a marker for memory T cells, was higher on CD3+ T cells from both non-dialysed and dialysed patients than that in controls (79·2 ± 1·7, 72·9 ± 1·7 and 40 ± 6, respectively).
Fig. 2.
Changes in the phenotype of T cells. Expression of CD95, CD45RO on peripheral (CD3+) T cells from 15 healthy volunteers, 20 non-dialysed ESRD patients and 20 dialysed patients. Data from FACS analysis are percentage (mean ±s.e.) increase.Statistical significance: **P < 0·001. □, Healthy controls; ▪, ESRD-predialysis;  , dialysis.
, dialysis.
Soluble CD95 and sTNFR1
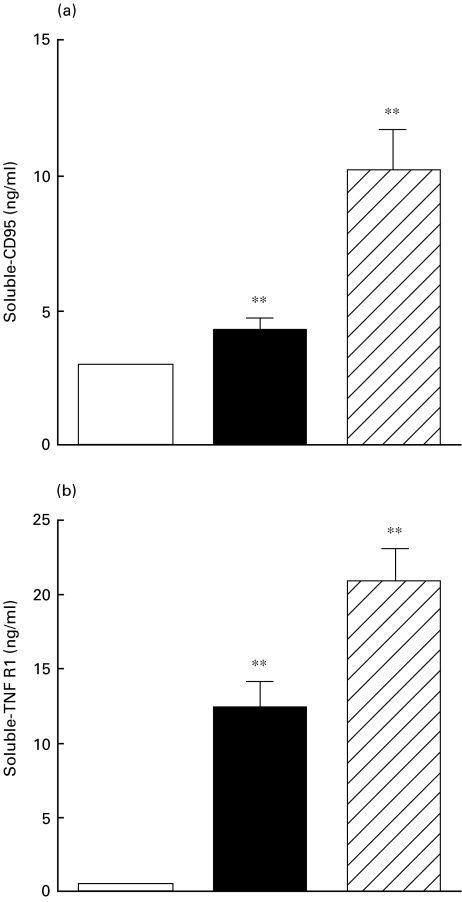
Translocation of phosphatidylserine, an early component of T cell apoptosis, occurs after ligation of CD95 receptor/Fas ligand, and this may lead to T cell activation, followed by antigen shedding and cell suicide. Therefore, we measured circulating levels of sCD95. As shown in Fig. 3a, the mean serum levels of sCD95 were significantly higher in both dialysed and non-dialysed patients versus controls (10·05 ± 1·3; 4·80 ± 0·26 and 2·90 ± 0·1, respectively, all P < 0·001).
Fig. 3.
Serum levels of soluble CD95 and tumour necrosis factor-α receptor type I (TNFR1). Serum samples were obtained from 15 healthy volunteers, 20 non-dialysed ESRD patients and 20 dialysed patients. The concentrations of sCD95 and sTNFR1 were measured by ELISA. The data are presented as mean (ng/ml ± s.e.). Statistical significance: **P < 0·001. □, Healthy controls; ▪, ESRD-predialysis;  , dialysis.
, dialysis.
Immune activation of lymphocytes and induction of apoptotic pathways can be initiated by TNF and results in TNFR1 shedding. To investigate the susceptibility of T cells to undergo increased activation-induced cell death (AICD) in dialysis versus non-dialysed patients, we measured circulating levels of sTNFR1. As can be seen from Fig. 3b the mean concentrations of sTNFR1 were significantly higher in both dialysed and non-dialysed patients versus controls (20·9 ± 2·2; 12·4 ± 1·8; and 0·53 ± 0·05, respectively, all P < 0·001).
Spontaneous T cell apoptosis in vivo
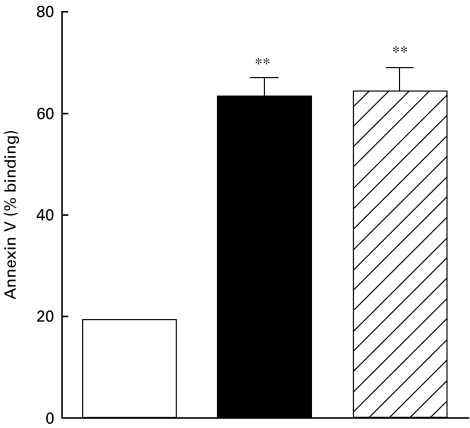
In further experiments we addressed the question of whether the elevated state of T cell activation is associated with increased percentage of peripheral T cells undergoing apoptosis. The results depicted in Fig. 4 show that CD3+ T cells from non-dialysed and dialysed patients had higher levels of annexin V binding than CD3+ T cells from controls (63·7 ± 4·25; 65·1 ± 3·85; 18·2 ± 1·0, respectively, all P < 0·001). In vivo dead cell exclusion was performed in all samples. Each group contained less than 0·5% 7-AAD positive cells.
