Abstract
Allergic reactions, such as urticaria, itching and anaphylactic shock, often complicate the course of cystic echinococcosis (CE). To investigate the role of the IgE-immunoreactive recombinant Echinococcus granulosus elongation factor-1 β/δ (EgEF-1 β/δ) in the allergic disorders during CE we determined humoral and cell-mediated responses to this antigen in patients with CE grouped according to the clinical presence or absence of allergic reactions. Immunoblotting analysis showed that serum IgE-binding reactivity to EgEF-1 β/δ differed significantly in patients with and without allergic reactions (38 of 42, 90% vs. 31 of 56, 56%; P < 10−4). EgEF-1 β/δ induced a proliferative response in 14 of 19 (74%) patients' peripheral blood mononuclear cells (PBMC) irrespective of the allergic manifestations and skewed Th1/Th2 cytokine activation towards a preferentially Th2 polarization. Epitope mapping identified an immunodominant epitope of 18 residues with 78% identity and 89% similarity with an IgE-immunoreactive Strongyloides stercoralis antigen. Overall these findings suggest that EgEF-1 β/δ is an allergenic molecule that may be a general marker of the intensity of CE immune response and that could lead to a deeper understanding of the specific antigen-induced mechanisms underlying allergic reactions in the human host.
Keywords: allergic reactions, E. granulosus, elongation factor, IgE immunodominant epitope Th1/Th2 cytokines
Introduction
Among the many common features that the host's immune response to parasitic helminths and the response of atopic individuals to extrinsic allergens share, the most prominent is probably the production of high levels of specific IgE antibodies [1,2]. In allergic individuals these antibodies, through their binding to the surface of basophils and mast cells, are largely responsible for initiating the hypersensitivity reactions typical of the acute allergic syndromes: asthma, allergic rhinitis and eczema. In helminth infections, IgE production may also be responsible for the protective immune response to the parasite or immune-mediated pathology or for both events. In particular, in Necator americanus, Ancylostoma caninum, Schistosoma mansoni and S. haematobium infections, the level of IgE correlates with the protected status of the host [2,3]. Conversely, in Echinococcus granulosus, Anisakis simplex and Strongyloides stercoralis infections the presence of IgE is associated with pathological allergic reactions [4–6].
Cystic echinococcosis (CE) is an infection by cestode larvae of E. granulosus that forms the hydatid cyst containing the protoscoleces [7]. CE shares with other helminthiases three responses typical of the immediate hypersensitivity reactions: elevated IgE/IgG4 antibody production, eosinophilia and mastocytosis [8–10]. The clinically observed immunological consequences of CE (both related and unrelated to cyst rupture) arise from acute hypersensitivity reactions, immunosuppressive action and complications associated with circulating immunological complexes. Hypersensitivity reactions vary widely, from benign urticaria and short episodes of shaking chills or fever, or both events, to potentially fatal bronchial spasms, angioneurotic oedema and anaphylactic shock. The latter occurs most frequently after the accidental rupture of the hydatid cyst or during surgery [4]. An understanding of the complex pathogenic mechanisms leading to an allergic reaction in CE requires information about the structure and function of allergenic proteins, namely antigens recognized by IgE. Ample evidence confirms that sera from patients with CE contain IgE specific to E. granulosus hydatid fluid, in particular to antigen 5 and B [11]. By screening an E. granulosus cDNA library with IgE from CE patients we isolated a conserved and constitutive protein homologous to the β/δ subunit of elongation factor-1, named EgEF-1 β/δ [12]. In immunoblotting (IB) analysis this antigen reacted exclusively with sera from patients with CE. The finding that 52% of these sera contained specific IgE as well as the lack of reactivity with IgE from atopic subjects, suggested a role of EgEF-1 β/δ in allergic reactions complicating CE.
In this study, to investigate the possible association between EgEF-1 β/δ and allergic disorders during CE, we evaluated the humoral and cell-mediated immune responses to this antigen in patients with CE with and without clinically evident allergic reactions. We used IB to evaluate the IgE antibody response in sera from patients with CE and peripheral blood mononuclear cell (PBMC) assay to determine EgEF-1 β/δ-induced cellular reactivity. To evaluate T helper cell activation (Th1/Th2), we examined IL-4 and IFN-γ production in PBMC cultures from CE patients. Finally, we sought to identify the immunodominant epitopes, first by analysing IgE- and IgG-reactivity to the N- and C-terminal subunits of EgEF-1 β/δ and secondly by determining by enzyme-linked immunosorbent assay (ELISA) the IgE binding epitopes.
