Abstract
We have previously shown that dendritic cells (DC), upon being pulsed in vitro with encephalitogenic myelin basic protein peptide 68–86 (MBP 68–86) and injected subcutaneously (s.c.) back to healthy Lewis rats, transfer immune tolerance to experimental allergic encephalomyelitis (EAE) induced by immunization with MBP 68–86 and Freund's complete adjuvant (FCA). We here assumed that DC become pulsed in EAE rats, and that expansion in vitro of such ‘in vivo pulsed EAE-DC’ might also have the capacity to induce immune tolerance to EAE, thereby eliminating the need for in vitro pulsing of DC with autoantigens which are still unknown in many autoimmune diseases in the human. In the present study, EAE-DC were generated from bone marrow of Lewis rats, with EAE induced with MBP 68–86 + FCA, and expanded in vitro by culture with GM-CSF and IL-4. In comparison with DC from normal rats, EAE-DC exhibited higher viability in the absence of growth factors, and presented specific antigen to naïve T cells in vitro. The DC derived from both EAE and healthy rats stimulated strong proliferation in an antigen-independent manner, lasting for 4 weeks after DC were s.c. injected into healthy rats. During this time, injection of EAE-DC did not induce clinical EAE. However, when these rats were immunized with MBP 68–86 + FCA, subsequent EAE was dramatically suppressed, and was associated with increased IFN-γ expression, nitric oxide production, gradually reduced proliferation and cell apoptosis, compared with PBS-injected control EAE rats. LPS-treated DC did not induce tolerance, suggesting that the tolerance is mediated by an immature stage of DC. These observations support the hypothesis that EAE-DC can transfer immune tolerance to EAE, thereby omitting the step of characterizing specific autoantigen. Omitting the step of loading DC with antigen not only eliminates the extremely complex procedure of defining pathogenically-relevant autoantigens, but also avoids the risk of inducing immunogenicity of DC in the treatment of autoimmune diseases.
Keywords: Dendritic cells, tolerance, experimental allergic encephalomyelitis, nitric oxide, apoptosis
Introduction
Accumulating evidence suggests that the process of determining the phenotype (immunity or tolerance) of immune responses is initiated by antigen-presenting cells (APC) [1–3]. Dendritic cells (DC), being the most potent professional APC, not only activate lymphocytes, but can also tolerize T cells to specific antigens, thereby minimizing autoimmune reactions [4]. In mice, in vivo treatment with Flt3L (a growth factor that expands DC in vivo), enhanced the induction of oral tolerance [5]. DC pre-cultured with IL-10 induced a state of antigen-specific anergy in CD4+ T cells [6] and CD8+ T cells [7]. Transfer of pancreas lymph node DC modulated autoimmunity and limited diabetes expression by the induction of regulatory cells in the NOD mouse [8]. Polarization of an immune response towards tolerance or immunity is dictated by the interactions between T cells and DC, which in turn are modulated by the expression of distinct surface molecules. FasL transfection of DC markedly augmented their capacity to induce apoptosis of Fas+ cells [9].
We previously generated bone marrow (BM) DC from healthy Lewis rats in the presence of GM-CSF and IL-4, pulsed the DC with the encephalitogenic MBP 68–86, and injected these DC subcutaneously (s.c.) back to healthy Lewis rats. These rats developed resistance to experimental allergic encephalomyelitis (EAE) induced by immunization with MBP 68–86 and Freund's complete adjuvant (FCA) [10]. Here, we hypothesize that DC from EAE rats may already have been pulsed in vivo with the specific antigen used for EAE induction. By expanding such ‘EAE-DC’ in vitro and injecting them back to healthy rats, tolerance to EAE might follow. If so, this could omit the most complex or, at present, almost impossible procedure of identifying specific antigens involved in several human autoimmune diseases, and could result in active or specific immunotherapy for MS and other autoimmune-related diseases. In the present study, we demonstrate that EAE-DC can present specific antigen to naive T cells in vitro, and induce immune tolerance to EAE when injected s.c. to Lewis rats prior to immunization with MBP 68–86 + FCA.
Materials and methods
Animals and reagents
Male Lewis rats, weighing 150–180 g, were obtained from Zentralinstitut fur Versuchstierzucht, Hannover, Germany. Guinea pig MBP 68–86 (YGSLPQKSQRSQDENPV) was produced in an automatic Tecan-Syro Synthesizer (Multisytech, Bochum, Germany). Monoclonal anti-rat MHC class II (OX-6) was purified from culture supernatant fluids of hybridomas [11]. Monoclonal anti-rat B7–1 and B7–2, FITC anti-rat IFN-γ and PE anti-rat IL-4 were from PharMingen (San Diego, CA). Monoclonal anti-rat IFN-γ (DB1) and polyclonal anti‐rat IFN-γ were from Innogenetics (Ghent, Belgium). Lipopolysaccharide (LPS) was from Sigma (St Louis, MO).