Fig. 4.
Spontaneous T cell apoptosis. PBMC from 15 healthy volunteers, 20 non-dialysed ESRD patients and 20 dialysed patients were stained for annexin V binding. Results from FACS analysis are presented as percentage (mean ±s.e.) of cell binding annexin V. Statistical significance: **P < 0·001. □, Healthy controls; ▪, ESRD-predialysis;  , dialysis.
, dialysis.
Susceptibility to activation-induced cell death after T cell receptor involvement
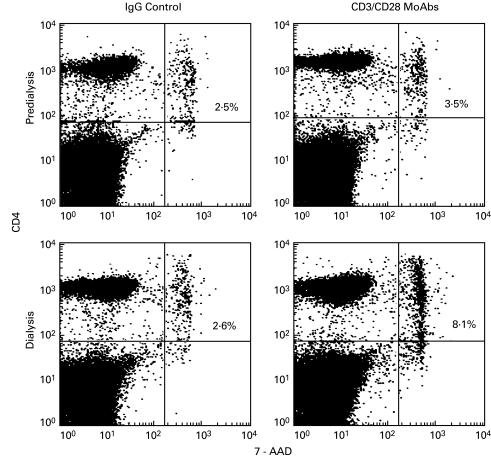
Since pre-activated T cells, which express CD95, are susceptible to activation-induced apoptosis after stimulation via the T cell receptor/CD3 complex, we investigated whether the observed defects in the proliferative responses of T cells in ESRD patients were related to activation-induced programmed cell death. As shown in Fig. 5, in a representative example of flow cytometry analysis 2·5% of resting CD4+ T cells from a non-dialysed patient expressed 7-ADD after 18 h of culture in medium with IgG control, and 3·5% after stimulation with anti-CD4/CD28 antibodies. When T cells from a dialysis patient were cultured for 18 h with IgG or anti-CD3/CD28 antibodies 2·6% and 8·1% of CD4+ cells were stained by 7-ADD, respectively (Fig. 5).
Fig. 5.
Antibody-induced T cell death. PBMC from a dialysis patient were cultured for 18 h with IgG control or anti-CD3/CD28 antibodies, and stained with fluorochrome-conjugated anti-CD4 and anti7-AAD monoclonal antibodies. Data from one representative experiment show the percentage flow cytometry analysis of CD4+ T cells that underwent activation-induced cell death (7-AAD staining). The percentage of cells stained by 7-AAD dye is indicated in the upper right quadrant.
Discussion
An aberrant state of T cell activation involving the CD95/Fas pathway and activation-induced CD4+ T cell death was found in ESRD patients. Defects in the cellular and humoral immune response may be responsible for the higher incidence of infection in haemodialysis patients, a major cause of increased morbidity and mortality rate [4–7]. In these patients, a functional T and B cell impairment may be accompanied by alloreactive T cell recognition of processed autoantigens, leading to production of autoantibodies specific for HLA molecules [22,23], cardiolipin, molondialdehyde-LDL, β2GPI and endothelial cells [24–26].
It has been shown previously that in ESRD patients T cells and macrophages, as well as other antigen-presenting cells (APC), are functionally activated, as defined by alteration of cytokine profile [27,28] and increased production of coagulation factors [28–30]. Furthermore, in patients undergoing haemodialysis or chronic ambulatory peritoneal dialysis, the macrophage biological functions such as adhesion, phagocytosis and killing and their capacity to present antigen to T-helper cells may be influenced markedly [5,31]. In ESRD patients, elevated sCD40 levels correlates strongly with creatinine concentration [32]. In addition, sCD23 levels correlated closely with the production of TNF-α and interleukin-6 [33]. These results indicate that patients with chronic renal failure often present an immunodeficiency state paradoxically exacerbated by haemodialysis with signs of B cell and monocyte activation. Since triggering via the T cell antigen receptor and the CD3 complex increases expression of CD95/Fas and its ligand (CD95L/FasL), subsequent T cell exposure to antigenic stimulation may result in activation-induced cell death, rather than the appropriate cell proliferative response, most probably because of interactions between CD95 and newly expressed CD95L.