Patients and methods
Blood samples
Blood samples were obtained from 98 patients (31 males and 67 females; mean age 51·4 years, range 24–69) with CE (85 with cysts in the liver, six with cysts in the lung, two with cysts in bone and five with cysts in multiple sites), from 28 subjects with atopic disorders as proven by results of skin prick tests (17 with polyspecific allergic reactions, five with monospecificity to Dermatophagoides farinae and six with monospecificity to Parietaria judaica), and from 30 non-atopic healthy donors. Patients with CE were grouped according to the presence of allergic reactions: 42 patients with allergic reactions (31 with skin manifestations characterized by intense itching and urticaria who reported negative skin prick tests for the major allergens and 11 patients with anaphylactic reactions due to traumatic or casual rupture of the cyst less than 6 months before the study); and 56 patients without allergic reactions. Twenty patients with allergic reactions had peripheral blood eosinophilia. CE was diagnosed on the basis of evidence from imaging techniques (ultrasonographic scanning or nuclear magnetic resonance or both), serological assays, surgery or adjuvant medical treatment. All procedures were approved by the local Ethical Committee and all subjects gave their informed consent to the study.
Antigen
Recombinant EgEF-1 β/δ was prepared as described previously [12]. In brief, the fusion protein was expressed in Escherichia coli SG130009 cells, purified by affinity of NI-NTA resin for the 6× histidine tag and eluted with urea under denaturing conditions according to the supplier's instructions (Qiagen, GmbH, Hilden, Germany). To remove urea before the cell cultures the protein was dialysed in phosphate-buffered saline (PBS) for 2 days at 4°C, divided into aliquots and kept at −80°C for subsequent use. The purified protein showed in 12% sodium dodecyl sulphate-polyacrilamide gel electrophoresis (SDS-PAGE) a single band of 33 kDa.
Preparation and purification of recombinant C- and N-terminal fragments
To obtain the N-and C-terminal amplified fragments we ran a polymerase chain reaction (PCR), using EgEF-1β/δ gene as a template and the following oligonucleotides with restriction sites as primers: N-terminal fragment: 5′ primer (BamHI restriction site) 5′GCATGGATCCTTTGATGCTCACTCTGTAT3′, 3′ primer (SacI restriction site) 5′GACTGAGCTCGACCGCCTTC AGCTGGCGG3′; C-terminal fragment: 5′ primer (BamHI restriction site) 5′GCATGGATCCGATGACGATGATATTG3′ and 3′ primer (SalI restriction site) 5′GACTGTCGACGATATGGTTAC AGTTTG3′. After amplification the fragments were run in 2% agarose gel, purified by Qiaex kit (Qiagen, GmbH, Hilden, Germany) following the manufacturer's instructions and after digestion with the restriction enzymes (Promega, Madison, WI, USA) were cloned in pQE-31 expression vector (Qiagen, GmbH, Hilden, Germany). The fusion proteins were expressed in E. coli SG130009 cells and purified in the same way as whole EgEF-1β/δ.
Preparation and purification of three recombinant N-terminal fragments
To identify the epitopes reacting with patients' IgE we divided the N-terminal subunits of 118 amino acids (aa) into three fragments of 60, 60 and 58 aa (1–60, 31–90, 61–118 aa, respectively) containing 30 overlapping aa. These fragments were obtained by amplifying the DNA for the N-terminal subunit with the following oligonucleotides: fragment 1: 5′ primer (BamHI restriction site) 5′GCATGGATCCTTTGATGCTCACTCTGTAT3′, 3′ primer (EcoRI restriction site) 5′GACTGAATTCACCATTACTTTCAA GTG3′; fragment 2: 5′ primer (BamHI restriction site) 5′GACTGGATCCTCTGCTACCGAACAA3′, 3′primer (EcoRI restriction site) 5′GACTGAATTCCAGACGTTGGATTTG3′; fragment 3: 5′ primer (BamHI restriction site) 5′GACTGGATCC TCGTGGAACGAGCGAATG3′ and 3′ primer (EcoRI restriction site) 5′GACTGAATTCACCGCTTCAGCTGGCGG3′. The three amplification products were cloned in pGEX2T expression vector (Amersham-Pharmacia-Biotech, Uppsala, Sweden). The Glutathione S-Transferase fusion proteins were expressed and purified by glutathione Sepharose 4B (Amersham-Pharmacia-Biotech) using the batch method following the manufacturer's instructions.
Multi-pin synthesis
Peptides were synthesized on polypropylene pins according to the instructions of the manufacturer (Chyron Mimotopes Phy Ltd, Australia). α-Fmoc-protected amino acids were purchased from Inalco (Milan, Italy). The 88–118 residues of EgEF-1 β/δ were covered by seven 12-residue peptides, with nine overlapping residues.