Induction of EAE
EAE was induced for two purposes: (i) to incite pulsing of DC in vivo with autoantigen and (ii) to observe the effects of EAE-DC-induced tolerance to EAE. Lewis rats were immunized in both hind footpads with 200 µl of inoculum containing 25 µg of MBP 68–86, 2 mg Mycobacterium tuberculosis (strain H37RA; Difco, Detroit, MI), 100 µl saline and 100 µl Freund's incomplete adjuvant (Difco). On day 7 post-immunization (p.i.), BM DC representing ‘in vivo pulsed DC’ were prepared.
For the clinical evaluation of EAE and DC-induced tolerance to EAE, clinical scores of EAE were graded as follows: 0, asymptomatic; 1, loss of distal half of tail tonicity; 2, loss of entire tail tonicity; 3, hindlimb paresis; 4, hindlimb paralysis; 5, tetraplegia. Clinical observations of EAE were made blind by at least two investigators. All immunized animals injected with PBS (group I) developed clinical signs of EAE, with maximum symptoms around day 14 p.i., followed by clinical improvement and recovery on day 20 p.i. (Fig. 2a).
Fig. 2.
EAE-DC protect Lewis rats from EAE induced by immunization with MBP 68–86 + FCA. (a) Mean clinical score and (b) mean body weight. Lewis rats were injected subcutaneously with bone marrow (BM) DC at 1 × 106 DC/rat prepared from EAE rats 7 days p.i. (group II). Since DC may induce immunity, the observation period after injection of EAE-DC and prior to immunization was extended over 4 weeks. No clinical signs of EAE were observed. After 4 weeks, the rats were immunized with MBP 68–86 + FCA. Rats receiving PBS (group I) or BM-DC from normal rats (group III) served as controls. The data were obtained from 6 rats of two independent experiments. • Group I; ▪ Group II; ▴ Group III.
Generation of DC from rat bone marrow
BM cells obtained from EAE rats on day 7 p.i. (group II) or normal rats (group III) were flushed from femurs and tibias, subsequently depleted of red cells by osmotic lysis, and then suspended in serum-free Dulbecco's modification of Eagle's medium (Gibco, Paisley, UK). Cells (5 × 106/ml) were seeded in 6-well plates (Costar, Fernwald, Germany). After 2 h, non-adherent cells were gently removed by swirling the plates and aspirating the medium. New medium supplemented with 10% fetal calf serum (FCS) (Gibco), rat rGM-CSF (10 ng/ml) (R & D Systems, Wiesbaden, Germany) and rIL-4 (10 ng/ml) (gift from Dr Peter van der Meide, Central Animal Laboratory, University of Utrecht, Utrecht, The Netherlands), 1% MEM amino acids (Gibco), 2 mm glutamine (Flow Lab., Irvine, UK), 50 IU/ml penicillin and 50 µg/ml streptomycin (Gibco), and 10 mm Hepes (Sigma), were added to the wells and exchanged 3–4 days later. After 7 days of culture, floating, non-adherent DC were collected by shaking and aspirating the medium, followed by harvesting adherent DC with a cell scrap. A restricted cell surface phenotype was measured in floating as well as adherent DC fractions, including CD11c (72% and 69%), MHC class II (87% and 84%), B7–1 (66% and 64%) and B7–2 (69% and 69%). There were no significant differences between the two DC fractions. CD3+ T cells amounted to <2·5%, and CD45RA + B cells <3%, in both floating and adherent DC fractions. The capacity of antigen presentation was lower in adherent DC than in floating DC, suggesting that adherent DC represent an immature stage of DC. Considering that immature DC may induce tolerance rather than immunity [12], adherent DC were used as effector cells in the present study. Cells were washed three times with serum-free medium, resuspended at 1 × 106 DC/ml in serum-free medium, and then injected s.c. in a volume of 1 ml. Control rats were injected s.c. with PBS (group I) or 1 × 106 DC from healthy Lewis rats in 1 ml (group III).
Functional comparison of BM DC from EAE and healthy rats
To define whether BM DC from EAE rats were activated and present specific antigen to naïve T cells, survival of DC in the absence of growth factors, as well as the capacity of antigen presentation to specific antigen, were detected. Viability of DC was detected by trypan blue exclusion at different times of culture.
The following procedure was used for antigen presentation. Spleens from normal rats were collected, and cell suspensions were attached to plastic plates for 2 h. After collecting non-adherent cells, T cell-enriched fractions were obtained through nylon wool columns and used as responder T cells in antigen presentation assays. BM DC from EAE and normal rats were exposed to 3000 rad γ‐irradiation, and 1 × 104 DC were co‐cultured with 2 × 105 responder T cells in 96-well round-bottomed plates in the presence of MBP 68–86 or irrelevant MBP 87–99 (10 µg/ml). After 60 h, proliferation was measured by 18 h uptake of [3H] added at 1 µCi/well.