The co-existence of defects in T cell-mediated immunity and prominent B-cell hyperreactivity in renal failure and dialysis patients is similar to HIV-1 infection [34]. Selective depletion of CD4+ T cells and immune dysfunction in AIDS patients accompanies a progressive increases in viral burden within APC, such as macrophages and dendritic cells [35,36]. One proposed mechanism to explain these findings is the inappropriate induction of apoptotic Th cell death, resulting from HIV-1-mediated interactions between CD95 and CD95L [37,38], suggesting the involvement of excessive T cell costimulation by virus-infected APC. However, by contrast with renal failure and dialysis initiation, the severe immune dysfunction associated with HIV-1 infections is multi-factorial, and apoptosis may be one of many immune defects induced by virus infection.
This paper demonstrates that patients undergoing heamodialysis present similar immune alterations compared to patients receiving a left ventricular assist device (LVAD) as a bridge to transplantation. LVAD recipients' T cells exhibit heightened susceptibility to AICD augmented by the CD95 pathway [16,17]. In both entities prolonged interaction between immunocompetent blood cell and polymers is prevalent. As LVAD patients are not under any immunosuppression we deduced previously that the chronically exposure to antigen, e.g. LVAD surface (polyurethane) or other polymers, such as silicone, PTFE or Dacron, is triggering a state of susceptibility towards programmed cell death [39,40]. We were able to demonstrate that commonly utilized polymers are able to act as superantigens, hence inducing nuclear factor of activated T cells (NFAT) and selective FasL/CD95L enhancement, as detected by immunoblotting analysis [39]. This observation is supported further by the induction of caspase 8 and 3 in naive PBMC exposed to biomaterials, indicating that a chemical compound leached from biopolymers initiates a pro-apoptotic pathway [40]. The increased pronicity of PBMCs from dialysis patients to undergo AICD is in line with recent data, demonstrating the presence of plastic softeners in dialysis patients and their immunosuppressive potential in vitro [41,42]. Our findings warrant a separate longitudinal study to address the relationship between CD95 and TNFR1 mediated T cell apoptosis, reduction in CD4+ T cell number and infectious complications in the cohort study.
Septicaemia remains a frequent cause of death of ESRD patients [4–7]. The results of our findings have two important implications. First, they identify an immunological activation pathway of T cells and suggest that CD95 and TNFR1 involvement relates to increased apoptosis in lymphocytes of ESRD patients. Secondly, this provides a mechanism to account for a progressive reduction in T cell counts, and suggests a genesis by which individuals become susceptible to infections. The data are consistent with the idea of reduced number of circulating T cells in dialysed and non-dialysed patients, shedding of CD95 and TNFR1 and increased T cell susceptibility to undergo activation-induced cell death triggered by augmentation of the T cell receptor and CD3 complex.
Acknowledgments
We thank Professor E. Tschachler for helpful discussion. We are indebted for the help from the out-patient ward and the dialysis unit CHD1, Department of Nephrology, of the General Hospital Vienna. Grant number 8920 of the Austrian National Bank supported this work. Dr Hendrik Jan Ankersmit designed and coordinated the study.