Conventional solid-phase synthesis
Peptides were prepared by standard Fmoc chemistry with a Vega Peptide synthesizer Model 1000. The synthesized peptides were cleaved and deprotected with trifluoroacetic acid/anisole (19:1), precipitated with cold ether, recovered by centrifugation, and purified by column chromatography or high pressure liquid chromatography (HPLC). The correct amino acid composition was assessed by amino acid analysis by HPLC/fluorescence after complete hydrolysis of 50 µg of samples with 6 N HCl at 108°C for 24–48 h. To search for sequence similarities we used the BlastP software with blitz post-processing in swissprot, swissall, swissnew, swall and trembl databases (Human Genome Center, Baylor College of Medicine, Texas, USA).
Proliferation assay
Proliferation was assayed by the established procedure [13]. In brief, triplicate cultures of PBMC were prepared at the concentration of 105 cells/well, by the addition of 180 µl of cell suspension and 20 µl of sterile antigen preparation (8 µg/ml of EgEF1 β/δ). In all experiments, cultures with phytohaemagglutinin (2 µg/ml) and cultures without antigen were also set up as positive and negative controls. After preliminary kinetic studies, an 8-day culture at 37°C in a humidified atmosphere containing 5% CO2 in air was chosen, the proliferative response being assessed by the addition of 20 µl containing 0·5 µCi [3H]-methyl-thymidine (specific activity 1 μCi/mmol) (Amersham, Life Science, UK) to each well. After a further 20 h at 37°C, cells were harvested on glass fibre filter paper (Wallac, EG & G Company, Turku, Finland), using an automatic cell harvester (Harvester 96, MACH III M, TOMTEC, Orange, CT, USA). [3H]-methyl-thymidine uptake into cell DNA was measured by reading samples in a β counter (1450 Microbeta Plus, Wallac EG & G Company). Net counts per minute (cpm) of triplicate cultures were measured and the proliferative response was expressed as stimulation indices (ratio between the mean cpm in stimulated cultures and mean cpm in unstimulated cultures). Mean value of stimulation indices in healthy blood donors, plus 2 s.d., was taken as the threshold level for positivity.
Cytokine assays
To test cytokine production 8 µg/ml of EgEF-1 β/δ were added as stimulus to PBMC cultured as described previously [14]. Five days later, supernatants were collected and analysed by Quantikine kit ELISA for quantitative determination of IFN-γ and by Quantikine High Sensitivity kit ELISA for quantitative determination of IL-4 (R&D Systems Inc., Minneapolis, USA). The ELISA kits yielded ranges of 15·6–1000 pg/ml for IFN-γ and 0·25–16 pg/ml for IL-4. Linear regression analysis was used to determine cytokine levels. The regression curve was constructed from the standard values and we considered IL-4 positive, samples having an OD > 0, even if this value was below the detection limit.
Serological tests
IB, after 12% SDS-PAGE in reducing conditions, was performed as previously described [12]. In brief, EgEF-1 β/δ and its subunits were used as antigens at the concentrations of 3 µg/lane and were revealed by human sera diluted 1:50 for IgG detection and 1:10 for IgE detection. A goat anti-human IgE peroxidase labelled serum (Cappel, Cochranville, PA, USA) and a goat anti-human IgG labelled serum (Biorad, Richmond, CA, USA) were used as second antibodies. Monoclonal antibody specific to the 6× histidine tail was purchased from Qiagen (Qiagen, GmbH, Germany) and used according to the manufacturer's instructions. Sera positive to EgEF-1 β/δ recognized the band at 33 kDa [12]. IgE specific for the peptides were determined by ELISA in disposable microplates (Dynex Technologies, Inc., Chantilly, Virginia, USA). Wells were filled with 50 µl of peptides diluted in 0·1 m carbonate–bicarbonate buffer, pH 9·6, to a content of 2 µg. After incubation overnight at 37°C plates were saturated with 5% milk in 0·015 m PBS, pH 7·2. Fifty µl of serum samples, diluted 1:10 in 3% milk in PBS, were added to each well and incubated overnight at 4°C. After the addition of 100 µl of goat anti-human IgE peroxidase conjugate (Cappel, Cochranville, PA, USA) the amount of peroxidase bound to the wells was determined by the addition of O-phenylenediamine dihydrochloride (Sigma Chemical Co., St Louis, MO, USA) as chromogen and absorbance was measured at 492 nm. Reactivity of human sera on pin-bound peptides was assessed by ELISA as reported previously [15].