Preparation of mononuclear cells
Peripheral blood obtained from different time points over the course of EAE was collected in tubes containing 0·13 m sodium citrate, and diluted with 4 volumes of 0·9% NaCl. The mononuclear cells (MNC) were separated by density gradient centrifugation on Lymphoprep (Nyegaard, Oslo, Norway). The cells from the interphase were collected and washed three times with serum-free medium. Cells were counted by trypan blue exclusion and then diluted to a cell concentration of 2 × 106/ml.
Proliferation assays
A radiometric proliferation assay based on [3H] thymidine incorporation was used. Briefly, 200 µl aliquots of MNC (2 × 106/ml) suspensions were applied to 96-well round-bottomed microtitre plates (Nunc, Copenhagen, Denmark) in the absence or presence of MBP 68–86 (10 µg/ml). After 60 h, cells were labelled for an additional 12 h with 10 µl aliquots containing 1 µCi of [3H] methylthymidine (Amersham, Little Chalfont, UK). Cells were harvested onto glass fibre filters and thymidine incorporation was measured. Cultures were run in triplicate and the results were expressed as mean cpm of the triplate cultures.
Assays of antibody isotype
Serum samples were collected on day 14 p.i. Isotypes of antibodies against MBP 68–86 were detected by ELISA using rabbit anti-rat IgG1 and IgG2a.
Assay of nitrite
As secreted NO quickly reacts with oxygen-yielding nitrite, the level of nitrite as a reflection of NO production in culture supernatant fluids was measured using modified Griess reagent (Sigma). MNC (4 × 105/200 µl) were cultured in 96-well round-bottomed microtitre plates (Nunc) in the absence or presence of MBP 68–86 (10 µg/ml). After 48 h, 100 µl of supernatant fluid from cultured cells were mixed with an equal volume of Griess reagent. After a 10 min reaction at room temperature (RT), absorbance at 540 nm was measured using an automated plate reader. Nitrite concentration was determined by comparison with a sodium nitrite standard curve in culture medium.
Assays of apoptotic cells
Apoptotic cells were determined using the Annexin-V-Fluos kit (Boehringer Mannheim, Mannheim, Germany). MNC (2 × 105) were washed twice in PBS and then incubated with Annexin-V-Fluos in Hepes buffer containing propidium iodide (PI) for 15 min at RT. After washing, with PBS, cells were analysed using a FACScan flow cytometer with Cell Quest software.
LDH assay
Lactate dehydrogenase (LDH) is a stable cytoplasmic enzyme present in all cells. It is rapidly released into the cell culture supernatant fluid upon damage of the plasma membrane. A diagnostic kit (Sigma) was used to measure LDH activity in supernatant fluids of cultures at 37°C, according to the manufacturer's instructions. One unit of LDH activity is defined as the amount of enzyme that will catalyse the formation of one micromole of reduced nicotinamide adenine dinucleotide per minute.
Intracellular analysis of cytokines
Intracellular cytokine staining was performed as described [13]. Cells were resuspended at 4 × 105/200 µl and stimulated with MBP 68–86 (10 µg/ml) for 24 h at 37°C. Cells were harvested, washed, fixed and stained with FITC anti-rat IFN-γ, PE anti-rat IL-4 or the respective isotype controls, for 30 min at RT. The antibodies were diluted in 1% BSA–PBS containing 0·15% saponin. These isotype-matched controls were used to set threshold markers on flow cytometric plots. Samples were analysed on a FACScan flow cytometer as described above.
Statistical analysis
Two-way anova and t-tests were performed to determine whether differences were significant. The level of significance was set to α =0·05. All tests were two-sided.
Results
EAE-DC exhibit higher viability and present specific antigen to naive T cells in vitro
Previous studies show that activated BM DC increase transient survival of DC in the absence of growth signals [14]. To determine whether BM DC from EAE rats were activated after immunization with MBP 68–86 + CFA, we compared survival of BM DC from EAE and normal rats. In comparison with BM DC from normal rats, BM DC from EAE rats on day 7 p.i. exhibited a significant enhancement (P < 0·05) of cell survival in the absence of GM-CSF + IL-4 at 7 days of culture (Fig. 1a). The results from antigen presentation demonstrated that BM DC from EAE rats present specific MBP 68–86, but not MBP 87–99, to naïve T cells (Fig. 1b). These data indicate that BM DC from EAE rats were in fact activated and pulsed with immunogenic peptide in vivo.
Fig. 1.
EAE-DC exhibit high survival and present specific MBP 68–86 to naïve T cells. BM DC from EAE and normal rats were obtained as described in Materials and methods. Viability of DC was detected by trypan blue exclusion at different time points (a) Antigen presentation was detected by co-culture of radiated-DC and T cells in the presence of MBP 68–86 and control MBP 87–99. (b) Results are expressed as the mean of quadruplicate wells ± s.d. *P < 0·05 when compared with Normal DC. • Normal DC; ▪ EAE-DC.