References
- 1.Jungers P, Khoa TN, Massy ZA, et al. Incidence of atherosclerotic arterial occlusive accidents in predialysis and dialysis patients: a multicentric study in the Ile de France district. Nephrol Dial Transplant. 1999;14:898–902. doi: 10.1093/ndt/14.4.898. [DOI] [PubMed] [Google Scholar]
- 2.Opartrny K., Jr Hemostasis disorders in chronic renal failure. Kidney Int. 1997;62(Suppl.):S87–9. [PubMed] [Google Scholar]
- 3.Manns BJ, Burgess ED, Parsons HG, Schaefer JP, Hyndman ME, Scott-Douglas NW. Hyperhomocysteinemia, anticardiolipin antibody status, and risk for vascular access thrombosis in hemodialysis patients. Kidney Int. 1999;55:315–20. doi: 10.1046/j.1523-1755.1999.00258.x. [DOI] [PubMed] [Google Scholar]
- 4.Bloembergen WE, Port FK. Epidemiological perspective on infections in chronic dialysis patients. Adv Ren Replace Ther. 1996;3:201–7. doi: 10.1016/s1073-4449(96)80022-7. [DOI] [PubMed] [Google Scholar]
- 5.Vanholder R, Ringoir S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: a review. J Am Soc Nephrol. 1993;39:1541–54. doi: 10.1681/ASN.V391541. [DOI] [PubMed] [Google Scholar]
- 6.US Renal Data System (USRDS) Annual data report. Bethesda, MD: The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1997. [Google Scholar]
- 7.Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9:869–76. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 8.Beaurain G, Naret C, Marcon L, et al. In vivo T cell preactivation in chronic uremic hemodialyzed and non-hemodialyzed patients. Kidney Int. 1989;36:636–44. doi: 10.1038/ki.1989.240. [DOI] [PubMed] [Google Scholar]
- 9.Sennesael JJ, Van der Niepen P, Verbeelen DL. Treatment with recombinant human erythropoietin increases antibody titers after hepatitis B vaccination in dialysis patients. Kidney Int. 1991;40:121–8. doi: 10.1038/ki.1991.189. [DOI] [PubMed] [Google Scholar]
- 10.Grooteman MP, Nube MJ, van Houte AJ, Schoorl M, van Limbeek J. Granulocyte sequestration in dialysers, a comparative eluation study of three different membranes. Nephrol Dial Transplant. 1995;10:1859–64. [PubMed] [Google Scholar]
- 11.Abrutyn E, Solomons NW, St Clair L, MacGregor RR, Root RK. Granulocyte function in patients with chronic renal failure: surface adherence, phagocytosis, and bactericidal activity in vitro. J Infect Dis. 1977;135:1–8. doi: 10.1093/infdis/135.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA, Lonnemann G, Bingel M, Koch KM, Shaldon S. Biological consequences of monocyte activation during hemodialysis. Contrib Nephrol. 1987;59:1–9. doi: 10.1159/000414608. [DOI] [PubMed] [Google Scholar]
- 13.Chatenoud L, Herbelin A, Beaurain G, Descamps-Latscha B. Immune deficiency of the uremic patient. Adv Nephrol Necker Hosp. 1990;19:259–74. [PubMed] [Google Scholar]
- 14.Martin P, Fabrizi F, Dixit V, et al. Epidemiology and natural history of hepatitis G virus infection in chronic hemodialysis patients. Am J Nephrol. 1999;19:535–40. doi: 10.1159/000013515. [DOI] [PubMed] [Google Scholar]
- 15.Huang CC. Hepatitis in patients with end-stage renal disease. J Gastroenterol Hepatol. 1997;12:S236–41. doi: 10.1111/j.1440-1746.1997.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 16.Ankersmit HJ, Edwards NM, Schuster M, et al. Quantitative changes in T-cell populations after left ventricular assist device implantation: relationship to T-cell apoptosis and soluble CD95. Circulation. 1999;100:II–211–15. doi: 10.1161/01.cir.100.suppl_2.ii-211. [DOI] [PubMed] [Google Scholar]
- 17.Ankersmit HJ, Tugulea S, Spanier T, et al. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550–5. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 18.Courtney PA, Crockard AD, Williamson K, McConnell J, Kennedy RJ, Bell AL. Lymphocyte apoptosis in systemic lupus erythematosus: relationships with Fas expression, serum soluble Fas and disease activity. Lupus. 1999;8:508–13. doi: 10.1191/096120399678840765. [DOI] [PubMed] [Google Scholar]
- 19.Pimentel-Muinos FX, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–93. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- 20.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 21.Jiang D, Zheng L, Leonardo MJ. Caspases in T-cell receptor-induced thymocyte apoptosis. Cell Death Diff. 1999;6:402–11. doi: 10.1038/sj.cdd.4400513. [DOI] [PubMed] [Google Scholar]