Statistical analysis
Differences between percentages were evaluated by Fisher's exact test. Differences between arithmetic means were evaluated by Student's t-test. Differences with a confidence interval of 95% or higher were considered statistically significant (P ≤ 0·05).
Results
Relation between IgE reactivity to EgEF-1 β/δ and presence of allergic reactions in patients with CE
Qualitative analysis by IB of IgE responses in patients' sera showed significantly higher binding reactivity to EgEF-1 β/δ in sera from patients with allergic reactions, in particular with urticaria, than in those without (P < 10−4) (Table 1). Of the 58 control subjects one serum from a subject with polyspecific atopic disorders and a healthy donor presented specific IgE to EgEF-1 β/δ protein.
Table 1.
Pattern of IgE reactivity to EgEF-1β/δ of 92 patients with cystic echinococcosis divided according to the presence of allergic reactions and 58 control subjects
| Serum samples | No. of serum samples | No. testing positive | % of positivity |
|---|---|---|---|
| Patients with cystic echinococcosis | |||
| With allergic reactions | 42 | 38 | 90* |
| Urticaria | 31 | 29 | 94§ |
| Anaphylactic shock | 11 | 9 | 82 |
| Without allergic reactions | 56 | 31 | 56*§ |
| Controls | |||
| Atopic subjects | 28 | 1 | 4 |
| Healthy subjects | 30 | 1 | 3 |
Statistically significant differences by Fisher's exact test: P < 10−4.
EgEF-1 β/δ-driven T-cell proliferation and cytokine production
In 14 of the 19 patients' PBMC (74%), EgEF-1 β/δ induced a proliferative response. Stimulation indices ranged from 8·4 to 31·7 irrespective of whether the patient had manifested allergic reactions (data not shown). In unstimulated cultures, PBMC from patients with allergies produced higher amounts of IL-4 than PBMC from those without, but similar amounts of IFN-γ. In patients without allergies EgEF-1 β/δ significantly increased IL-4 production (P = 0·05), and in both groups it slightly increased IFN-γ production (Table 2). The IFN-γ/IL-4 ratio clearly confirmed that EgEF‐1 β/δ induced in PBMC cultures from patients without allergies similar to in vitro Th2 polarization present in stimulated and unstimulated PBMC cultures from patients with allergies.
Table 2.
Cytokine production in peripheral blood mononuclear cell cultures from 14 patients with cystic echinococcosis divided into two groups according to the presence of allergic reactions
| Patients | |||||||
|---|---|---|---|---|---|---|---|
| With allergic reactions (n = 7) | Without allergic reactions (n = 7) | ||||||
| Stimulus | Cytokine | No. testing positive (%) | Mean concentrations pg/ml (range) | IFN-γ/IL-4 | No.testing positive (%) | Mean concentrations pg/ml (range) | IFN-γ/IL-4 |
| None | IL-4 | 4 (57) | 0·09 (0·0–0·9) | 522 | 3 (43) | 0·02 (0·0–0·08)* | 2000 |
| IFN-γ | 5 (71) | 47·0 (0·0–208·0) | 5 (71) | 40·0 (0·0–97·0) | |||
| EgEF-1 β/δ | IL-4 | 4 (57) | 0·11 (0·0–0·5) | 620 | 3 (43) | 0·14 (0·0–0·34)* | 436 |
| IFN-γ | 6 (86) | 62·0 (0·0–192·0) | 7 (100) | 61·0 (7·8–141·0) | |||
Statistically significant differences by Student's t-test: P = 0·05. Note that the regression curve was constructed from the standard values, and samples having an OD > 0, even if this value was below the detection limit, were considered positive. Positive values for IFN-γ were always above the detection limit. Only 3/7 antigen-stimulated patients (43%) had IL-4 values below the detection limit (0·25 pg/ml).
Serological reactivity of the N- and C-terminal subunits of EgEF-1 β/δ
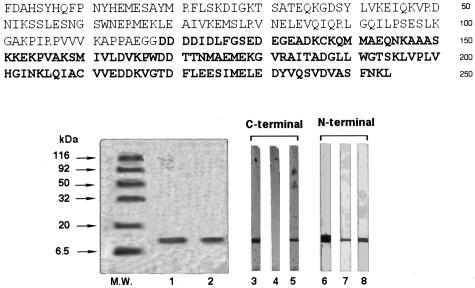
The N- and C-terminal subunits of EgEF-1 β/δ of 118 and 126 aa in 12% SDS-PAGE showed a single band of 14 kDa and in IB were recognized by IgG or IgE of CE patients' sera, or both (Fig. 1).
Fig. 1.
Amino acid sequence, SDS-PAGE and immunoblotting of N- and C-terminal subunits of EgEF-1β/δ. The two subunits were subjected to 12% SDS-PAGE and stained with Coomassie Brilliant Blue (lanes 1 and 2) or transferred to nitrocellulose and revealed with the monoclonal antibody specific to the 6× histidine (lanes 3, 6), human serum IgE (lanes 4, 7) and human serum IgG (lanes 5, 8). Molecular weights (MW) are indicated on the left.
All patients whose serum IgE reacted to whole EgEF-1 β/δ were IgE-positive in IB exclusively to the N-terminal subunit; 35 of the 38 (92%) patients with allergic reactions and 30 of the 31 (97%) patients without allergic reactions were also IgG-positive to the N-terminal subunit. Regardless of whether they had allergic reactions, only few patients (32%) resulted IgG-positive to the C-terminal subunit (Table 3).
Table 3.
Immunoblotting pattern of IgE and IgG reactivity against the N- and C-terminal subunits of EgEF-1 β/δ in 69 patients with cystic echinococcosis divided into two clinical groups according to the presence of allergic reactions
| Patients | ||||
|---|---|---|---|---|
| With allergic reactions (n = 38) | Without allergic reactions (n = 31) | |||
| Antigen | No. sera IgE positive (%) | No. sera IgG positive (%) | No. sera IgE positive (%) | No. sera IgG positive (%) |
| N-terminal subunit | 38 (100) | 35 (92) | 31 (100) | 30 (97) |
| C-terminal subunit | 0 | 12 (32) | 0 | 10 (32) |
Epitope mapping and peptide homology analysis
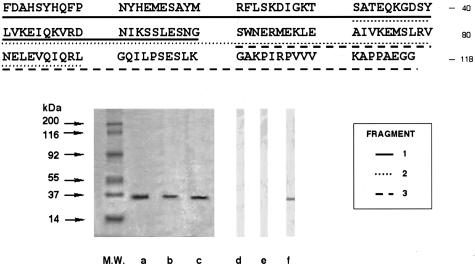
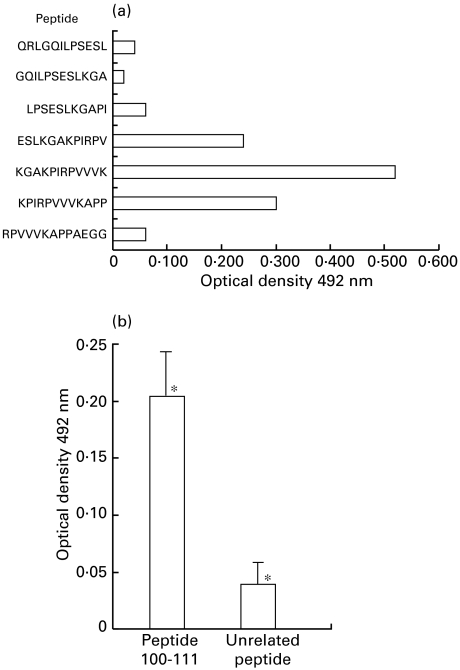
In IB with the three EgEF-1 β/δ fragments (60, 60 and 58 aa), a pool of seven sera from allergic patients exclusively recognized the 58aa fragment, indicating that IgE reactivity arose from the last 28aa (Fig. 2). ELISA with 12-mer pin-bound peptides covering residues 87–118 identified 100–111 (KGAKPIRPVVVK) as the most reactive region (Fig. 3a). This peptide containing 12 residues was specifically recognized in ELISA by IgE from 15 patients with allergic reactions (Fig. 3b). The 101–109 residues (GAKPIRPVV) showed in databases 78% identity and 89% similarity with the 5 A IgE reactive antigen, residues 50–58, of Strongyloides stercoralis (GPKPIRPIV) [16].
Fig. 2.
Amino acid sequence, SDS-PAGE and immunoblotting of the three following fragments of EgEF-1 β/δ N-terminal subunit: (1) 1–60 aa; (2) 31–90 aa; and (3) 61–118 aa. Lanes a, b and c, 12% SDS PAGE stained with Coomassie Brilliant Blue of the three fragments 1, 2 and 3, respectively. Lanes d, e and f, immunoblotting analysis of the three fragments revealed with a pooled serum IgE from seven patients with cystic echinococcosis and allergic reactions. Molecular weights (MW) are reported on the left.
Fig. 3.
Epitope mapping. (a) Epitope profiles obtained by 12-mer pin bound peptides in IgE-ELISA with a pool of 7 sera from patients with allergic reactions. Data represent the means of duplicate experiments. (b) Specific IgE reactivity in 15 patients with cystic echinococcosis and allergic reactions by ELISA with the 100–111 peptide and a unrelated peptide (IRYPLTFGWCYK) as antigen. Data represent means ±s.d. of triplicate experiments. *Statistically significant differences by Student's t-test: P = 10−4.
Discussion
To clarify the IgE response in patients with CE and extend findings obtained by investigating total IgE or IgE specific to the main hydatid fluid antigens (antigen 5 and B), in this study we used a different recombinant antigen and grouped patients according to the presence of allergic reactions [9,11]. Our results clearly suggest an association between IgE specific to EgEF-1 β/δ and allergic disorders related to CE. The high percentage of patients with urticaria or anaphylactic shock who were IgE-positive to EgEF-1 β/δ implies that these allergic reactions could be caused by E. granulosus antigens released during the course of the CE infection. EgEF-1 β/δ is a conserved constitutive protein, located predominantly in the endoplasmic reticulum and probably released into the hydatid fluid only after degeneration of the protoscoleces due to ageing, calcification or drug treatment [12]. In this study, the high percentage of sera specific to EgEF-1 β/δ detected in patients who had experienced an anaphylactic shock suggest that EgEF-1 β/δ, usually hidden within the cyst, could be suddenly released, thus participating in the development of anaphylactic shock. The occasional release of EgEF-1 β/δ from the cyst could explain the typically intermittent episodes of itching and urticaria commonly seen during CE.
In this study we evaluated by IB only the qualitative IgE response specific to EgEF-1 β/δ without considering the outcome of the disease. Previously we observed a more frequent humoral immune response against EgEF-1 β/δ in patients with calcified inactive cysts than in patients with active cysts [12]. Further studies should investigate whether the quantitative determination by ELISA of IgE antibodies specific to EgEF-1 β/δ might be useful in clinical follow-up to predict the outcome of the disease or the allergic manifestations, or both. A quantitative analysis could also determine an association between allergic manifestations and quantitative changes in specific IgE.
Control of IgE synthesis, in response to an allergen, depends essentially on Th cells. Th cell subsets, Th1 and Th2, counter regulate each other through the secretion of cytokines that either switch on (Th2, IL-4) or switch off (Th1, IFN-γ) B cell IgE synthesis. T lymphocytes of the Th2 phenotype are important in the pathophysiology of both allergic disease and helminthic infections [3,17,18]. Because only some of the proteins of an allergen source are allergenic, the allergen itself might possibly deviate the immune response in favour of Th2 dominance and IgE production. In this study we found that EgEF-1 β/δ can induce cellular reactivity and skew the Th1/Th2 cytokine balance towards a preferentially Th2 polarization in patients with CE. The ability of EgEF-1 β/δ to manipulate the Th balance is demonstrated by the decrease in the IFN-γ:IL-4 ratio that this recombinant antigen achieved in patients without allergic reactions. In previous in vivo and in vitro studies we found that sheep hydatid fluid, a crude mixture that contains various antigens, can promote activation of Th1 and Th2 cells, and that Th2 polarization is associated with susceptibility to CE [19]. Our results here suggest for EgEF-1 β/δ a double pathological role, inducing allergic reactions and interfering on the susceptibility to human CE.
In our experiments designed to identify the immunodominant epitopes in the conserved (the C-terminus) or variable (the N-terminus) regions of EgEF-1 β/δ, we found that IgE epitopes were localized exclusively in the variable N-terminal regions of the EgEF-1 β/δ. This location contrasts with data demonstrating that many allergens are evolutionary conserved proteins [20]. Interestingly, patients' serum IgG reacted with both the N- and C-terminal subunits even though the percentage of positivity differed markedly. Patients' serum IgG that reacted with the EgEF-1 β/δ C-terminal subunit reacted also with a recombinant human C-terminal EF-1 (data not shown). Whether this cross-reaction causes autoimmune reactions needs further investigation. In the β and δ subunits of EF-1 the function of GDP-GTP exchange is localized in the C-terminal subunit [21]. Our observation that the human humoral response is mainly directed to the N-terminal region of EgEF-1 β/δ suggests that E. granulosus could manipulate the host immune response by addressing IgE and IgG antibodies to non-functional epitopes present in this region.
Even though the method we used mapped only linear epitopes, epitope mapping of the N-terminal EgEF-1 β/δ subunit with patients' serum IgE allowed us to identify an 12aa immunodominant epitope (100–111). This epitope contained nine amino acids with 78% identity and 89% similarity with a S. stercoralis recombinant antigen recognized by IgG and IgE from patients with strongyloidiasis [16]. Like CE, strongyloidiasis infections manifest commonly with cutaneous symptoms and urticaria. The fact that the IgE immunodominant epitope of EgEF-1 β/δ is homologous with a parasitic IgE immunoreactive antigen suggests that in helminthic infections the IgE-immune response is addressed to a limited number of conserved epitopes. Recent studies have revealed that the IgE-binding epitopes of a Schistosoma japonicum protein exhibit similarities to the IgE-binding epitopes of known allergens [22]. The conserved structure of the IgE-binding peptides might be the common denominator between the immune responses against parasites and allergens.
Even though patients with CE commonly have positive skin-tests, elevated total and specific IgE and degranulation of basophils with hydatid antigens, clinicians tend to underestimate the importance of the allergic manifestations reported during the course of disease [9,23,24]. In our clinical experience, about 20% of patients report a history of allergic manifestations of unknown origin during the infection. A recent report suggests that some urticarias diagnosed as idiopathic may actually be associated with hydatid cysts [25]. Our results here, indicating the possible association between allergic reactions during CE and the presence of IgE specific to a conserved E. granulosus protein, could be useful in clarifying the host–parasite relationship and the origin of the allergic reactions during the infection. Future research should also investigate the functional activity of EgEF-1 β/δ in the in vivo induction of basophil- and mast cell-degranulation.
The allergic reactions that occur during the course of E. granulosus infection undoubtedly play a crucial part in the pathogenesis of this parasitic disease. Questions remain concerning the physiological role of IgE and the development of IgE-mediated pathologies.
Studies investigating immunotherapy using small synthetic peptides of immunodominant allergen-derived T cell epitopes open the way to new and interesting therapeutic advances in allergic disorders [26]. Before successful immunotherapy can be developed for patients with CE who have allergic manifestations, research needs to identify a Th1 skewing peptide.
Overall, our findings suggest that EgEF-1 β/δ is an allergenic molecule of potential clinical importance that could lead to a deeper understanding of the specific antigen-induced mechanisms underlying allergic reactions in the human host.
Acknowledgments
This work was supported partially by Italian Ministry of Health grants (‘Surveillance project on emerging and re-emerging infectious disease’ and ‘Allergic diseases: development of diagnostic and therapeutic tools and evaluation of their suitability for the management of the allergic patient’) from the ISS (Italian Superior Institute of Health) (art. 502/12).
References
- 1.Scott ST. Parasites and asthma/allergy: what is the relationship? J Allergy Clin Immunol. 2000;105:205–10. doi: 10.1016/s0091-6749(00)90067-8. [DOI] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. Immunology of human helminth infection. Int Arch Allergy Immunol. 1996;109:3–10. doi: 10.1159/000237225. [DOI] [PubMed] [Google Scholar]
- 3.Hagan P. IgE and protective immunity to helminth infections. Parasite Immunol. 1993;15:1–4. doi: 10.1111/j.1365-3024.1993.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 4.Pawlowski ZS. Critical points in the clinical management of cystic echinococcosis: a revised review. In: Andersen FL, Ouhelli H, Kachani M, editors. Compendium on cystic echinococcosis. Provo, UT, USA: Brigham Young University Print Services; 1997. pp. 199–35. [Google Scholar]
- 5.Daschner A, Alonso-Gomez A, Cabanas R, Suarez-de-Parga JM, Lopez-Serrano MC. Gastroallergic anisakiasis: borderline between food allergy and parasitic disease-clinical allergologic evaluation of 20 patients with confirmed acute parasitism by Anisakis simplex. J Allergy Clin Immunol. 2000;105:176–81. doi: 10.1016/s0091-6749(00)90194-5. [DOI] [PubMed] [Google Scholar]