EAE-DC do not induce clinical EAE, but cause immune tolerance to EAE
The following study was performed in two independent experiments. Six of six control rats (group I) injected s.c. with PBS (Fig. 2a) developed typical EAE upon immunization with MBP 68–86 + FCA, with a mean maximal clinical score of 3·5 (range from 3 to 4) and a mean duration of 10 days (range day 11–20 p.i.).
Six rats (group II) were injected s.c. with EAE-DC (1 × 106 DC/rat) and failed to develop clinical EAE during an observation period of 4 weeks (Fig. 2a). They were then immunized with MBP 68–86 + FCA. During subsequent observation for an additional 40 days p.i., this group of rats showed tolerance to EAE as reflected by delayed onset, marked reduction of clinical severity (mean clinical score of 0·6 with range of 0·5–1·5; P < 0·05 when compared with group I) and short duration. This group of rats injected s.c. with EAE-DC (group II) also showed only a slight body weight loss compared with groups I and III (P < 0·05) (Fig. 2b).
Six rats injected s.c. with DC (1 × 106 DC/rat) from normal rats (group III) developed EAE with similar onset, severity of clinical signs (P > 0·05 when compared with group I) and duration as the rats injected with PBS (Fig. 2a).
In order to study antigen specificity of DC-mediated immune tolerance to EAE, we compared the effect of DC from EAE rats immunized with encephalitogenic MBP peptide 68–86 or non-encephalitogenic MBP peptide 87–99 + FCA. When BM DC (1 × 106/rat) from EAE rats immunized with 87–99 were injected by the same route and time point, we did not observe immune tolerance against EAE rats upon immunization with MBP 68–86 + FCA (Fig. 3a) (P > 0·05 when compared with group I). We also cultured BM DC derived from healthy rats and pulsed these DC with different peptides (MBP68–86; MOG-35–55 or PLP 139–151) in vitro. Such DC did not induce immune tolerance against EAE rats upon immunization with MBP 68–86 + FCA (data not shown). The results demonstrate that DC from EAE rats carry a specific antigen signal.
Fig. 3.
Comparison of different BM-DC injection in Lewis EAE rats induced by immunization with MBP 68–86 + FCA. EAE 87–99-DC, BM-DC were obtained from rats immunized with MBP 87–99 + FCA; following DC were obtained from rats immunized with MBP 68–86 + FCA. EAE-adDC, adherent DC from EAE rats (EAE 68–86-DC = group II); EAE-flDC, floating DC from EAE rats; EAE-LPS-DC, adherent DC from EAE rats were treated with LPS. Lewis rats were injected subcutaneously with the differently prepared DC at 1 × 106 DC/rat obtained from EAE rats 7 days p.i. (group II). After 4 weeks, such rats were immunized with MBP 68–86 + FCA. Rats receiving PBS (group I) or BM-DC from EAE (group II) served as controls. Arrows represent day of immunization. The data were obtained from 4–6 rats of two independent experiments. (a) • Control EAE (Group I); ▪ EAE 68–86-DC (Group II); ▴ EAE 87–99-DC. (b) • Control EAE (Group I); ▪ EAE-adDC (Group II); □ EAE-flDC; × EAE-LPS-DC.
Immune tolerance is mediated by immature stage of DC
As the capacity of antigen presentation was lower in adherent DC than in floating DC, we proposed that immune tolerance by EAE-DC may be related to the maturation status of the DC. Compared with adherent DC, floating DC from EAE rats did not mediate immune tolerance (P > 0·05 when compared with group) (Fig. 3b). LPS can induce DC into the mature stage. In an extended experiment in which adherent EAE-DC were treated with LPS (1 µg/ml) for 48 h, LPS-treated EAE-DC also did not induce immune tolerance in Lewis rats (P > 0·05 when compared with group I) (Fig. 3b). These results suggest that a different stage of DC influences tolerance or immunity.
DC derived from both EAE and healthy rats promote antigen-independent proliferation
DC derived from both EAE and healthy rats induced strong antigen-independent proliferation after a single injection in healthy rats (Fig. 4a,c) (P < 0·001). Proliferation was slightly lower in rats that had received DC from healthy rats compared with rats injected with EAE-DC (P < 0·05). Expression of MHC class II, B7–1 and B7–2 by blood MNC on days 7, 14, 21 and 28 after injection did not differ between the three groups of rats (data not shown).
Fig. 4.
Spontaneous (a and c) and MBP 68–86-induced (b and d) proliferation of blood MNC at different time points. Groups I (□), II (▪) and III ( ), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results are expressed as mean ± s.d. for 4–6 rats per group.
), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results are expressed as mean ± s.d. for 4–6 rats per group.
Proliferation in rats injected with DC from healthy rats declined rapidly by about 55% (from 15 475 to 6865 cpm in spontaneous proliferation) and by 50% (from 16 746 to 8340 cpm in MBP 68–86-induced proliferation) on day 3 p.i. In rats that had received EAE-DC, the proliferative response was gradually reduced by about 24% (from 17 734 to 13 400 cpm in spontaneous proliferation and 24%, from 19 575 to 14 900 cpm in MBP 68–86-induced proliferation) compared with proliferation on day 0 p.i. (Fig. 4b,d).
EAE-DC induce IFN-γ expression and IgG2a production
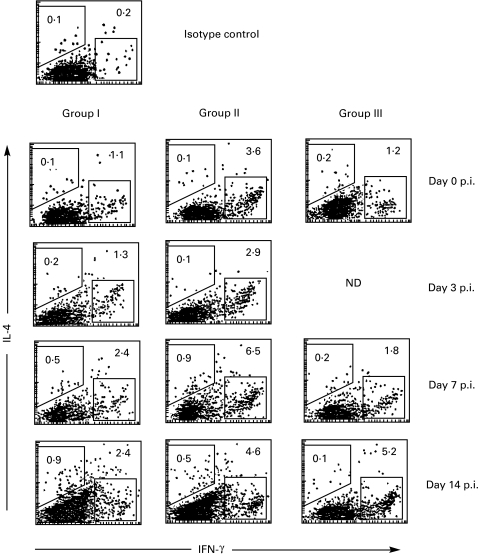
High levels of IFN-γ have previously been reported in antigen-stimulated splenocytes upon adoptive transfer of ovalbumin-pulsed DC [15]. Here, we observed that s.c. injection of EAE-DC was also associated with upregulation of IFN-γ. Levels of IFN-γ expressing blood MNC were higher on days 0, 3 and 7 p.i. in the rats of group II than groups I and III (Fig. 5).
Fig. 5.
MBP 68–86-induced levels of IFN-γ and IL-4 expression by blood MNC obtained at different time points. Groups I, II and III, respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Data are representative of two independent experiments. ND, not done.
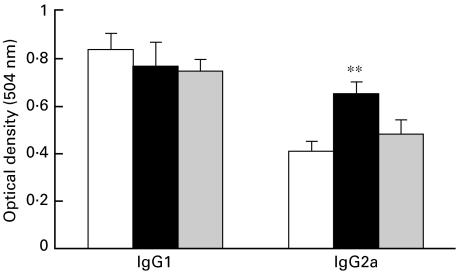
Serum antibody isotype profiles may serve as relative indicators of Th subset activities [16,17]. We used ELISA to determine IgG1 and IgG2a antibodies against MBP 68–86, which may reflect the preferential production of IL-4 and IFN-γ, respectively. Serum from EAE-DC-treated rats contained high levels of the Th1-dependent IgG2a compared with the other two groups (Fig. 6). These results show that EAE-DC can upregulate Th1 subset activities.
Fig. 6.
Serum antibody isotypic profiles in sera from different group of rats on day 14 p.i., as determined by ELISA. Groups I (□), II (▪) and III ( ), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results represent mean ± s.d. for 4 rats per group. **P < 0·01 when compared with Group I.
), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results represent mean ± s.d. for 4 rats per group. **P < 0·01 when compared with Group I.
EAE-DC increase NO production
As IFN-γ can induce NO production [18], we examined NO production as reflected by nitrite concentration, an end-product of NO. Rats injected s.c. with EAE-DC showed augmented nitrite concentrations in blood MNC on day 0 p.i., and in particular on day 3 p.i., regardless of whether spontaneous or MBP 68–86-induced stimulation was examined (Fig. 7).
Fig. 7.
Spontaneous (a) and MBP 68–86-induced (b) nitrite production by blood MNC obtained at different time points. Groups I (□), II (▪) and III ( ), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results are expressed as mean ± s.d. for 6 rats per group. ***P < 0·001 when compared with Group I.
), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results are expressed as mean ± s.d. for 6 rats per group. ***P < 0·001 when compared with Group I.
EAE-DC increased cell apoptosis
We have previously shown that high levels of NO induce apoptosis of CD4+ T cells in EAE [19]. Since s.c. injection of EAE-DC results in augmented IFN-γ expression (Fig. 5) and NO production (Fig. 7), we next examined cell apoptosis in the three groups of rats. Rats injected s.c. with EAE-DC (group II) had higher numbers of apoptotic cells among blood MNC on days 3 and 7 p.i. compared with groups I and III (Fig. 8, upper panels). Addition of the iNOS inhibitor nitrol-l-arginine methylester (NAME, Sigma) effectively inhibited cell apoptosis, indicating that apoptosis of blood MNC from rats of group II is NO dependent. In accordance with the enhanced NO production and cell apoptosis, the numbers of viable cells were lower in rats of group II than groups I and III on day 3 p.i. (Fig. 9a). The levels of LDH in supernatant fluids of MBP 68–86-stimulated MNC from rats of group II were also higher compared with groups I and III on days 3 and 7 p.i. (Fig. 9b). These results imply that NO-mediated cell apoptosis during the initial phase of the immune response may be an important mechanism for tolerance induced by EAE-DC.