- 22.Hakim RM, Milford E, Himmelfarb J, Wingard R, Lazarus JM, Watt RM. Extracorporeal removal of anti-HLA antibodies in transplant candidates. Am J Kidney Dis. 1990;16:423–31. doi: 10.1016/s0272-6386(12)80054-0. [DOI] [PubMed] [Google Scholar]
- 23.Barocci S, Valente U, Gusmano R, et al. Autoreactive lymphocytotoxic IgM antibodies in highly sensitized dialysis patients waiting for a kidney transplant: identification and clinical relevance. Clin Nephrol. 1991;36:12–20. [PubMed] [Google Scholar]
- 24.George J, Aron A, Levy Y, et al. Anti-cardiolipin, anti-endothelial-cell and anti-malondialdehyde-LDL antibodies in uremic patients undergoing hemodialysis: relationship with vascular access thrombosis and thromboembolic events. Hum Antibodies. 1999;9:125–31. [PubMed] [Google Scholar]
- 25.Fabrizi F, Sangiorgio R, Pontoriero G, et al. Antiphospholipid (aPL) antibodies in end-stage renal disease. J Nephrol. 1999;12:89–94. [PubMed] [Google Scholar]
- 26.Valeri A, Joseph R, Radhakrishnan J. A large prospective survey of anti-cardiolipin antibodies in chronic hemodialysis patients. Clin Nephrol. 1999;51:116–21. [PubMed] [Google Scholar]
- 27.Descamps-Latscha B, Chatenoud L. T cells and B cells in chronic renal failure. Semin Nephrol. 1996;16:183–91. [PubMed] [Google Scholar]
- 28.Zamauskaite A, Perez-Cruz I, Yaqoob MM, Madrigal JA, Cohen SB. Effect of renal dialysis therapy modality on T cell cytokine production. Nephrol Dial Transplant. 1999;14:49–55. doi: 10.1093/ndt/14.1.49. [DOI] [PubMed] [Google Scholar]
- 29.Krebs M, Kaun C, Lorenz M, Haag-Weber M, Geiger M, Binder BR. Protease dependent activation of endothelial cells by peritoneal dialysis effluents. Thromb Haemost. 1999;82:1334–41. [PubMed] [Google Scholar]
- 30.Boaz M, Matas Z, Biro A, et al. Comparison of hemostatic factors and serum malondialdehyde as predictive factors for cardiovascular disease in hemodialysis patients. Am J Kidney Dis. 1999;34:438–44. doi: 10.1016/s0272-6386(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 31.Brauner A, Lu Y, Hallden G, Hylander B, Lundahl J. Difference in the blood monocyte phenotype between uremic patients and healthy controls: its relation to monocyte differentiation into macrophages in the peritoneal cavity. Inflammation. 1998;22:55–66. doi: 10.1023/a:1022395723972. [DOI] [PubMed] [Google Scholar]
- 32.Schwabe RF, Engelmann H, Hess S, Fricke H. Soluble CD40 in the serum of healthy donors, patients with chronic renal failure, haemodialysis and chronic ambulatory peritoneal dialysis (CAPD) patients. Clin Exp Immunol. 1999;117:153–8. doi: 10.1046/j.1365-2249.1999.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Soluble CD23 as an effector of immune dysregulation in chronic uremia and dialysis. Kidney Int. 1993;43:878–84. doi: 10.1038/ki.1993.123. [DOI] [PubMed] [Google Scholar]
- 34.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–9. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 36.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo. Virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 37.Safrit JT, Koup RA. The immunology of primary HIV infection: which immune responses control HIV replication? Curr Opin Immunol. 1995;7:456–61. doi: 10.1016/0952-7915(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 38.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immune deficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–40. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ankersmit J, Schuster M, Hoffman M, et al. Polymeric biomaterials induce T cell apoptosis and defects in cellular immunity via increased cellular levels of NFAT and CD95 ligand. J Heart Lung Transpl. 2000;78:19. (Abstract) [Google Scholar]
- 40.Ankersmit J, Hoffman M, Olson K, et al. Polymeric biomaterials act as superantigens, causing T cell apoptosis via induction of CD95 ligand expression and defects in cellular immunity. Circulation. 1999;100:1–802. (Abstract) [Google Scholar]
- 41.Fischer FP, Machleidt C, Rettenmeier AW, Kuhlmann U, Mettang T. Plasticizers and inhibition of leukocyte function in vitro. Perit Dial Int. 1998;18:620–5. [PubMed] [Google Scholar]
- 42.Dine T, Luyckx M, Gressier B, et al. A pharmacokinetic interpretation of increasing concentrations of DEHP in haemodialysed patients. Med Eng Phys. 2000;22:157–65. doi: 10.1016/s1350-4533(00)00022-9. [DOI] [PubMed] [Google Scholar]