- 6.Robinson J, Ahmed Z, Siddiqui A, et al. A patient with persistent wheezing, sinusitis, elevated IgE and eosinophilia. Ann Allergy Asthma Immunol. 1999;82:144–9. doi: 10.1016/S1081-1206(10)62588-4. [DOI] [PubMed] [Google Scholar]
- 7.Rausch RL. Echinococcus granulosus: biology and ecology. In: Andersen FL, Ouhelli H, Kachani M, editors. Compendium on cystic echinococcosis. Provo, UT, USA: Brigham Young University Print Services; 1997. pp. 18–53. [Google Scholar]
- 8.Aceti A, Pennica A, Teggi A, et al. IgG subclasses in human hydatid disease: prominence of the IgG4 response. Int Arch Allergy Immunol. 1993;102:347–51. doi: 10.1159/000236582. [DOI] [PubMed] [Google Scholar]
- 9.Afferni C, Pini C, Misiti-Dorello P, Bernardini L, Conchedda M, Vicari G. Detection of specific IgE antibodies in sera from patients with hydatidosis. Clin Exp Immunol. 1984;55:587–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JF. Recent advances in the immunology of cestode infections. J Parasitol. 1979;65:337–49. [PubMed] [Google Scholar]
- 11.Siracusano A, Ioppolo S, Lastilla M, Notargiacomo S, Ortona E, Rigano' R. Detection of IgE antibodies against antigens 5 and B of Echinococcus granulosus by immunoblotting. G Malattie Infet Parassit. 1993;45:286–8. [Google Scholar]
- 12.Margutti P, Ortona E, Vaccari S, et al. Cloning and expression of a cDNA encoding an elongation factor 1 β/δ protein from Echinococcus granulosus with immunogenic activity. Parasite Immunol. 1999;21:485–92. doi: 10.1046/j.1365-3024.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 13.Siracusano A, Teggi A, Quintieri F, Notargiacomo S, De Rosa F, Vicari G. Cellular immune responses of hydatid patients to Echinococcus granulosus antigens. Clin Exp Immunol. 1988;72:400–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Riganò R, Profumo E, Di Felice G, Ortona E, Teggi A, Siracusano A. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin Exp Immunol. 1995;99:433–9. doi: 10.1111/j.1365-2249.1995.tb05569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Modugno F, Rosano' L, Castelli M, Chersi A. Isolation, characterization, and comparison of antipeptide and antiprotein rabbit antibodies to the p-isoform of glutathione S-transferase. Z Naturforsch. 1998;53C:902–10. doi: 10.1515/znc-1998-9-1020. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran S, Thompson RW, Gamm AA, Neva FA. Recombinant cDNA clones from immunodiagnosis of strongyloidiasis. J Infect Dis. 1998;177:196–203. doi: 10.1086/513817. [DOI] [PubMed] [Google Scholar]
- 17.Del Prete GF, De Carli M, D'Elios M, et al. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993;23:1445–52. doi: 10.1002/eji.1830230707. [DOI] [PubMed] [Google Scholar]
- 18.Abbas AK, Murphy KM, Sher A. Funtional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 19.Riganò R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol. 1995;102:281–5. doi: 10.1111/j.1365-2249.1995.tb03778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virtanen T, Zeiler T, Rautiainen J, Mantyarvi R. Allergy to lipocalins: a consequence of misguided T-cell recognition of self and nonself? Immunol Today. 1999;9:398–400. doi: 10.1016/s0167-5699(99)01515-7. [DOI] [PubMed] [Google Scholar]
- 21.Liljas A, Garber M. Ribosomal proteins and elongation factors. Curr Opin Struct Biol. 1995;5:721–7. doi: 10.1016/0959-440x(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 22.Santiago ML, Hafalla JCR, Kurtis JD, et al. Identification of the Schistosoma japonicum 22.6-kDa antigen as a major target of the human IgE response: similarity of IgE-binding epitopes to allergen peptides. Int Arch Allergy Immunol. 1998;117:94–104. doi: 10.1159/000023995. [DOI] [PubMed] [Google Scholar]
- 23.Casoni T. La diagnosi dell'echinococcosi umana mediante l'introdermoreazione. Folia Clin Chim Microscop. 1911;4:5. [Google Scholar]
- 24.Aceti A, Celestino D, Teggi A, et al. Histamine release test in the diagnosis of human hydatidosis. Clin Exp Allergy. 1989;19:335–9. doi: 10.1111/j.1365-2222.1989.tb02392.x. [DOI] [PubMed] [Google Scholar]
- 25.Veraldi S, Miadonna A. Chronic ‘idiopatic’ urticaria and hydatid disease. Allergy. 1998;53:1234–5. doi: 10.1111/j.1398-9995.1998.tb03856.x. [DOI] [PubMed] [Google Scholar]
- 26.Janssen EM, van Oosterhout AJM, van Rensen AJM, van Eden W, Nijkamp FP, Wauben MAHM. Modulation of Th2 responses by peptide analogues in a murine model of allergic asthma: amelioration or deterioration of the disease process depends on the Th1 or Th2 skewing characteristics of the therapeutic peptide. J Immunol. 2000;164:580–8. doi: 10.4049/jimmunol.164.2.580. [DOI] [PubMed] [Google Scholar]