Fig. 8.
Apoptotic cells among blood MNC obtained on days 3 and 7 p.i. MNC (1 × 105) were stained with Annexin-V-FITC and PI for 15 min at RT. Samples were analysed by a FACScan flow cytometer using Cell Quest software. Results are expressed as percentage of Annexin-V-FITC-positive cells. Plots shown in the upper panels are representative of two independent experiments. The lower panels shows inhibitory effects of NAME on cell apoptosis. Untreated cells (–) and cells treated with 500 µm NAME (+) were stained with Annexin-V-FITC and PI for 15 min at RT.
Fig. 9.
Numbers of viable cells (a) and LDH release (b) measured in supernatant fluids of cultures of blood MNC in the presence of MBP 68–86 at different time points. Groups I (□), II (▪) and III ( ), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results are expressed as mean ± ±s.d. Data are representative of two independent experiments. **P < 0·01 when compared with Group I.
), respectively, represent rats injected s.c. with PBS, EAE-DC or normal rat DC, 4 weeks prior to immunization. Results are expressed as mean ± ±s.d. Data are representative of two independent experiments. **P < 0·01 when compared with Group I.
Discussion
The main finding of our study is that BM-derived EAE-DC prevent the development of clinical EAE. In preliminary experiments, we found that expression of MHC class II, B7–1 and B7–2 by EAE-DC was increased on days 3, 5 and 7 p.i., and then rapidly declined to healthy rat levels. Based on these results, we chose DC obtained from EAE rats on day 7 p.i. This omits the difficult step of identifying autoantigens, which are still unknown in many autoimmune diseases. Induction of tolerance by DC might thus become a reality in the future therapy of MS and other autoimmune-related diseases.
The mechanisms behind DC-mediated tolerance are not completely understood. It has been proposed that deficiency or blockade of co-stimulatory molecules such as CD40, B7–1 or B7–2 may be related to DC tolerogenicity, both in allograft models [20] and experimental autoimmune disease [21]. Lespagnard et al. [22] reported that inhibition of B7–1 and B7–2 did not lead to tolerance. In the present study, we did not find any difference in expression of B7–1, B7–2 and MHC class II among the three groups of rats, indicating that deficiency or blockade of co-stimulatory molecules is not necessary for EAE-DC-mediated tolerance.
In our study, the suppression of EAE was accompanied by considerable induction of IFN-γ and NO. In view of the established Th1-mediated autoimmune pathogenesis of EAE, the induction of a Th1 cytokine after EAE-DC injection is unexpected in the course of EAE suppression. However, this traditional view has been challenged recently by a number of studies which describe unexpected disease-ameliorating effects of proinflammatory cytokines [23–26]. Shinomiya et al. [27] reported that transfer of DC treated with IFN-γ suppressed autoimmune diabetes in non-obese diabetic mice. Consistent with our observation, previous studies have also revealed that DC can upregulate IFN-γ production [28–30]. Dillon et al. [31] reported that long-term T-cell responses and the production of IFN-γ can be generated using a single inoculation of antigen-pulsed DC. In the present study, we did not observe significant differences in levels of IL-4. However, it has recently been reported that immature DC led to tolerance by differentiation of T-regulatory cells by producing high levels of IL-10 and low IL-4 [32,33].
We next attempted to elucidate the mechanism by which IFN-γ upregulation might mediate tolerance to EAE. IFN-γ strongly upregulates NO production that can cause apoptosis. NO has the potency to eliminate autoreactive T cells via apoptosis [34], and induces apoptosis of myelin-reactive T cells [35], mouse splenic T cells and mouse thymocytes [36]. Downing et al. [28] proposed that a nitrergic mechanism is responsible for DC-mediated T-cell elimination. Antigenic stimulation of T cells resulted in production of IFN-γ and TNF-α. These cytokines induced cells to express iNOS, and to produce NO that mediated lethal growth inhibition of co-cultured T cells. In fact, this inhibition of T cells by NO appears to be a specific impairment of Th1 CD4+ T cells while sparing Th2 cells [37]. Because EAE is a Th1 cell-mediated autoimmune disease, the increase in NO may selectively limit the encephalitogenic effector population by inducing cell apoptosis in the periphery. In the present study, we observed that EAE-DC can cause a strong and rapid induction of NO on day 3 p.i. A possible explanation would be that injection of DC results in augmented proliferation, and that proliferating cells may be reactivated to produce NO when they encounter specific antigen after immunization. Augmented NO exhibited an anti-proliferative effect on day 14 p.i. compared with the proliferation measured before immunization. Due to lack of specific antigen signal, these proliferating cells in rats that had received DC from healthy rats could not be reactivated to expand and produce NO on days 3 and 7 p.i. When such rats were immunized with MBP 68–86 + FCA, immune responses were re-established and resulted in the development of EAE. In the present study, we did not provide direct evidence that EAE-DC-mediated immune tolerance is antigen-specific. An alternative experiment demonstrates that DC from rats immunized with MBP-87–99 did not induce immune tolerance against EAE rats immunized with MBP 68–86, revealing EAE-DC to have antigen specificity after immunization with MBP 68–86 + FCA.
The demonstration in this study of immune tolerance in rats that had received EAE-DC are in context with those reported elsewhere in several autoimmune diseases [38,39]. One potential explanation for the difference between our results and those of other studies is the route of DC injection. Dittel et al. adopted intravenous injection while we used subcutaneous injection. Because functional differences may result from the different stages of DC to be used, we compared the properties of adherent and floating DC. Adherent DC exhibited a low capacity of antigen presentation compared with floating DC. Importantly, LPS-treated adherent DC did not induce immune tolerance against EAE. These data suggest that the adherent DC used in the present study may represent an immature stage of DC.
It is clear that DC can prime and expand antigen-specific T-cell responses in vivo and in vitro [40]. TNF-α-treated DC constitute a stronger stimulator for proliferation of both allogeneic cord blood lymphocytes and peripheral blood lymphocytes [41]. After culture with GM-CSF + IL-4, DC also stimulated vigorous proliferation of allogeneic T cells [42]. These results suggest that DC not only induce antigen-specific proliferation, but also promote antigen-independent proliferation. By which mechanism DC induce spontaneous proliferation remains to be resolved.
In summary, the present study shows that BM-DC from EAE rats can present specific antigen to naive T cells in vitro, revealing that they had been pulsed in vivo after immunization with MBP 68–86. EAE-susceptible rats injected s.c. with ‘in vivo pulsed’ EAE-DC did not develop clinical EAE. Instead, such DC can induce tolerance to EAE. EAE-DC-mediated tolerance was associated with increased IFN-γ expression and NO production by blood MNC. High levels of NO promote cell apoptosis at a critical time point. Use of ‘in vivo pulsed DC’ by-passes the need for identification of the target autoantigen. It may be a useful strategy for achieving a safe and specific procedure for the therapy of patients with autoimmune diseases.
Acknowledgments
This work has been supported by grants from the Swedish MS Society (NHR), the Swedish Medical Research Council and Karolinska Institute Research Funds.
References
- 1.Pulendran B, Smith JL, Caspary G, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalinski P, Hilkens CMU, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Viney JL, Mowat AM, O'Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–25. [PubMed] [Google Scholar]
- 6.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 7.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8 (+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 8.Clare-Salzier MJ, Brooks J, Chai A, van Herle K, Anderson C. Prevention of diabetes in nonobetic mice by dendritic cell transfer. J Clin Invest. 1992;90:741–8. doi: 10.1172/JCI115946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min WP, Gorczynski R, Huang XY, et al. Dendritic cells engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. J Immunol. 2000;164:161–7. doi: 10.4049/jimmunol.164.1.161. [DOI] [PubMed] [Google Scholar]
- 10.Huang YM, Yang JS, Xu LY, Link H, Xiao BG. Autoantigen-pulsed dendritic cells induce tolerance to experimental allergic encephalomyelitis in Lewis rats. Clin Exp Immunol. 2000;122:437–44. doi: 10.1046/j.1365-2249.2000.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmdahl R, Olsson T, Moran T, Klareskog L. In vivo treatment of rats with monoclonal anti-T cell antibodies. Immunohistochemical and functional analysis in normal rat and experimental allergic neuritis. Scand J Immunol. 1985;22:157–69. doi: 10.1111/j.1365-3083.1985.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 12.Labeau MS, Roters B, Pers B, et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–75. [PubMed] [Google Scholar]
- 13.Openshaw P, Murphy EE, Hosken NA, et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–9. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 15.Stumbles PA, Thomas JA, Pimm CL, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med. 1998;188:2019–31. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman FD, Holmes J. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 17.Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–7. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 18.Hertz CJ, Mansfield JM. IFN-γ-dependent nitric oxide production is not linked to resistance in experimental African trypanosomiasis. Cell Immunol. 1999;192:24–32. doi: 10.1006/cimm.1998.1429. [DOI] [PubMed] [Google Scholar]
- 19.Xiao BG, Huang YM, Xu LY, Ishikawa M, Link H. Mechanisms of recovery from experimental allergic encephalomyelitis induced with myelin basic protein peptide 68–86 in Lewis rats: a role for dendritic cells in inducing apoptosis of CD4+ T cells. J Neuroimmunol. 1999;97:25–36. doi: 10.1016/s0165-5728(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 20.Thomson AW, Lu L, Murase H, Demetris AJ, Rao AS, Starzl TE. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622–39. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury SJ, Gallon L, Verburg RR, et al. Ex Vivo treatment of antigen-presenting cells with CTLA-4Ig and encephalitogenic peptide prevents experimental autoimmune encephalomyelitis in the Lewis rat. J Immunol. 1996;157:3700–5. [PubMed] [Google Scholar]
- 22.Lespagnard L, Mettens P, De Smedt T, et al. The immune response induced in vivo by dendritic cells is dependent on B7–1 or B7–2, but inhibition of both signals does not lead to tolerance. Int Immunol. 1998;10:295–304. doi: 10.1093/intimm/10.3.295. [DOI] [PubMed] [Google Scholar]
- 23.Weishaupt A, Jander S, Bruck W, et al. Molecular mechanisms of high-dose antigen therapy in experimental autoimmune encephalomyelitis: rapid induction of Th1-type cytokines and inducible nitric oxide synthase. J Immunol. 2000;165:7157–63. doi: 10.4049/jimmunol.165.12.7157. [DOI] [PubMed] [Google Scholar]
- 24.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–8. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J Immunol. 2000;164:2759–68. doi: 10.4049/jimmunol.164.5.2759. [DOI] [PubMed] [Google Scholar]
- 26.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–86. [PubMed] [Google Scholar]
- 27.Shinomiya M, Fazle Akbar SM, Shinomiya H, Onji M. Transfer of dendritic cells vivo stimulated with interferon-gamma down-modulates autoimmune diabetes in non-obese diabetic mice. Clin Exp Immunol. 1999;117:38–43. doi: 10.1046/j.1365-2249.1999.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downing JE, Virag L, Perry ME. Nitrergic mechanism of DC-mediated T-cell elimination. Immunol Today. 1998;19:190–1. doi: 10.1016/s0167-5699(97)01223-1. [DOI] [PubMed] [Google Scholar]
- 29.Sciorati C, Rovere P, Ferrarini M, Heltai S, Manfredi AA, Clementi E. Autocrine nitric oxide modulates CD-95-induced apoptosis in gammadelta T lymphocytes. J Biol Chem. 1997;272:23211–5. doi: 10.1074/jbc.272.37.23211. [DOI] [PubMed] [Google Scholar]
- 30.Wilkes DS, Thompsom LK, Cummings OW, Bragg S, Heidler KM. Instillation of allogeneic lung macrophages and dendritic cells cause differential effects on local IFN-gamma production, lymphocytic bronchitis, and vasculitis in recipient murine lungs. J Leukoc Biol. 1998;64:578–86. doi: 10.1002/jlb.64.5.578. [DOI] [PubMed] [Google Scholar]
- 31.Dillon SM, Griffin JF, Hart DN, Watson JD, Baird MA. A long-lasting IFN-γ response is induced to a single inoculation of antigen-pulsed dendritic cells. Immunology. 1998;95:132–40. doi: 10.1046/j.1365-2567.1998.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–F9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams MS, Noguchi S, Henkart PA, Osawa Y. Nitric oxide synthase plays a signaling role in TCR-triggered apoptotic death. J Immunol. 1998;161:6526–31. [PubMed] [Google Scholar]
- 35.Zettl UK, Mix E, Zielasek J, Stangel M, Hartung HP, Gold R. Apoptosis of myelin-reactive T cells induced by reactive oxygen and nitrogen intermediates in vitro. Cell Immunol. 1997;178:1–8. doi: 10.1006/cimm.1997.1113. [DOI] [PubMed] [Google Scholar]
- 36.Fehsel K, Kroncke KD, Meyer KL, Huber H, Wahn V, Vand Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–65. [PubMed] [Google Scholar]
- 37.Taylor-Robinson AW, Liew FY, Severn A, et al. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24:980–4. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 38.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J Immunol. 1999;163:32–9. [PubMed] [Google Scholar]
- 40.Schlienger K, Craighead N, Lee KP, Levine BL, June CH. Efficient priming of protein antigen-specific human CD4+ T cells by monocyte-derived dendritic cells. Blood. 2000;96:3490–8. [PubMed] [Google Scholar]
- 41.Zheng Z, Takahashi M, Narita M, et al. Generation of dendritic cells from adherent cells of cord blood by culture with GM-CSF, IL-4 and TNF-α. J Hematother Stem Cell Res. 2000;9:453–64. doi: 10.1089/152581600419116. [DOI] [PubMed] [Google Scholar]
- 42.Agger R, Peterson MS, Toldbod HE, et al. