Abstract
Cardiac surgery with cardiopulmonary bypass (CPB) leads to a systemic inflammatory response with secretion of cytokines (e.g. IL-6, TNF-α, IL-1β and sIL-2R). The objective of the following study was to investigate in vitro and in vivo cytokine responses and white blood cell counts (WBC) of patients with high versus low cytokine secretion after a coronary artery bypass grafting (CABG) procedure. Twenty male patients undergoing elective CABG surgery with CPB under general anaesthesia were enrolled in the study. On the day of surgery (postoperatively), serum levels of TNF-α and IL-1β were significantly higher in patients of the high IL-6 level group compared to the respective values in the patient group with low IL-6 levels. The inter-individual differences in IL-6 release in patients undergoing CABG surgery with CPB were accompanied by differences in the release of other cytokines, such as TNF-α, IL-1β and sIL-2R. To understand whether genetic background plays a role in influencing cytokine plasma levels under surgical stress, we examined the distribution of polymorphic elements within the promoter regions of the TNF-α and IL-6 genes, and determined their genotype regarding the BAT2 gene and TNF-β intron polymorphisms. Our preliminary data suggests that regulatory polymorphisms in or near the TNF locus, more precisely the allele set 140/150 of the BAT2 microsatellite marker combined with the G allele at −308 of the TNF-α gene, could be one of the genetic constructions providing for a less sensitive response to various stimuli. Our results suggest: (1) close relationships between cytokine release in the postoperative period, and (2) inter-individually varying patterns of cytokine release in patients undergoing CABG surgery with CPB.
Keywords: BAT2, cardiac surgery, cytokine, TNF-α, IL-6, IL-1β, sIL-2R, inter-individual differences, polymorphism, TNF-α, TNF-β, IL-6 gene
Introduction
A systemic inflammatory response with secretion of cytokines and activation of leucocytes and endothelial cells is induced by cardiac surgery involving a cardiopulmonary bypass (CPB) [1,2].
Pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 are released into the circulation in response to cardiac surgery and have been suggested to play an important role in the pathogenesis of myocardial dysfunction in ischaemia-reperfusion injury [3–6]. TNF-α is well known as an early mediator of the inflammatory cascade [7], and can stimulate the release of IL-1β [8,9]. Moreover, IL-1β can stimulate the synthesis of IL-6 [10]. Common postoperative problems associated with CPB, i.e. coagulopathy, increased capillary permeability, fever and multi-system organ failure, have been assumed to be manifestations of the profoundly altered immune functions following cardiac surgery [11]. Alterations in serum concentrations of cytokines, especially IL-6, have been shown to be of prognostic significance [12–14]. High IL-6 levels have been reported to be associated with poor outcome in critically ill patients [14]. In human septic shock, the systemic release of large amounts of cytokines was associated with fatal outcome [15]. Reports on the cytokine response during and after cardiac surgery with CPB have shown inconsistent results [16]. This may be due to the fact that the perioperative release of cytokines during and following cardiac surgery with CPB is influenced by many factors and processes such as tissue damage, contact activation with non-endothelial surfaces, reperfusion injury, perioperatively administered drugs and haemodilution [5,17–20]. Another factor that may influence the degree of the inflammatory response under surgical injury — the individual-specific reactivity of the cytokine system — has not yet been fully characterized.
Only scant information is available concerning the potential inter-individual differences in cytokine response during and following cardiac surgery. Evidence for a relationship between genetic variations at the HLA and/or cytokine gene loci, and the expression of cytokines have been reported elsewhere [21–23]. The regulation of the expression of a number of cytokine genes has been suggested to be genetically determined in part [24,25]. To understand whether genetic background plays a role in influencing cytokine plasma levels and the degree of inflammatory response under surgical stress (e.g. with cardiopulmonary bypass), we examined the distribution of polymorphic elements within the promoter regions of the TNF-α and IL-6 genes and TNF-β intron in our samples, and determined their genotype regarding the BAT2 gene.
The objectives of the following study were: (1) to compare the perioperative cytokine responses and white blood cell counts (WBC) of patients with high versus low IL-6 secretion after a coronary artery bypass grafting (CABG) procedure involving CPB; (2) to assess inter-individual variations in IL-6 and TNF-α production after LPS stimulation of whole blood cells; and (3) to assess polymorphisms of the IL-6 and TNF-α genes as well as the BAT2 microsatellite marker and TNF-β intron.
Patients and methods
Following approval by the ethics committee, written informed consent was obtained from all study participants.
Subjects
Male patients scheduled for elective CABG surgery without any known immune or HPA-axis dysfunctions were enrolled in the study (study I: n = 20; study II: n = 15). Patients with a history of myocardial infarction during the 6 weeks before surgery were excluded. Other exclusion criteria included: congestive heart failure, exogenous hormone therapy, chronic renal failure, history of malignancy, signs of acute infection or inflammation, malnutrition or diabetes mellitus type I. Those patients enrolled in study I (n = 20) were classified into two study groups according to the median IL-6 concentration (on the day of surgery) of the entire group, i.e. 10 patients with IL-6 release below the median (group 1) and 10 patients with IL-6 release higher than the median (group 2). Five patients from the first could not be reviewed in our second study (one of them died, one of them suffered cerebral dysfunction, and we lost contact with the other three). Therefore, only 15 patients from study I could participate in study II.
Study design
Premedication consisted of oral flunitrazepam (1 mg) on the evening before surgery and upon reporting to the operating room (OR). Anaesthesia was induced with midazolam (0·05 mg/kg), sufentanil (1 µg/kg) and etomidate (0·2 mg/kg). Following relaxation with pancuronium (0·1 mg/kg), the patient's trachea was intubated. Anaesthesia was maintained using continuous infusions of sufentanil (0·1 µg/kg/h) and midazolam (0·03 mg/kg/h). End-tidal concentrations of isoflurane were titrated between 0·4 vol% and 0·8 vol% depending on the clinical situation. Repetitive doses of pancuronium (0·03 mg/kg) were given on an hourly basis to maintain adequate neuromuscular blockade. Controlled mechanical ventilation (CMV) with an air in oxygen mixture (inspired oxygen concentration, FIO2, between 0·33 and 1·0) was used. Following surgery, all patients remained intubated and artificially ventilated in the intensive care unit (ICU) until they regained sufficient spontaneous respiration. During this time, sufentanil infusion was continued at 0·01–0·02 µg/kg/h for sedation. All extubations took place when patients showed stable cardiovascular and respiratory conditions. During the sampling period, patients received no steroid-containing medication.
Cardiopulmonary bypass
CPB was conducted using a membrane oxygenator (Stöckert Instruments, Mutz an der Knatter, Germany) with non-pulsatile flow at 28–36°C. The pump prime consisted of 1000 ml of Ringer's solution, 250 ml of human albumin (5%) and 250 ml of mannitol solution (20%). Pump flow was maintained at 2·4 l/m2 body surface area (BSA)/min. In order to achieve cardiac arrest, 600 ml St Thomas's Hospital crystalloid cardioplegic solution was injected into the aortic root immediately after cross-clamping of the aorta. Additional cardioplegic solution was administered at 200-ml increments every 30 min to maintain cardiac standstill.
Blood samples
The time and route of blood sample collections in study I were as follows. The first blood sample (T1) was taken preoperatively between 6 and 8 p.m. on the evening before surgery. The second sample (T2) was taken intra-operatively 90 min after incision, on bypass. The third sample (T3) was taken between 6 and 8 p.m. postoperatively, on the day of surgery (DOS). The fourth sample (T4) was taken between 6 and 8 p.m. on the first postoperative day. The fifth sample (T5) was taken between 6 and 8 p.m. on the second postoperative day. Since most of our CABG patients left the hospital on the second or third postoperative day to undergo cardiac rehabilitation programmes elsewhere, blood sampling was only possible up to the second postoperative day at most (for 16 patients), while for the remainder, blood sampling was possible up to the first postoperative day.
Blood samples were obtained via an i.v. sampling catheter. For cytokine measurements, blood was spun down for 10 min in a centrifuge (1000 g, 4°C) after withdrawal. The serum was stored at −80°C until it was assayed. For determining blood cell counts, whole blood was collected in EDTA tubes and analysed within 3 h.
Measurement of serum cytokines
Serum concentrations of IL-6, TNF-α, IL-1β and sIL-2R were measured using quantitative sandwich enzyme immunoassay techniques (ELISA). Only commercial kits were used (Quantikine, R&D Systems, Abingdon, UK).
According to information provided by the manufacturer, sensitivities for the assays were: TNF-α < 180 fg/ml, IL-6 assay = 0·70 pg/ml, IL-1β assay <100 fg/ml, and sIL-2R assay = 24 pg/ml. Intra-assay coefficients were 5·6–6·1% for TNF-α, 8·0–11·8% for IL-1β, 3·2–8·5% for IL-6 and 4·6–6·1% for IL-2R. Interassay coefficients were 7·5–10·4% for TNF-α, 7·1–11·1% for IL-1β, 3·5–8·7% for IL-6 and 6·0–7·2% for IL-2R.
For taking perioperative plasma volume changes into account, simultaneous determinations of cytokines, WBC, neutrophils and packed cell volume (PCV) were performed. WBC and neutrophils were processed using the Technicon H1 (Technicon Instruments, Terrytown, USA) with standard procedures. PCV was computed from the erythrocyte count and the mean cellular volume. The effect of haemodilution on cytokine values and blood cell counts was accounted for using a formula described by Taylor et al. [26]: PCV-corrected value (concentration unit) = absolute value (concentration unit) × preoperative PCV/PCV at the time of sampling.
In vitro cytokine measurements
Blood samples were collected aseptically into a pyrogen-free lithium-heparin containing system (Sarstedt S-Monovette, Nümbrecht, Germany) and processed within 7 h after samples were taken as described by Brand et al. [27].
Blood samples were diluted 1:10 in 5-ml tubes (Greiner Labortechnik GmbH, Frickenhausen, Germany) with culture medium RPMI 1640 (Biowhittaker Europe, Vervies, Belgium) enriched with 2 mm glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. Blood suspension, 950 µl, was supplemented with 50 µl lipopolysaccharide (LPS) solution (20 µg/ml; Sigma, Deisenhofen, Germany), negative controls were supplemented with 50 µl culture medium RPMI 1640. Blood culture tubes were incubated in a humidified atmosphere at 37°C in 5% CO2. After 24 h of incubation, the cell-free culture supernatants were transferred to cryo vials (Greiner, Frickenhausen, Germany) and stored at −80°C until assay. Culture supernatant concentrations of IL-6 and TNF-α were measured by quantitative sandwich enzyme immunoassay techniques (ELISA) using commercial kits (Quantitine R&D Systems, Abingdon, UK).
Method of genotyping
DNA extraction and polymerase chain reaction (PCR)
Genomic DNA was extracted from 5 ml peripheral blood leucocytes using the QIAmap Blood Kit (Qiagen GmbH, Hilden, Germany).
Ready-To-Go PCR Beads (Amersham Pharmacia Biotech GmbH, Freiburg, Germany) were used for all PCR reactions. In a final volume of 25 µl PCR mixture (containing 1·5 units of Taq DNA polymerase, 10 mm Tris-HCl, 50 mm KCl, 1·5 mm MgCl2, 200 µm of each dNTP and 0·4 µm of each primer) approximately 50 ng DNA template was added. The reaction was carried out in a GeneAmp PCR System 2400 (Perkin Elmer, PE Applied Biosystems GmbH, Weiterstadt, Germany) thermal cycler. The amplification conditions were optimized by changing the annealing temperature and time. In general, after an initial melting time of 5 min, samples were subjected to 30 cycles of 94°C for 30 s, 58°C for 45 s and 72°C for 30 s. A final elongation at 70°C for 3 min was added to the programme. The size of the PCR products was checked on an agarose gel.
Restriction-fragment-length-polymorphism (RFLP) markers
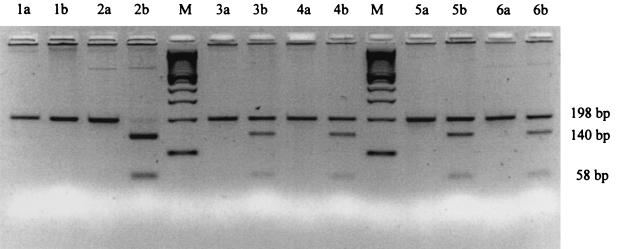
Amplification of the TNF-α promoter region polymorphism at the −308 and −238 positions was performed using primers of TNFA-NcoI and TNFA-BamHI [28]. Primer IL6-SfaNI was used to amplify the polymorphism at position −174 of the IL-6 gene promoter region [29]. Primer TNF-β NcoI was used to amplify the polymorphism at the intron of the TNF-β gene [30]. The PCR products were digested at 37°C overnight using specific restriction enzymes. The TNF-α −308, −238 and TNF-β intron polymorphism was specifically detected using NcoI and BamHI (Roche Diagnostics GmbH, Mannheim, Germany), and the IL-6 G/A polymorphism was confirmed by digestion with the SfaNI restriction enzyme (New England Biolabs GmbH, Frankfurt am Main, Germany). After ethidium bromide staining, the allele size could be observed on a 3·5% agarose gel (Fig. 1)
Fig. 1.
IL-6 SfaNI restriction pattern. M = 100 bp ladder; Lanes 1–6 are patient samples. a: PCR products before SfaNI restriction. b: PCR products after SfaNI restriction. Lane 1 homozygous for the absence of the SfaNI restriction site (198 bp); Lane 2: homozygous for the presence of the SfaNI restriction site (140/58 bp); Lane 3: heterozygous for the presence and absence of the SfaNI restriction site (198 and 140/58 bp).
Microsatellite marker
The microsatellite marker used for the BAT2 gene is a dinucleotide repeat on chromosome 6 (6p21.3) with 15 alleles. Primer BAT2-misa was used for PCR [31]. The forward prime was 5′ end-labelled with a fluorescent dye (6-Fam). PCR products were analysed on an ABI Prism 377 automated fluorescent fragment analyser (Perkin Elmer, PE Applied Biosystems GmbH, Weiterstadt, Germany). The PCR product (2 µl) was added to 2 µl deionized formamide, and 1·5 µl loading buffer containing Genescan-350 ROX internal lane standard. The loading mix was then denatured at 95°C for 3 min and placed on ice before electrophoresis at 40 W on a 6% polyacrylamide sequencing gel. Genescan software (Perkin Elmer, GeneScan™ Analysis 2.0.2) was used for allele sizing.
Statistical analysis
We used the Statistical Package for the Social Sciences (SPSS) for analysing the data. Data are presented as means ±s.e.m. In study I, serial data from each group were evaluated by analysis of variance for repeated measurements. Post-hoc comparisons were performed using t-tests. Pearson correlation coefficients were computed for determining associations between measurements. In study II, the chi-squared test was performed. The significance level was set at α = 0·05.
Results
Descriptions of the study participants from both studies as well as the details of surgery are given in Table 1.
Table 1.
Description of the study participants
| Group 1 (n = 10) | Group 2 (n = 10) | |
|---|---|---|
| Gender | Male | Male |
| Age (years)* | 57·9 ± 7·5 | 62·0 ± 7·6 |
| Duration of anaesthesia (min)* | 288·5 ± 50·3 | 271·5 ± 32·7 |
| Duration of surgery (min)* | 203·2 ± 47·4 | 195·8 ± 27·4 |
| Duration of bypass (min)* | 76·1 ± 28·0 | 80·9 ± 20·9 |
| Temperature during bypass (°C)* | 31·3 ± 1·7 | 30·8 ± 1·9 |
Group 1, patients with low IL-6 secretion; Group 2, patients with high IL-6 secretion.
Values are given as mean ±s.d.
Individual specific variability of perioperative cytokine production (study I)
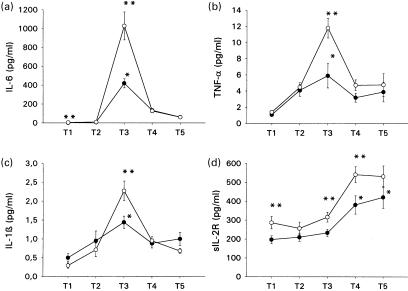
There were no significant differences between both groups concerning duration of anaesthesia, surgery and cardiopulmonary bypass times. Peak concentrations of IL-6, TNF-α and IL-1β were found postoperatively on the day of surgery in both groups. On the day of surgery (postoperatively), serum levels of IL-6, TNF-α and IL-1β were significantly elevated compared to preoperative measurements in both groups. On the first postoperative day, serum concentrations of sIL-2R were significantly increased compared to preoperative values in both groups (Fig. 2).
Fig. 2.
Perioperative serum concentrations of IL-6 (a) TNF-α (b), IL-1β (c) and sIL-2R (d) in patients undergoing coronary artery bypass grafting surgery. (T1) Preoperatively at 6–8 p.m. on the evening before surgery, (T2) 90 min following incision, (T3) at 6–8 p.m. postoperatively on the day of surgery, (T4) at 6–8 p.m. on the first postoperative day ans (T5) at 6–8 p.m. on the second postoperative day. Results are expressed as means ±s.e.m. *Significant differences compared to preoperative values; **significant differences between both groups. Group 1, patients with IL-6 secretion at T3 below the median of the study group. Group 2, patients with IL-6 secretion at T3 above the median of the study group. •, Group 1; ○, group 2.
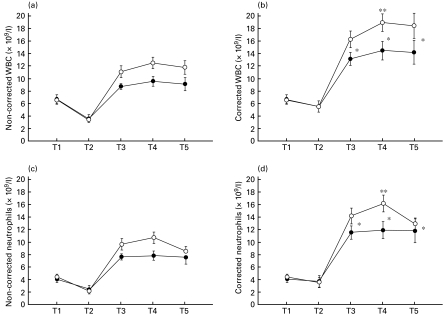
Intra-operatively and postoperatively, leucocyte and neutrophil counts corrected for haemodilution were significantly higher compared to noncorrected values. On the day of surgery (postoperatively) and on the first and second postoperative days, leucocyte and neutrophil counts corrected for haemodilution were significantly higher compared to preoperative measurements (Fig. 3)
Fig. 3.
Perioperative white blood cell counts (a) and neutrophils (c) without and with (b,d) correction for haemodilution in patients undergoing coronary artery bypass grafting surgery. (T1) Preoperatively at 6–8 p.m. on the evening before surgery, (T2) 90 min following incision, (T3) at 6–8 p.m. postoperatively on the day of surgery, (T4) at 6–8 p.m. on the first postoperative day and (T5) at 6–8 p.m. on the second postoperative day. Results are expressed as means ±s.e.m. *Significant differences compared to preoperative values; **significant differences between both groups. Group 1, patients with IL-6 secretion at T3 below the median of the study group. Group 2, patients with IL-6 secretion at T3 above the median of the study group. •, Group 1; ○, group 2.
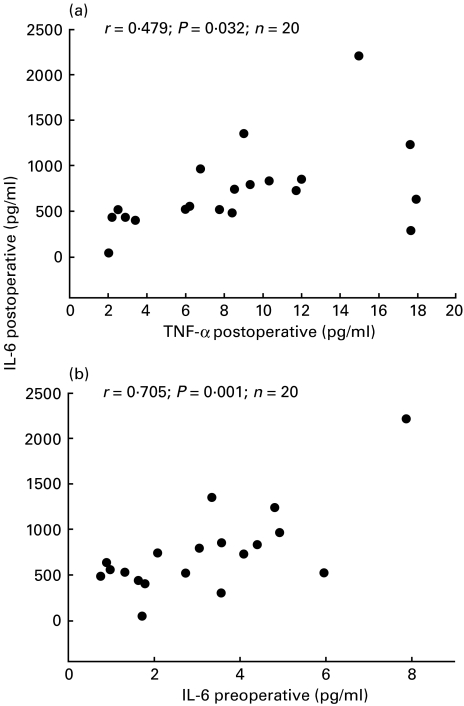
On the first postoperative day, leucocyte and neutrophil counts were significantly increased in patients of group 2 compared to those of group 1 (Fig. 3b,d). Postoperatively, on the day of surgery and preoperatively (3·9 ± 1·9 pg/ml of group 2 versus 2·2 ± 1·5 pg/ml of group 1), serum concentrations of IL-6 in group 2 patients were significantly higher compared to those of group 1 (Fig. 2a). Preoperatively, on the day of surgery (postoperatively), and on the first postoperative day, serum concentrations of sIL-2R in patients of group 2 patients were significantly higher than those of group 1 (Fig. 2d). On the day of surgery (postoperatively), serum levels of TNF-α and IL-1β were significantly higher in patients of group 2 compared to the respective values of group 1 patients (Fig. 2b,c). On the day of surgery (postoperatively), serum levels of IL-6 in the entire study group were significantly correlated with serum concentrations of IL-1β, sIL-2R (rDOS IL-6/IL-1β = 0·474; P = 0·035, rDOS IL-6/sIL-2R = 0·449; P = 0·049) and TNF-α (rDOS IL-6/TNF-α = 0·479; P = 0·032; Fig. 4). Preoperatively, serum levels of IL-6 in the study group were significantly correlated with serum concentrations of IL-6 on the day of surgery (postoperatively) (rpreop/DOS IL-6 = 0·705; P = 0·001; Fig. 4).
Fig. 4.
Pearson correlation coefficients between postoperative IL-6 concentrations, postoperative TNF-α concentrations (a) and preoperative IL-6 levels (b) of 20 patients undergoing coronary artery bypass grafting surgery.
Results of study II
For both study groups, in vitro secretion of IL-6 and TNF-α, preoperative and postoperative IL-6 levels and G/C polymorphism of the IL-6 gene at position −174 are all given in Table 2. All of the 15 patients investigated in the second study were successfully genotyped (Table 2 and Table 3). Six out of seven patients with preoperative low serum secretion of TNF-α (n = 7) were homozygous G/G and one patient showed G/A at position −308 of the TNF-α gene. Four patients from the group with a high preoperative serum secretion of TNF-α showed homozygous G/G (n = 4) and the other half were heterozygous G/A (n = 4) at position −308 of the TNF-α gene (Table 3). There was no significant difference between the two groups (P = 0·143). Two patients from the group with a high preoperative serum secretion of TNF-α (n = 8) showed 1/1 and the other six patients were heterozygous (1/2) at the intron of the TNF-β gene (Table 3).
Table 2.
Preoperative and postoperative IL-6 levels of the individual patients of the study groups, in vitro secretion of IL-6 (pg/ml) and TNF-α (pg/ml), and G/C polymorphism of the IL-6 gene at position −174
| IL-6 concentration (pg/ml) | ||||||
|---|---|---|---|---|---|---|
| Subject | Preoperative | Postoperative | IL-6 promoter region −174 Sfa NI | IL-6 concentration in vitro (pg/ml) | TNF-α concentration in vitro (pg/ml) | |
| Low | 1 | 1·79 | 398·85 | G/C | 2255·7 | 960·5 |
| 2 | 5·96 | 515·43 | C/C | 4462·7 | 948·5 | |
| 3 | 1·33 | 520·00 | C/C | 4305·3 | 1029·9 | |
| 4 | 1·61 | 432·04 | G/C | 2181·0 | 907·4 | |
| 5 | 1·72 | 42·59 | G/C | 3872·4 | 657·2 | |
| 6 | 0·77 | 479·74 | G/C | 3093·8 | 760·8 | |
| 7 | 3·56 | 294·09 | G/C | 4083·7 | 658·6 | |
| High | 1 | 4·92 | 958·57 | G/C | 4464·0 | 1796·5 |
| 2 | 4·08 | 720·48 | C/C | 3807·0 | 578·2 | |
| 3 | 2·08 | 734·56 | G/C | 3233·7 | 647·1 | |
| 4 | 4·80 | 1232·53 | G/C | 2761·6 | 422·5 | |
| 5 | 3·34 | 1343·57 | G/C | 2349·9 | 339·6 | |
| 6 | 3·05 | 787·86 | G/C | 4229·0 | 1318·3 | |
| 7 | 7·87 | 2202·52 | G/G | 3719·2 | 865·3 | |
| 8 | 0·89 | 632·00 | G/C | 2664·3 | 2500·8 | |
Low, patients with postoperative low secretion of IL-6; high, patients with postoperative high secretion of IL-6.
Table 3.
Serum TNF-α concentrations according to TNF-α −238 G/A, −308 G/A genotypes, BAT2 microsatellite and TNF-β intron polymorphism in 15 patients with cardiac surgery and CPB from study I
| TNF-α concentration (pg/ml) | RFLP marker of TNF‐α promoter region | ||||||
|---|---|---|---|---|---|---|---|
| Subject | Preoperative | Postoperative | −238 BamHI | −308 NcoI | Microsatellite marker of HLA region BAT2 | TNF-β intron NcoI | |
| Low | 1 | 1·00 | 3·40 | G/G | G/G | 140/150 | 1/2 |
| 2 | 1·05 | 5·96 | G/G | G/A | 138/140 | 1/2 | |
| 3 | 1·30 | 2·89 | G/G | G/G | 140/146 | 1/2 | |
| 4 | 0·72 | 2·03 | G/G | G/G | 140/150 | 1/2 | |
| 5 | 1·00 | 2·49 | G/G | G/G | 140/150 | 1/2 | |
| 6 | 1·28 | 8·51 | G/G | G/G | 140/150 | 1/2 | |
| 7 | 1·05 | 14·99 | G/G | G/G | 142/142 | 1/2 | |
| High | 1 | 1·40 | 8·39 | G/G | G/G | 140/150 | 1/2 |
| 2 | 1·91 | 17·87 | G/G | G/G | 140/142 | 1/2 | |
| 3 | 1·40 | 17·66 | G/G | G/A | 138/138 | 1/2 | |
| 4 | 1·64 | 11·72 | G/G | G/G | 138/142 | 1/2 | |
| 5 | 1·40 | 17·60 | G/G | G/A | 140/152 | 1/2 | |
| 6 | 1·46 | 9·00 | G/G | G/A | 138/142 | 1/1 | |
| 7 | 1·50 | 9·32 | G/G | G/A | 140/140 | 1/1 | |
| 8 | 1·40 | 6·76 | G/A | G/G | 138/140 | 1/2 | |
Low, patients with preoperative low secretion of TNF-α; high, patients with preoperative high secretion of TNF-α.
At position −174 of the IL-6 gene, six out of eight patients with low postoperative IL-6 secretion were heterozygous G/C, and the other two were homozygous C/C. Five out of seven patients with postoperative high IL-6 secretion were heterozygous G/C, while one was C/C homozygous and the other G/G homozygous.
The BAT2 microsatellite marker is a dinucleotide repeat and contains 15 alleles. In our samples, seven alleles ranging from 138 bp to 150 bp were determined. The genotyping results from 15 patients are shown in Table 3. Four out of seven patients with a preoperative low secretion of TNF-α showed the genotype 140/150. Only one out of eight patients with a high preoperative secretion of TNF-α showed the genotype 140/150 (Table 3). Statistical analysis showed no significant difference between the two groups (P = 0·232).
Discussion
Serum IL-6, TNF-α, IL-1β and sIL-2R concentrations for patients of study I (undergoing elective CABG surgery) were determined at five sampling points. In patients from study II, in vitro cytokine secretion after LPS-stimulation, G/C polymorphism of the IL-6 gene at position −174, G/A polymorphism of the TNF-α gene at position −308 and −238, the microsatellite BAT2 marker and TNF-β intron were all investigated.
There were no significant differences between the study groups concerning demographic factors, the surgical procedure performed, anaesthetic technique, duration of surgery or CPB.
Consistent with our previous reports on perioperative cytokine release and the results of other studies, peak concentrations of IL-6, TNF-α and IL-1β were found on the day of surgery after operation [32–34]. On the first and second postoperative days, serum concentrations of sIL-2R were significantly elevated compared to pre‐ and intra-operative values. This result is consistent with the findings of others who reported an increase in sIL-2R during the postoperative period [35].
In accordance with our previous reports concerning the effects of perioperative haemodilution on cytokine measurements in patients undergoing cardiac surgery, we have now also documented a significant effect of haemodilution on total WBC and neutrophil counts [17]. Consistent with the findings of others, leucocytes and neutrophils in our study were significantly increased after cardiac surgery [36–38].
Preoperatively, serum concentrations of IL-6 and sIL-2R in group 2 patients were significantly higher compared to group 1 patients. On the day of surgery (postoperatively), serum concentrations of IL-6, TNF-α, IL-1β and sIL-2R were significantly higher in group 2 patients compared to group 1 patients. On the first postoperative day, serum concentrations of sIL-2R as well as WBC and neutrophil counts were significantly higher in patients of group 2 compared to those of group 1. On the day of surgery (postoperatively), serum levels of IL-6 in the entire study group were significantly correlated with serum concentrations of TNF-α, IL-1β and sIL-2R.
Our results indicate that the postoperative release of IL-6 is positively related to the release of TNF-α, IL-1β and sIL-2R. This is consistent with the reports of others who have shown that the up-regulation of IL-6 synthesis is influenced by the release other inflammatory cytokines [5]. In critically ill patients at risk of inflammatory organ injury, IL-6 is a marker of disease severity [13]. Earlier studies by Oka et al. [39] on postoperative changes in cancer patients suggested that circulating IL-6 is a clinically useful marker for the detection and prediction of postoperative complications [39]. Until now it has not been possible to predict individual cytokine responses to surgical stress [40]. Our result showing that postoperative IL-6 release was significantly correlated with preoperative IL-6 measurements suggests that there may be a significant relationship between preoperative IL-6 release and postoperative IL-6 secretion. The biological relevance of our result remains to be established and has to be considered with caution due to our small sample size.
The main findings of our study are that the postoperative release of IL-6 (on the day of surgery) is accompanied by a higher secretion of other cytokines such as IL-1β, TNF-α and sIL-2R, and higher leucocyte and neutrophil counts. These results suggest that inter-individually there may be different patterns of cytokine release in patients undergoing cardiac surgery with CPB, and underline the role of IL-6 as a marker of inflammation. Our results indicate that there might be inter-individual differences in the inflammatory response to cardiac surgery in the investigated patients.
Inter-individual differences in the reactivity of cytokine release imply that there may be individual-specific reactions of the cytokine system to cardiac surgery. Individual-specific, uncontrolled cytokine responses after anaesthesia and surgery have been described elsewhere [40]. This has led to the hypothesis of different ‘responder’ types to inflammatory stimuli, such as surgical interventions. High responders show a greater amount of cytokine secretion in a certain stimulus situation, whereas low responders show an opposite reaction pattern [40]. The molecular mechanisms underlying the low responder reaction are not yet fully understood [41].
Tumour necrosis factor α is regulated both transcriptionally and post-transcriptionally. Sequence polymorphisms have been identified that could play a part in the transcriptional regulation of the gene. In the first LT-α (TNF-β) intron there is a NcoI RFLP polymorphism, named TNFB*1 in the presence of the restriction-site and TNFB*2 in its absence. TNFB*2 is the more frequent allele and seems to be correlated with higher TNF production [42]. The G/A polymorphism within the TNF-α gene promoter region of position −308 was reported to affect the ability of nuclear factors to bind to the −308 region [43]. The A allele may be responsible for a general increase in transcriptional activity compared to the G allele at −308. In other words, the A allele at −308 is associated with higher constitutive and inducible levels of TNF-α [44].
In our study, four heterozygous patients of genotype G/A at −308 were observed in the ‘high TNF-α group’(n = 8) and only one subject showed the G/A genotype in the ‘low TNF-α group’ (n = 7). There were no statistically significant differences between these two groups (P = 0·143), possibly because of the limited sample size. Our data agreed with the results reported by other authors, and suggested that the inter-individual differences in cytokine release in our patients undergoing cardiac surgery with CPB could in part be genetically determined. There was no evidence of any difference in G/A polymorphism at −238 in either group. The real function of any variation at this position remains unclear [45].
Since the TNF-α gene is located within the major histocompatibility complex (MHC) region, there has been much speculation surrounding a potential genetic association between TNF/HLA gene alleles and disease susceptibility. Several authors have reported an association between −308 polymorphism of the TNF-α gene and the HLA haplotype [46,47]. In our study, a 15 allelic polymorphic microsatellite marker BAT2, also out of the MHC, was used to investigate the relationship to the TNF-α production. The BAT2 micosatellite marker lies between the HLA-B locus at the telomeric end of 6p21.3 and the HLA-DR locus at its centromeric end [31]. It has been reported that the BAT2 and TNF-α microsatellites are in a useful location with regards to a part of the MHC which contains an important set of inducible, inflammatory genes [31]. Our preliminary data shows that the genotype 140/150 seems to be more frequent in the group with lower TNF-α levels – four out of seven subjects had the 140/150 genotype. In the group with higher TNF-α levels, the 140/150 genotype appeared only once (n = 8). The four patients with low TNF-α secretion who also exhibited a 140/150 genotype for the BAT2 marker showed a homozygous G at −308 of the TNF-α promoter region (Table 3). More genotypes need to be investigated in order to confirm this observation. The TNF-α microsatellite marker is a highly polymorphic marker with 13 alleles [48]. We were not able to acquire sufficient allelic information to interpret the genotyping results of the TNF-α microsatellite marker (data not presented) because of our limited sample size.
Fishman [49] reported that the G/C polymorphism of the IL-6 gene at position −174 is associated with different transcription rates, and that subjects homozygous for the C allele at position −174 showed lower serum IL-6 levels compared to G/C or G/G subjects. Our data could not confirm Fishman's observation. A median level of IL-6 release was observed in subjects with a C/C genotype at −174 of the IL-6 gene. A single subject with the G/G genotype showed a very high serum IL-6 concentration. Most of our subjects were heterozygous G/C at −174. The cytokine network is complex, and it is well known that the inflammatory response to cardiac surgery with CPB is a multifactorial phenomenon. Clinically, the serum concentration of a particular cytokine is a product of the influence of many factors and interactions. Whether homozygous C at −174 of the IL-6 gene is really associated with lower serum IL-6 levels needs further studies.
In summary, the inter-individual differences in IL-6 release among patients undergoing CABG surgery with CPB are accompanied by differences in the release of other cytokines such as TNF-α, IL-1β and sIL-2R. The genetic disposition might partly determine a subject's response to an inflammatory stimulus. The close relationships between the releases of IL-6, TNF-α, sIL-2R and IL-1β in the postoperative period suggest that there is a partly individual-specific pattern of cytokine release in patients undergoing CABG surgery with CPB. Further studies are necessary to clarify the stimulation characteristics, and the complex interactions between the individual inflammatory responses, of patients undergoing cardiac surgery.
Acknowledgments
We would like to thank Mrs Bianca Sternberg, Institute of Immunology and Transfusion Medicine, Medical University of Luebeck and Mrs A. Daher for the processing of the cytokine assays, Professor Dr H. H. Sievers, Chairman of the Department of Cardiac Surgery, who gave permission to perform the study in his department, PD Dr H. J. Friedrich, Associate Professor of Biomedical Statistics, for his help with the statistical analysis of the data and Mrs Angret Daher, for her help with the manuscript.
References
- 1.Asimakopoulos G. Mechanisms of the systemic inflammatory response. Perfusion. 1999;14:269–77. doi: 10.1177/026765919901400406. [DOI] [PubMed] [Google Scholar]
- 2.Herskowitz A, Mangano DT. Inflammatory cascade. A final common pathway for perioperative injury? Anesthesiology. 1996;85:957–60. doi: 10.1097/00000542-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Cain BS, Meldrum DR, Dinarello CA, et al. Tumor necrosis factor-α and interleukin-1β synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–18. doi: 10.1097/00003246-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Hennein HA, Ebba H, Rodriguez JL, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–35. [PubMed] [Google Scholar]
- 5.Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766–82. doi: 10.1097/00000539-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Abe K, Nishimura M, Sakakibara T. Interleukin-6 and tumour necrosis factor during cardiopulmonary bypass. Can J Anaesth. 1994;41:876–7. doi: 10.1007/BF03011610. [DOI] [PubMed] [Google Scholar]
- 7.Udelsman R, Holbrook NJ. Endocrine and molecular responses to surgical stress. Curr Prob Surg. 1994;31:653–720. [PubMed] [Google Scholar]
- 8.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 9.Kaushansky K, Broudy VC, Harlan JM, Adamson JW. Tumor necrosis factor-α and tumor necrosis factor-β (lymphotoxin) stimulate the production of granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, and IL-1 in vivo. J Immunol. 1988;141:3410–5. [PubMed] [Google Scholar]
- 10.Zhang Y, Lin JX, Yip YK, Vilcek J. Stimulation of interleukin-6 mRNA levels by tumor necrosis factor and interleukin-1. Ann NY Acad Sci. 1989;557:548–9. [Google Scholar]
- 11.Sawa Y, Shimazaki Y, Kadoba K, et al. Attenuation of cardiopulmonary bypass-derived inflammatory reactions reduces myocardial reperfusion injury in cardiac operations. J Thorac Cardiovasc Surg. 1996;111:29–35. doi: 10.1016/S0022-5223(96)70398-7. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77:110–7. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JL, Moore EE, Tamura DY, Zallen G, Biffl WL, Silliman CC. Interleukin-6 augments neutrophil cytotoxic potential via selective enhancement of elastase release. J Surg Res. 1998;76:91–4. doi: 10.1006/jsre.1998.5295. [DOI] [PubMed] [Google Scholar]
- 14.Torpy DJ, Bornstein SR, Chrousos GP. Leptin and interleukin-6 in sepsis. Horm Metab Res. 1998;30:726–9. doi: 10.1055/s-2007-978967. [DOI] [PubMed] [Google Scholar]
- 15.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–6. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 16.Misoph M, Babin-Ebell J. Interindividual variations in cytokine levels following cardiopulmonary bypass. Heart Vessels. 1997;12:119–27. doi: 10.1007/BF02767129. [DOI] [PubMed] [Google Scholar]
- 17.Roth-Isigkeit A, Borstel T, Seyfarth M, Schmucker P. Perioperative serum levels of tumour-necrosis-factor alpha (TNF-α), IL-1ß, IL-6, IL-10 and soluble IL-2 receptor in patients undergoing cardiac surgery with cardiopulmonary bypass without and with correction for haemodilution. Clin Exp Immunol. 1999;118:242–6. doi: 10.1046/j.1365-2249.1999.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler J, Pillai R, Rocker GM, Westaby S, Parker D, Shale DJ. Effect of cardiopulmonary bypass on systemic release of neutrophil elastase and tumor necrosis factor. J Thorac Cardiovasc Surg. 1993;105:25–30. [PubMed] [Google Scholar]
- 19.Westaby S. Organ dysfunction after cardiopulmonary bypass. A systemic inflammatory reaction initiated by the extracorporeal circuit. Intens Care Med. 1987;13:89–95. doi: 10.1007/BF00254791. [DOI] [PubMed] [Google Scholar]
- 20.Marti F, Munoz J, Peiro M, et al. Higher cytotoxic activity and increased levels of IL-1β, IL-6, and TNF-α in patients undergoing cardiopulmonary bypass. Am J Hematol. 1995;49:237–9. doi: 10.1002/ajh.2830490310. [DOI] [PubMed] [Google Scholar]
- 21.Tang GJ, Huang SL, Yien HW, et al. Tumor necrosis factor gene polymorphism and septic shock in surgical infection. Crit Care Med. 2000;28:2733–6. doi: 10.1097/00003246-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Abraham LJ, Kroeger KM. Impact of the −308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J Leukocyte Biol. 1999;66:562–6. doi: 10.1002/jlb.66.4.562. [DOI] [PubMed] [Google Scholar]
- 23.Mira JP, Cariou A, Grall F, et al. Association of TNF2, a TNF-α promoter polymorphism, with septic shock susceptibility and mortality. A multicenter study. JAMA. 1999;282:561–8. doi: 10.1001/jama.282.6.561. [DOI] [PubMed] [Google Scholar]
- 24.Louis E, Franchimont D, Piron A, et al. Tumour necrosis factor (TNF) gene polymorphism influences TNF-α production in lipopolysaccharide (LPS) –stimulated whole blood cell culture in healthy humans. Clin Exp Immunol. 1998;113:401–6. doi: 10.1046/j.1365-2249.1998.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99:303–10. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor KM, Bain WH, Jones JV, Walker MS. The effect of hemodilution on plasma levels of cortisol and free cortisol. J Thorac Cardiovasc Surg. 1976;72:57–61. [PubMed] [Google Scholar]
- 27.Brand JM, Kirchner H, Poppe C, Schmucker P. Cytokine release and changes of peripheral blood mononuclear cells by general anaesthesia. Anaesthesist. 1998;47:379–86. doi: 10.1007/s001010050573. [DOI] [PubMed] [Google Scholar]
- 28.Skoog T, van'T Hooft FM, Kallin B, et al. A common functional polymorphism (C→A substitution at position −863) in the promoter region of the tumour necrosis factor-α (TNF-α) gene associated with reduced circulating levels of TNF-α. Hum Mol Genet. 1999;8:1443–9. doi: 10.1093/hmg/8.8.1443. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Real JM, Broch M, Vendrell J, et al. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49:517–20. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- 30.Shimura T, Hagihara M, Takebe K, et al. The study of tumor necrosis factor beta gene polymorphism in lung cancer patients. Cancer. 1994;73:1184–8. doi: 10.1002/1097-0142(19940215)73:4<1184::aid-cncr2820730410>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher G, Eskdale J, Miller S. A highly polymorphic microsatellite marker in the human MHC class III region, close to the BAT2 gene. Immunogenetics. 1997;46:357–8. doi: 10.1007/s002510050285. [DOI] [PubMed] [Google Scholar]
- 32.Roth-Isigkeit A, Schwarzenberger J, v Borstel T, et al. Perioperative cytokine release during coronary artery bypass grafting in patients of different ages. Clin Exp Immunol. 1998;114:26–32. doi: 10.1046/j.1365-2249.1998.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denizot Y, Lorgeot V, Cornu E, Nathan N. Plasma leukaemia inhibitory factor, interleukin 6 and soluble interleukin 6 receptor levels during cardiopulmonary bypass with extracorporeal circulation. Cytokine. 1998;10:303–6. doi: 10.1006/cyto.1997.0285. [DOI] [PubMed] [Google Scholar]
- 34.Cruickshank AM, Fraser WD, Burns HJG, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci. 1990;79:161–5. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- 35.Lahat N, Shtiller R, Zlotnick AY, Merin G. Early IL-2/sIL-2R surge following surgery leads to temporary immune refractoriness. Clin Exp Immunol. 1993;92:482–6. doi: 10.1111/j.1365-2249.1993.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diegeler A, Tarnok A, Rauch T, et al. Changes of leukocyte subsets in coronary artery bypass surgery: cardiopulmonary bypass versus ‘off-pump’ techniques. Thorac Cardiovasc Surg. 1998;46:327–32. doi: 10.1055/s-2007-1010247. [DOI] [PubMed] [Google Scholar]
- 37.Hiesmayr MJ, Spittler A, Lassnigg A, et al. Alterations in the number of circulating leukocytes, phenotype of monocyte and cytokine production in patients undergoing cardiothoracic surgery. Clin Exp Immunol. 1999;115:315–23. doi: 10.1046/j.1365-2249.1999.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu SH, Hu RH, Lee YC, Chen KT. Changes of white blood cells, immunosuppressive acidic protein, and interleukin-2 receptor after open heart surgery. Thorac Cardiovasc Surg. 1995;43:94–8. doi: 10.1055/s-2007-1013778. [DOI] [PubMed] [Google Scholar]
- 39.Oka Y, Murata A, Nishijima J, et al. Circulating interleukin 6 as a useful marker for predicting postoperative complications. Cytokine. 1992;4:298–304. doi: 10.1016/1043-4666(92)90070-8. [DOI] [PubMed] [Google Scholar]
- 40.Masterson GR, Hunter JM. Does anaesthesia have long-term consequences? Br J Anaesth. 1996;77:569–71. doi: 10.1093/bja/77.5.569. [DOI] [PubMed] [Google Scholar]
- 41.Schraut W, Wendelgass P, Calzada-Wack JC, Frankenberger M, Ziegler-Heitbrock HW. TNF gene expression in monocytes of low and high responder individuals. Cytokine. 1997;9:206–11. doi: 10.1006/cyto.1996.0155. [DOI] [PubMed] [Google Scholar]
- 42.Rink L, Kirchner H. Recent progress in the tumor necrosis factor-α field. Int Arch Allergy Imm. 1996;111:199–209. doi: 10.1159/000237369. [DOI] [PubMed] [Google Scholar]
- 43.Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–9. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 44.Wilson AG, Gordon C, di Giovine FS, et al. A genetic association between systemic lupus erythematosus and tumor necrosis factor alpha. Eur J Immunol. 1994;24:191–5. doi: 10.1002/eji.1830240130. [DOI] [PubMed] [Google Scholar]
- 45.Pociot F, D'Alfonso S, Compasso S, Scorza R, Richiardi PM. Functional analysis of a new polymorphism in the human TNF alpha gene promoter. Scand J Immunol. 1995;42:501–4. doi: 10.1111/j.1365-3083.1995.tb03686.x. [DOI] [PubMed] [Google Scholar]
- 46.Jongeneel CV, Briant L, Udalova IA, Sevin A, Nedospasov SA, Cambon-Thomsen A. Extensive genetic polymorphism in the human tumor necrosis factor region and relation to extended HLA haplotypes. Proc Natl Acad Sci USA. 1991;88:9717–21. doi: 10.1073/pnas.88.21.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mascher B, Schmitt W, Csernok E, et al. Polymorphisms in the tumor necrosis factor genes in Wegener's granulomatosis. Exp Clin Immunogenet. 1997;14:226–33. [PubMed] [Google Scholar]
- 48.McDonnell GV, Kirk CW, Middleton D, et al. Genetic association studies of tumour necrosis factor α and β and tumour necrosis factor receptor 1 and 2 polymorphisms across the clinical spectrum of multiple sclerosis. J Neurol. 1999;246:1051–8. doi: 10.1007/s004150050511. [DOI] [PubMed] [Google Scholar]
- 49.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]