Abstract
We have examined normal T-cells and T-cell lines with respect to expression of various somatostatin receptor subtypes (SSTR1–5) using RT-PCR and PCR. To evaluate the function of these receptors we have further studied the effects of subtype specific signalling on T-cell adhesion using somatostatin analogs specific for various receptors as probes. Human T-lymphocytes showed SSTR expression related to activation and stage of differentiation. Normal T-cells (peripheral blood, T-cell clone) and T-leukaemia cell lines expressed SSTR2, SSTR3 and SSTR4. Normal T-cells expressed SSTR1 and SSTR5 while T-leukaemia lines did not. SSTR5 was selectively expressed in activated normal T-cells. T-lymphocytes produced no somatostatin themselves. Somatostatin and somatostatin analogs specific for SSTR2 and/or SSTR3 enhanced adhesion of T-cells to fibronectin (FN), and to a certain extent, also to collagen type IV (CIV) and laminin (LAM). T-lymphocytes express multiple SSTR and somatostatin may therefore regulate lymphocyte functions via distinct receptor subtypes as shown here for adhesion to extracellular matrix components (ECM) via SSTR2 and SSTR3. SSTR expression also distinguishes normal and leukaemic T-cells. Our findings suggest that SSTR subtypes may be useful targets for therapy during inflammatory diseases and malignancies affecting lymphocytes.
Keywords: T-cells, somatostatin receptors, lymphocyte adhesion, extracellular matrix, neuropeptides
Introduction
Somatostatin-producing cells are widely distributed in the central and peripheral nervous system, in the endocrine pancreas and gut, but they also occur in the thyroid, prostate, placenta, adrenals, kidneys and skin [1,2]. Somatostatin induces inhibitory effects on two key cellular processes, secretion and cell proliferation [2,3]. Somatostatin functions as a neurotransmitter in the brain and inhibits the release of growth hormone and thyroid stimulating hormone (TSH) [4,5]. In the gastrointestinal tract somatostatin inhibits the release of virtually all hormones and it has a generalized inhibitory effect on gut exocrine secretion [1,2]. Its synthetic analogue octreotide, which has a more prolonged duration of action, is used in clinical practice for the suppression of the symptoms of neuroendocrine tumours, and to prevent complications after pancreatic surgery [6]. Somatostatin has been shown to suppress the inflammatory reaction [7], and somatostatin has been used in several clinical trials to treat inflammatory disorders, such as psoriasis [8] and rheumatoid arthritis [9]. Antineoplastic activity of somatostatin has been demonstrated in several tumour models [10,11]. New potent and more selective somatostatin analogs effectively inhibit proliferation and induce apoptosis in different cancer and leukaemia cell lines [12–14]. Consequently various somatostatin analogs have also been evaluated in clinical trials on malignancies that are known to express receptors for somatostatin, including breast cancer [15], hepatocellular cancer [16], pancreatic cancer [17] and lymphoma [18].
There is increasing evidence that the neuroendocrine and immune systems communicate with each other [19]. Somatostatin receptors (SSTR) and receptors for vasoactive intestinal peptide and substance P have been found in lymphoid tissues [20]. Somatostatin inhibits lymphocyte proliferation induced by mitogens, antibody production and cytokine production in CD4+ T-cells [2,21]. The functional roles of neuropeptides in the immune system, however, is far from clear. Reports on nonlymphoid cells have shown that the most widely studied neuropeptide, somatostatin, binds to five distinct receptors [22]. These receptors have recently been cloned and their function characterized using selective ligands for individual receptor subtypes [23–28]. Some data show that the various receptor subtypes may mediate different actions of somatostatin.
Somatostatin is present in normal and pathological lymphoid tissues [29]. Macrophages from granulomas express mRNA for preprosomatostatin [30]. Rat B- and T-lymphocytes have been reported to synthesize and secrete somatostatin [31]. Indirect evidence indicates that lymphocytes express somatostatin receptors. Lymphocyte proliferation increases markedly after removal of somatostatin and this suggests that somatostatin is a paracrine/autocrine inhibitor of lymphocyte proliferation [32]. Adhesive interaction with ECM components is of pivotal importance to migration and proliferation of cells of the immune system [33]. It is interesting in this context that leukaemic T-cells seem to exhibit defective cell substrate adhesion [34]. Recently neuropeptides have been reported to augment binding of peripheral blood T-cells to FN [35]. This somatostatin-induced adhesion to ECM components is dependent on β1-integrins [35].
In view of the fact that somatostatin seems to affect several fundamental lymphocyte functions, very few direct studies have been done on the expression of various somatostatin receptors on lymphocytes [36,37]. In the present paper we have directly tackled the question whether T-lymphocytes produce somatostatin. We have also studied the expression of various somatostatin receptor subtypes in normal and leukaemic T-cells. To determine the function of these receptors we used somatostatin analogs specific for distinct SSTR subtypes as probes of SSTR-mediated enhancement of T-lymphocyte adhesion to ECM components [38]. The adhesion response of T-lymphocytes to somatostatin analogs specific for various SSTR subtypes enabled conclusions about the function of individual SSTR subtypes. The experiments were made using eight human leukaemia T-cell lines in various stages of differentiation, with a human T-cell clone and normal blood T-cells, differing in their adhesion to ECM components.
Materials and methods
Chemicals
Table 1 lists the somatostatin analogs used: somatostatin-14 (Sigma Chemical Co., St. Louis, MO and Peninsula Laboratories, Belmont CA); somatostatin-28 and RC-160 (Peninsula Laboratories); octreotide acetate (gift from Novartis Laboratories, Basel, Switzerland); and NC 8–12 (Affinity Research Products LTD, Exeter, UK). Human FN was purified, as described elsewhere [39]; CIV, LAM, bovine serum albumin (BSA) and phorbol 12-myristate 13-acetate (TPA) were purchased from the Sigma Chemical Co. We used the following reagents for RT-PCR and PCR: Taq polymerase, MuLV reverse transcriptase, RNasin, dNTP, 25 mm MgCl2 and 10× PCR buffer (Promega, Madison, WI, USA); random hexamers (Perkin Elmer Cetus, Norwalk, CT, USA). Human leukaemic T-cell lines CCRF-HSB2, CCRF-CEM, DND-CCRF, Jurkat, Molt-4, P30, Peer and HUT-78 were obtained from the American Type Culture Collection, Manassas, VA, USA, and the human T-cell clone AF 24 from ALK Research Laboratories, Hoersholm, Denmark.
Table 1.
Binding selectivity of somatostatin analogs to the somatostatin receptor subtypes*
| Peptide | Structure | Receptor selectivity |
|---|---|---|
| Somatostatin 14 | Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys) | SSTR 1 2 3 4 5 |
| Somatostatin 28 | Ser-Ala-Asn-Ser-Asn-Pro-Ala-Met-Ala-Pro-Arg-Glu-Arg-Lys-Ala-Gly-c(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys) | SSTR 1 2 3 4 5 |
| Octreotide | D-Phe-c(Cys-Phe-D-Trp-Lys-Thr-Cys)-Thr(ol) | SSTR 2 3 5 |
| RC 160 | D-Phe-c(Cys-Tyr-D-Trp-Lys-Val-Cys)-Trp-NH2 | SSTR 2 5 (3 4) |
| NC 8–12 | D-Phe-c(Cys-Tyr-D-Trp-Lys-Abu-Cys)Nal-NH2 | SSTR 2 3 |
The somatostatin agonist binding selectivity, as determined by Patel and Srikant on SSTR 1–5 expressing CHO-K1 cells (38).
Analysis of somatostatin and somatostatin receptor mRNA expression
Isolation of cellular RNA
RNA was isolated from the human leukaemic T-cell lines CCRF-HSB2, CCRF-CEM, DND-CCRF, Jurkat, Molt-4, P30 and Peer (representing various stages of differentiation), the Sézary cell line HUT-78, the normal T-cell line AF 24 and from nonactivated and TPA-activated CD2-positive human blood T-cells [40]. Cells from the decidua and trophoblasts (T220) were kindly provided by Lucia Mincheva-Nilsson (University of Umeå), and used as a positive control. 10 × 106 cells were collected by centrifugation, washed in 1 × 10 ml phosphate-buffered saline (PBS) and 2 × 10 ml DEPC-treated PBS before being dissolved in 1 ml of solution D2 (4 m guanidine isothiocyanate, 25 mm sodium citrate pH 7·0, 0·5% N-lauroyl sarcosine and 0·1-β-mercaptoethanol). RNA was isolated from each of the cell lines using 500 µl of a cell suspension (= 5 × 106 cells). The following steps were carried out on ice in an RNase free area; 100 µl sodium acetate, 500 µl TE saturated phenol (mix), and 200 µl chloroform/isoamyl alcohol (24:1) were added. The mixture was placed on ice for 15 min. After centrifugation (25 min, 14000 r.p.m. at 4°C), the upper phase, containing RNA, was carefully removed and transferred to new tubes. An equal volume of isopropanol (500 µl) was added, and the tubes were kept at −20°C overnight. Centrifugation (20 min, 14000 r.p.m. at 4°C) was carried out to pellet RNA. The supernatant was removed and the pellets resuspended in 400 µl of solution D2. 800 µl of 100% ethanol was added, and the tubes were kept at −20°C overnight. RNA pellets were collected by centrifugation (20 min, 14000 r.p.m. at 4°C) and washed 2 × 1 ml in 70% ethanol. After the final wash, the tubes were allowed to air-dry on the bench for 45 min. The pellets were then resuspended in 30 µl of DEPC-treated water + 0·5 µl RNasin. RNA solutions were stored at −70°C).
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR from 0·5 µl of purified RNA was done in the presence of RNasin. Temperature cycling for the reaction was: step 1 42°C, 15 min; step 2 99°C, 5 min.
Polymerase chain reaction (PCR)
We used the following primers for PCR [41]: somatostatin (223 bp) 5′primer GTTTCTG CAGAAGTCCCTGG, 3′primer AATTCTTGCAGCCAGCTTTG; SSTR1(656 bp) 5′primer AAGGTAGTAAACCTGGGCGTG, 3′primer GGCTCAGAGCGTCGTGATCCG; SSTR2 (626 bp) 5′primer ATGATCACCATGGCTGTGTGG, 3′primer GATACT GGTTTGGAGGTCTCC; SSTR3 (402 bp) 5′ primer CTTCATCC ATCATCGGTGTCC; 3′primer TCATGACAGTCAGGCAGAAT AT; SSTR4 (635 bp) 5′primer AAGCTCATCAACCTGGGCGTG, 3′primer GGGTTCTGGTTGCAGGGCTTC; SSTR5 (599 bp) 5′primer CTGTCTCTGTGCATGTCGCTG, 3′primer TCACAGC TTGCTGGTCTGCAT.
The temperature cycling for all reactions was: step 1, 94°C for 1 min; step 2, 55°C for 1 min; and step 3, 72°C for 2 min. Steps 1 through 3 were repeated 35 times, followed by 72°C for 10 min [42]. Amplified products were run on 1·5% agarose gels with 0·5 µg/ml ethidium bromide. Bands were visualized on a UV-table and photographed using a CCD-camera.
Cell preparations and culture conditions
Mononuclear cells were isolated from 10 healthy blood donors by centrifuging heparinized venous blood on a Ficoll gradient (Lymphoprep, Nycomed, Oslo, Norway). Recovered cells in the interface were carefully washed in PBS and remaining erythrocytes were lysed with a buffer containing 0·15 m NH4Cl, 0·01 m KHCO3 and 0·1 m EDTA. The remaining mononuclear cells were then treated with carbonyl iron and phagocytic cells removed by a magnet. The CD2+ blood T-cells for preparation of RNA were purified using anti-CD2-coated Dynabeads (Dynal, Norway). Recovered cells were resuspended in RPMI 1640 (Life Technologies Inc., Paisley, Scotland) supplemented with 2 mm l-glutamine, 0·16% sodium bicarbonate, 100 IU/ml benzylpenicillin, 100 µg/ml streptomycin and 10% FCS. The cells were cultured in Nunclon Bottles (Nunc, Delta, Denmark) in a humidified CO2 incubator at 37°C and the cell attachment assays were done the following day. We also performed experiments on the human leukaemic T-cell lines P30, CCRF-HSB2, CCRF-CEM, DND-CCRF, Jurkat, Molt-4, HUT-78 (Sézary) and the normal human T-cell clone AF 24. The cell lines were cultured in complete RPMI supplemented with 10% FCS as described above.
Cell attachment assays
In the cell attachment assays, plastic Petri dishes (60 mm, Becton Dickinson, Mountain View, CA) were coated with FN, CIV, LAM or BSA 10 µg/ml in a buffer containing 0·02 m NaH2PO4 and 0·15 m NaCl (pH 7·4) at 22°C overnight. After coating with the ECM proteins, the dishes were washed in the coating buffer. A total of 2 × 106/ml of cells were washed in RPMI supplemented with 10% FCS, resuspended in complete medium and used in the adhesion experiments. The T-cells were preincubated with somatostatin-14 10−4 to 10−8 m (in some experiments 10−4 to 10−14 m), with one of the somatostatin analogs listed in Table 1 or with a negative control, in a humidified CO2 incubator for 2 h at 37°C. Somatostatin was also present during the adhesion assay. Unbound cells were removed after 1 h by gentle aspiration. Bound cells were fixed in cold glutaraldehyde 2·5% in PBS for 10 min and the adherent cells were counted using an inverted microscope (IMT-2, Olympus). Cells from five microscope fields were counted and the mean of the adherent cells was determined.
Statistical methods
The findings were analysed using one-way analysis of variance (anova) with repeated measures. Post hoc comparison of means was done with the Tukey HSD test. The distribution of some of the variables was skewed and the data have therefore been log-transformed to meet requirements for an adequate anova.
Results
Lack of somatostatin mRNA expression in human normal and leukaemic T-cells
The possible expression of somatostatin mRNA in the human leukaemic T-cell lines CCRF-HSB2, CCRF-CEM, HUT-78, Jurkat, Molt-4, P30 and Peer, the normal T-cell clone AF24, and in nonactivated and activated CD2+ blood T-cells was analysed with RT-PCR. The somatostatin primers were designed to detect mRNA for both somatostatin-14 and somatostatin-28. Human decidua and trophoblast cells, which express somatostatin, were used as a positive control (Fig. 1) [43]. We found no somatostatin mRNA expression in the leukaemic T-cell lines (Fig. 1), in blood lymphocytes and AF24 (not shown).
Fig. 1.
Expression of somatostatin mRNA in human leukaemic T-cell lines. RT-PCR and PCR were performed on RNA from the various human leukaemic T-cell lines using a primer specific for somatostatin. Cells from decidua and trophoblasts (T220) were used as positive controls. The products were run on an agarose gel containing ethidium bromide, and visualized on a UV table. The amplified products measured 223 bp.
mRNA expression of SSTR subtypes in human leukaemic and normal T-cells
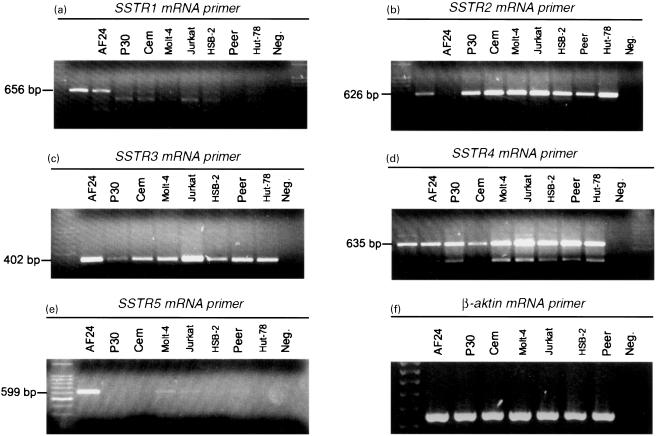
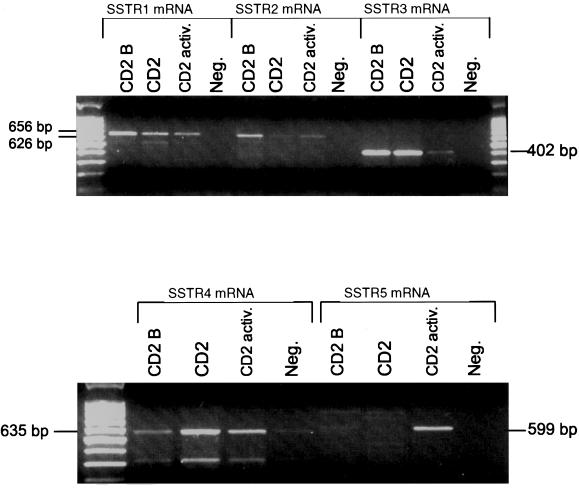
The expression of mRNA in the five SSTR subtypes by the human leukaemic T-cell lines CCRF-HSB2, CCRF-CEM, HUT-78, Jurkat, Molt-4, P30 and Peer, the human T-cell clone AF 24, and in nonactivated and TPA-activated CD2+ blood T-cells was analysed using RT-PCR and PCR (Table 2). The leukaemic T-cell lines expressed mRNA for SSTR2, 3 and 4, of which the amplified products for SSTR2 and SSTR4 were pronounced in all cell lines (Fig. 2). The expression of mRNA for SSTR1 and 5 was very weak or undetectable in the leukaemic cell lines (Fig. 2). SSTR1 was expressed in resting and activated CD2 positive T-cells and in the T-cell clone AF24, while SSTR5 was expressed selectively in activated CD2 positive T-cells and AF24. In the T-cell clone AF 24, mRNA for all five receptors could be detected, with the strongest expression for SSTR5 (Fig. 3). Nonactivated CD2+ cells from healthy blood donors expressed mRNA for SSTR1–4 of which the amplified product for SSTR3 showed the highest intensity, while activated CD2+ cells showed an intense SSTR5 band, but a weak expression of SSTR1–4 (Fig. 3). These findings show that normal blood T-cells and T-cell clones express SSTR1 and 5, while the leukaemic T-cell lines show weak or negligible expression of these receptors. It is also obvious that SSTR2, 3 and 4 are expressed in all T-cell lines, but vary in expression in normal T-cells in relation to activation.
Table 2.
Expression of mRNA for somatostatin receptor subtypes by human T-lymphocytes*
| Cell type | SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 |
|---|---|---|---|---|---|
| P30 | – | + + + | + | + + | – |
| CCRF-CEM | – | + + + | + | + | – |
| Molt-4 | – | + + + | + | + + | + |
| Jurkat | – | + + + | + + + | + + | + |
| CCRF-HSB2 | – | + + + | + | + + | – |
| Peer | – | + + + | + + | + + | – |
| HUT-78 | – | + + + | + | + + | – |
| AF 24 | + + | + | + + | + + | + + + |
| CD2 | + | + | + + + | + | – |
| CD2(activated) | + | + | + | + | + + |
Relative expression of mRNA for somatostatin receptor subtypes in human leukaemic T-cell lines (P30, CCRF-CEM, Molt-4, Jurkat, CCRF-HSB2, Peer and HUT-78), the normal T-cell clone AF 24 and normal nonactivated and activated CD2+ blood T-cells was determined by RT-PCR and PCR (Figs 1–3). The findings from two identical experiments are summarized here: – no expression; + weak signal; ++ moderate to strong signal; ++ + strong signal.
Fig. 2.
Expression of somatostatin receptor (SSTR) subtypes in a normal T-cell clone (AF 24) and in human leukaemic T-cell lines. RNA from the T-cell lines was reverse-transcribed and subjected to PCR amplification with primers specific for SSTR1 (a), SSTR2 (b), SSTR3 (c), SSTR4 (d), SSTR5 (e) and β-aktin (f). The products were visualized using agarose electrophoresis. The amplification gave products of the following sizes: SSTR1: 656 bp; SSTR2: 626 bp; SSTR3: 402 bp; SSTR4: 635 bp; SSTR5: 599 bp; β-aktin: 540 bp.
Fig. 3.
Somatostatin receptor (SSTR) expression in normal human blood T-cells. RNA from resting (CD2 and CD2 B, two donors) and TPA-activated (CD2 activ.) CD2 fractions from blood T-cells were reverse-transcribed and subjected to PCR amplification with primers specific for SSTR1 (656 bp), SSTR2 (626 bp), SSTR3 (402 bp), SSTR4 (635 bp) and SSTR5 (599 bp). The products were visualized using agarose electrophoresis.
Somatostatin enhances adhesion of T-cells to ECM proteins
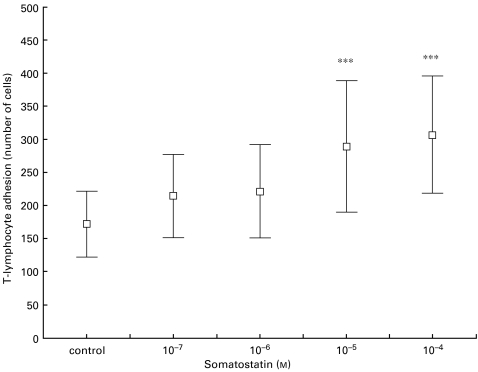
To determine whether the expression of SSTR could exert functional effects on T-lymphocytes, we evaluated the influence of somatostatin-14 on the adhesion of T-cells to surfaces coated with the ECM proteins FN, CIV and LAM. Somatostatin at high concentration (10−4 m and 10−5 m) significantly increased the adhesion of peripheral blood T-lymphocytes from healthy donors (n = 10) to FN (P < 0·001) (Fig. 4). Somatostatin at concentrations of 10−4 to 10−6 m tended to increase T-lymphocyte adhesion also to CIV and LAM (data not shown). Treatment with somatostatin also increased adhesion of TPA-activated blood T-lymphocytes to ECM proteins.
Fig. 4.
Somatostatin-induced adhesion of blood T-cells to fibronectin (FN). T-lymphocytes from 10 blood donors in separate experiments were allowed to adhere to FN-coated wells in the presence of somatostatin-14. After fixation, we determined the mean of five microscopic fields. Significant differences between the control and somatostatin-treated cells are indicated (***P < 0·001).
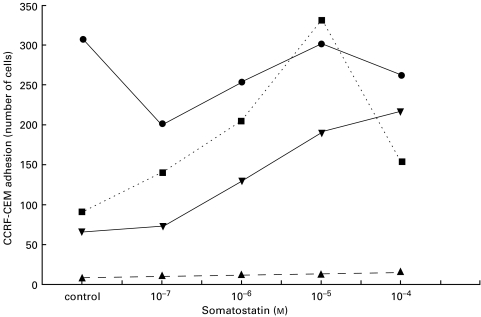
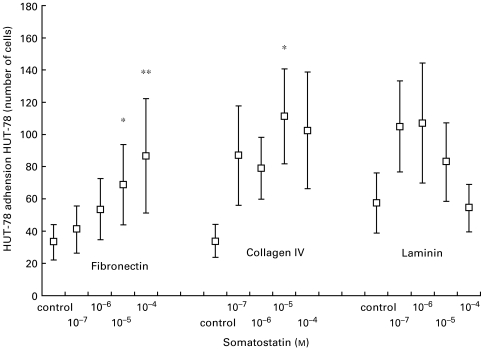
Adhesion experiments with somatostatin were also performed using the human leukaemic T-cell lines CCRF-HSB2, CCRF-CEM, DND-CCRF, Jurkat, Molt-4, P30 and Peer, the Sezary cell line HUT-78 and the human T-cell clone AF 24. The leukaemic T-cell lines showed a high spontaneous adhesion to FN, which was only slightly augmented by somatostatin. The leukaemic T-cell lines showed weak ‘spontaneous’ adhesion to CIV and LAM. However, somatostatin markedly increased the adhesion of the T-cell lines to these ECM components as exemplified by representative experiments with CCRF-CEM and HUT shown in Figs 5 and 6.
Fig. 5.
Somatostatin-induced adhesion of CCRF-CEM T-cells to various ECM components. Note that this T-cell line shows poor adhesion to CIV and LAM in the absence of somatostatin. CCRF-CEM cells were allowed to adhere to FN, CIV, LAM and BSA-coated wells in the presence of somatostatin-14. After fixation the number of adherent cells in five microscopic fields were counted and the mean determined. The graph shows the results of one representative experiment. The spontaneous adhesion to FN is high and not affected by somatostatin. The adhesion to BSA is not influenced by somatostatin. • FN; ▾ LAM; ▪ CIV; ▴ BSA.
Fig. 6.
Somatostatin-induced adhesion of HUT-78 T-cells to various ECM components. Note that this T-cell line in the absence of somatostatin shows poor or negligible substrate adhesion to all the three ECM components FN, CIV and LAM. The Sézary cell line HUT-78 was allowed to adhere to FN, CIV or LAM-coated wells in the presence of somatostatin-14. After fixation, we determined the mean of five microscopic fields. Mean values and standard error of eight experiments are shown. Significant difference between control and somatostatin-treated cells are indicated (*P < 0·05, **P < 0·01).
In repeated experiments with HUT, somatostatin caused a significant increase in the adhesion to FN in concentrations of 10−4 m (P < 0·01) and 10−5 m (P < 0·05), and also to CIV at 10−5 m (P < 0·05) (Fig. 6). The T-cell clone AF 24 showed a high ‘spontaneous’ adhesion to FN, CIV and LAM, and no further increase occurred after somatostatin treatment.
Adhesion experiments with somatostatin analogs having different receptor affinities
The RT-PCR studies showed that all T-cell lines in the adhesion experiments and normal T-cells expressed mRNA for SSTR2, 3 and 4 (Table 2; Figs 2, 3). The leukaemic T-cell lines showed negligible expression of mRNA for SSTR1 and 5 (Figs 2, 3).
Somatostatin-14 has a high affinity for all five SSTR which would suggest that it can enhance adhesion via all these receptors. To determine whether a specific SSTR subtype is mainly responsible for the increased T-cell adhesion to ECM components induced by somatostatin, we did experiments with somatostatin analogs selective for various SSTR (Table 1) [38]. NC 8–12 (highly selective for SSTR2 and 3) in a concentration of 10−4 m, increased T-cell adhesion to FN, CIV and LAM more effectively than the other analogs. The somatostatin analogs octreotide (specific for SSTR2, 3, 5), RC 160 (specific for SSTR2, 5; in higher concentrations also to SSTR3, 4), and somatostatin-28 (specific for SSTR1–5), increased T-cell adhesion to the same extent as somatostatin-14, although the effect of somatostatin-14 was stronger in most experiments.
Thus, NC 8–12 and octreotide, which both have a high affinity for SSTR2 and 3, effectively increased T-cell adhesion. This finding, taken together with the fact that all the T-cell lines and normal T-cells as shown by RT-PCR expressed SSTR2 and 3, supports the conclusion that the enhancement of T-cell adhesion to ECM induced by somatostatin is probably mediated via SSTR2 and/or 3.
Discussion
Although somatostatin probably has an important functional role for the immune system, and new somatostatin analogs are under development as putative drugs in the treatment of inflammatory diseases and malignancies, there is relatively little previous information concerning the expression of somatostatin receptor subtypes on lymphocytes. This study presents new information regarding somatostatin receptor expression and function of T-cells, and provides evidence that SSTR expression distinguishes normal and leukaemic T-cells. By using RT-PCR, we have mapped the mRNA expression of five different somatostatin receptors in normal blood T-lymphocytes, in eight human leukaemic T-cell lines and in a human T-cell clone. The expression of somatostatin receptors by T-lymphocytes has also been compared to the effect of signals exerted via these receptors on adhesion of T-cells to FN, CIV and LAM. To evaluate the function of signals via the separate somatostatin receptors, we used somatostatin analogs with known specificity according to binding assays (Table 1) [38]. The experiments show that SSTR may have a profound effect on fundamental lymphocyte functions.
Normal T-lymphocytes as well as a T-cell clone and all the eight T-cell lines studied were found to be negative with respect to somatostatin mRNA expression. Therefore, although T-cells can respond to somatostatin, they do not seem to produce the neuropeptide themselves. The finding that lymphocytes produced somatostatin in a previous study may have been due to use of a cell suspension from rat spleen which could have contained macrophages/monocytes [32].
The normal T-lymphocytes, including peripheral blood T-cells and a T-cell clone, and the leukaemic T-cell lines, showed some consistent differences and similarities with respect to SSTR expression. SSTR2, 3 and 4 were generally present in all the normal and leukaemic T-cells examined. The normal T-cells expressed mRNA for SSTR1 and SSTR5 while the leukaemic cell lines did not have SSTR1 and 5. The leukaemic T-cell lines showed a strong expression of SSTR2 while the normal T-cells had a relatively weak SSTR2 expression at the mRNA level. Two studies have previously reported the expression of somatostatin receptors on cells of the immune system. The receptors have been detected in normal lymphoid tissue and in pathological tissues, e.g. Hodgkin and non-Hodgkin lymphomas, rheumatoid arthritis and sarcoidosis [19,44]. Tsutsumi et al. [36] and Cardoso et al. [37] analysed somatostatin receptor expression at the mRNA level in Jurkat cells, which were also examined in the present study. Cardoso et al. reported expression of SSTR3 but not the other subtypes in Jurkat, while Tsutsumi et al. detected mRNA for SSTR3,4 and 5 but not SSTR1 and 2. In our experiments, Jurkat clearly expressed SSTR2,3 and 4. The expression of SSTR3 was very strong in our study, which may be consistent with the data of Cardoso et al. A plausible explanation why Cardoso et al. did not detect the other SSTR subtypes may be the use of different primers.
The fact that the individual T-cell lines express multiple SSTR may suggest that the different receptors operate in concert rather than as individual receptors. However, there is some evidence from other test systems for a subtype selectivity of SSTR effects in that SSTR1, 2, 4, and 5 can arrest cell growth, but SSTR3 is cytotoxic [45–47]. A comparison of the SSTR expression in various leukaemic T-cell lines and of the responsiveness of the same cells to somatostatin and its analogs in adhesion tests as described in ′results', suggests that SSTR2 and/or 3 are responsible for the enhancement of adhesion to extracellular matrix components. Functional diversity of the different SSTR, however, can not be excluded [46,48]. Such a diversity might explain our finding that SSTR2 and 3 seem to be the most likely inducers of the augmented capacity to adhere to ECM components.
The present findings are relevant to understanding the functional role of neuropeptides for the immune system. Neuropeptides are probably present in sufficient amounts in tissues to affect various lymphocyte functions such as adhesion [3,20]. Neuropeptides, including somatostatin, can be released from nerve endings and secreting cells in lymphoid tissues and in most major peripheral nonlymphoid organs. For example, in the gut, where lymphocytes are found in large numbers, somatostatin may act as an important regulator of gastrointestinal physiology and function of the immune system. However, it is not yet possible to present a unifying concept of the specific actions of neuropeptides and their receptors in the function of the immune system.
T-lymphocytes express multiple SSTR and somatostatin may therefore regulate cell functions via distinct receptor subtypes. SSTR expression also distinguishes normal and leukaemic T-cells. It is now an important task to identify the exact functional role of each SSTR subtype on T-lymphocytes. The development of selective SSTR receptor antagonists in the future will provide further information in this regard. The present findings and particularly the distinct SSTR expression in normal and leukaemic T-cells suggest that SSTR subtypes may be useful targets for specific somatostatin analogs in the treatment of inflammatory diseases and lymphocyte malignancies.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (16X-08295), the Swedish Cancer Society (1940), the Children's Cancer Foundation, the Welander-Finsen Foundation and the Swedish Psoriasis Association.
References
- 1.Patel YC. General aspects of the biology and function of somatostatin. In: Weil C, Muller EE, Thorner MO, editors. Basic and Clinical Aspects of Neuroscience. Vol. 4. Berlin: Springer-Verlag; 1992. pp. 1–16. [Google Scholar]
- 2.Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–501. 1556–63. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 3.Payan DG, Hess CA, Goetzl EJ. Inhibition by somatostatin of the proliferation of T lymphocytes and molt-4 lymphoblasts. Cell Immunol. 1984;84:433–8. doi: 10.1016/0008-8749(84)90117-5. [DOI] [PubMed] [Google Scholar]
- 4.Brazeau P, Vale WW, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic peptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–9. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 5.Epelbaum J, Dornauad P, Fodor M, Viollet C. The neurobiology of somatostatin. Crit Rev Neurobiol. 1994;153:1180–6. [PubMed] [Google Scholar]
- 6.Editorial. Octreotide steaming ahead. Lancet. 1992;339:837–9. [PubMed] [Google Scholar]
- 7.Karalis K, Mastorakos G, Chrousos GP, Tolis G. Somatostatin analogues suppress the inflammatory reaction in vivo. J Clin Invest. 1994;93:2000–6. doi: 10.1172/JCI117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matt LH, Kingston TP, Lowe NJ. Treatment of severe psoriasis with intravenous somatostatin. J Dermatol Treatm. 1989;181:81–2. [Google Scholar]
- 9.Fioravanti A, Govoni M, La Montagna G, et al. Somatostatin 14 and joint inflammation: evidence for intraarticular efficacy of prolonged administration in rheumatoid arthritis. Drugs Exp Clin Res. 1995;21:97–103. [PubMed] [Google Scholar]
- 10.Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocrine Rev. 1991;12:450–82. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- 11.Pollak MN, Scally AV. Mechanisms of antineoplastic action of somatostatin analogs. Proc Soc Exp Biol Med. 1998;217:143–52. doi: 10.3181/00379727-217-44216. [DOI] [PubMed] [Google Scholar]
- 12.Qin Y, Ertl T, Groot K, Horvath J, Cai R-Z, Schally AV. Somatostatin analog RC-160 inhibits growth of CFPAC-1 human pancreatic cancer cells in vitro and intracellular production of cyclic adenosine monophosphate. Int J Cancer. 1995;60:694–700. doi: 10.1002/ijc.2910600521. [DOI] [PubMed] [Google Scholar]
- 13.Keri GY, Érchegyi J, Horvath A, et al. A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo antitumor activity. Proc Natl Acad Sci USA. 1996;93:12513–8. doi: 10.1073/pnas.93.22.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianetti N, Enjalbert A, Krantic S. Somatostatin analog SMS 201995 inhibits proliferation in human leukemia T-cell line: Relevance of the adenyl cyclase stimulation. J Cell Biochem. 2000;78:666–73. doi: 10.1002/1097-4644(20000915)78:4<666::aid-jcb15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.O'Byrne KJ, Dobbs N, Propper DJ, et al. Phase II study of RC-160 (vapreotide), an octapeptide analogue of, somatostatin, in the treatment of metastatic breast cancer. Br J Cancer. 1999;79:1413–8. doi: 10.1038/sj.bjc.6690226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raderer M, Hejna MH, Muller C, et al. Treatment of hepatocellular cancer with the long acting somatostatin analog lanreotide in vitro and in vivo. Int J Oncol. 2000;16:1197–201. doi: 10.3892/ijo.16.6.1197. [DOI] [PubMed] [Google Scholar]
- 17.Sulkowski U, Buchler M, Pederzoli P, et al. A phase II study of high-dose octreotide in patients with unresectable pancreatic carcinoma. Eur J Cancer. 1999;35:1805–8. doi: 10.1016/s0959-8049(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 18.Witzig TE, Letendre L, Gerstner J, et al. Evaluation of a somatostatin analog in the treatment of lymphoproliferative disorders: results of a phase II North Central Cancer Treatment Group Trial. J Clin Oncol. 1995;13:2012–5. doi: 10.1200/JCO.1995.13.8.2012. [DOI] [PubMed] [Google Scholar]
- 19.Van Hagen PM. Somatostatin receptor expression in clinical immunology. Metab. 1996;45:86–7. doi: 10.1016/s0026-0495(96)90092-x. [DOI] [PubMed] [Google Scholar]
- 20.Reubi JC, Horisberger U, Kappeler A, Laissue JA. Localization of receptors for vasoactive intestinal peptide, somatostatin, and substance P in distinct compartments of human lymphoid organs. Blood. 1998;92:191–7. [PubMed] [Google Scholar]
- 21.Blum AM, Metwali A, Mathew RC, Elliott D, Weinstock JV. Substance P and somatostatin can modulate the amount of IgG2a secreted in response to schistosome egg antigens in murine schistosomiasis mansoni. J Immunol. 1993;151:6994–7004. [PubMed] [Google Scholar]
- 22.Raynor K, Reisine T. Analogs of somatostatin selectively label distinct subtypes of somatostatin receptors in rat brain. J Pharmacol Exp Therapeut. 1989;251:510–7. [PubMed] [Google Scholar]
- 23.Rens-Domiano S, Law SF, Yamada Y, Seino S, Bell GI, Reisine T. Pharmacological properties of two cloned somatostatin receptors. Mol Pharmacol. 1992;42:28–34. [PubMed] [Google Scholar]
- 24.Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI. Cloning of a novel somatostatin receptor SSTR3, coupled to adenylcyclase. J Biol Chem. 1992;267:20422–8. [PubMed] [Google Scholar]
- 25.Raynor K, O'Carrol A-M, Kong H, Yasuda K, Mahan LC, Bell GI, Reisine T. Characterization of cloned somatostatin receptors SSTR4 and SSTR5. Mol Pharmacol. 1993;44:385–92. [PubMed] [Google Scholar]
- 26.Raynor K, Coy DC, Reisine T. Analogues of somatostatin bind selectively to brain somatostatin receptor subtypes. J Neurochem. 1992;59:1241–50. doi: 10.1111/j.1471-4159.1992.tb08433.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel YC. Molecular pharmacology of somatostatin receptor subtypes. J Endocrinol Invest. 1997;20:348–67. doi: 10.1007/BF03350317. [DOI] [PubMed] [Google Scholar]
- 28.Hocart SJ, Jain R, Murphy WA, Taylor JE, Morgan B, Coy DH. Potent antagonists of somatostatin: synthesis and biology. J Med Chem. 1998;41:1146–54. doi: 10.1021/jm970730q. [DOI] [PubMed] [Google Scholar]
- 29.Aguila MC, Dees WL, Haensly WE, McCann SM. Evidence that somatostatin is localized and synthesized in lymphoid organs. Proc Natl Acad Sci USA. 1991;88:11485–9. doi: 10.1073/pnas.88.24.11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott DE, Blum AM, Li J, Metwali A, Weinstock JV. Preprosomatostatin messenger RNA is expressed by inflammatory cells and induced by inflammatory mediators and cytokines. J Immunol. 1998;160:3997–4003. [PubMed] [Google Scholar]
- 31.Aguila MC, Solar-Rodriguez AM. Somatostatin inhibits the lipopolysaccharide induced somatostatin messenger ribonucleic acid levels from lymphocytes in culture. Annu Meeting Endocrine Soc. 1993:443. (Abstract 1570) [Google Scholar]
- 32.Aguila MC, Rodriguez AM, Aguila-Mansilla HN, Lee WT. Somatostatin antisense oligodeoxynucleotide-mediated stimulation of lymphocyte proliferation in culture. Endocrinol. 1996;137:1585–90. doi: 10.1210/endo.137.5.8612489. [DOI] [PubMed] [Google Scholar]
- 33.Hauzenberger D, Klominek J, Bergström SE, Sundqvist KG. T-lymphocyte migration: The influence of interactions via adhesion molecules, the T cell receptor and cytokines. Crit Rev Immunol. 1995;15:285–16. doi: 10.1615/critrevimmunol.v15.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 34.Sundqvist KG, Mellstedt H, Otteskog P. Motility and infiltration capacity of lymphoid tumour cells: disturbance of motile behaviour in TCCL-lymphocytes. J Clin Lab Immunol. 1987;23:71–5. [PubMed] [Google Scholar]
- 35.Levite M, Cahalon L, Hershkoviz R, Steinman L, Lider O. Neuropeptides via specific receptors, regulate T cell adhesion to fibronectin. J Immunol. 1998;160:993–1000. [PubMed] [Google Scholar]
- 36.Tsutsumi A, Takano H, Ichikawa K, Kobayashi S, Koike T. Expression of somatostatin receptor subtype 2 mRNA in human lymphoid cells. Cell Immunol. 1997;181:44–9. doi: 10.1006/cimm.1997.1193. [DOI] [PubMed] [Google Scholar]
- 37.Cardoso A, Gaultron JP, Horvat B, Gautier N, Enjalbert A, Krantic S. Somatostatin increases mitogen-induced IL-2 secretion and proliferation of human Jurkat T cells via sst3 receptor isotype. J Cell Biochem. 1998;68:62–73. doi: 10.1002/(sici)1097-4644(19980101)68:1<62::aid-jcb6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 38.Patel YC, Srikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1–5) Endocrinol. 1994;136:2814–7. doi: 10.1210/endo.135.6.7988476. [DOI] [PubMed] [Google Scholar]
- 39.Klominek J, Robért K-H, Sundqvist KG. Chemotaxis and hapothaxis of malignant mesothelioma cells: effects of fibronectin, laminin, collagen IV and an autocrine motility factor-like substance. Cancer Res. 1993;53:4376–82. [PubMed] [Google Scholar]
- 40.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidine thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C-Y, Yokogoshi Y, Yoshimoto K, Fujinaka Y, Matsumoto K, Saito S. Point mutation of the somatostatin receptor 2 gene in the human small cell lung cancer cell line COR-L103. Biochem Biophys Res Commun. 1995;210:805–15. doi: 10.1006/bbrc.1995.1730. [DOI] [PubMed] [Google Scholar]
- 42.Takeba Y, Suzuki N, Takeno M, Asai T, Tsuboi S, Hoshino T, Sakane T. Modulation of synovial cell function by somatostatin in patients with rheumatoid arthritis. Arthr Rheum. 1997;40:2128–38. doi: 10.1002/art.1780401206. [DOI] [PubMed] [Google Scholar]
- 43.Watkins WB, Yen SS. Somatostatin in cytotrophoblast of the immature human placenta: Localization by immunoperoxidase cytochemistry. J Clin Endocrinol Metab. 1980;50:969–71. doi: 10.1210/jcem-50-5-969. [DOI] [PubMed] [Google Scholar]
- 44.Van Hagen PM, Krenning EP, Kwekkeboom DJ, Reubi JC, Anker-Lugtenburg PJ, Löwenberg B, Lamberts SWJ. Somatostatin and the immune and haematopoietic system; a review. Eur J Clin Invest. 1994;24:91–9. doi: 10.1111/j.1365-2362.1994.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 45.Florio T, Yao H, Carey KD, Dillon TJ, Stork PJS. Somatostatin activation of mitogen-activated protein kinase via somatostatin receptor 1 (SSTR1) Mol Endocrinol. 1999;13:24–37. doi: 10.1210/mend.13.1.0224. [DOI] [PubMed] [Google Scholar]
- 46.Sharma K, Patel YC, Srikant CB. Subtype selective induction of p53-dependent apoptosis but not cell cycle arrest by human somatostatin receptor 3. Mol Endocrinol. 1996;10:1688–96. doi: 10.1210/mend.10.12.8961277. [DOI] [PubMed] [Google Scholar]
- 47.Sharma K, Patel YC, Srikant CB. C-terminal region of human somatostatin receptor 5 is required for induction of Rb and Gi cell cycle arrest. Mol Endocrinol. 1999;13:82–90. doi: 10.1210/mend.13.1.0220. [DOI] [PubMed] [Google Scholar]
- 48.Patel YC, Greenwood M, Panetta R, Hukovic N, Grigorakis S, Robertson LA, Srikant CB. Molecular biology of somatostatin receptor subtypes. Metab. 1996;45:31–8. doi: 10.1016/s0026-0495(96)90076-1. [DOI] [PubMed] [Google Scholar]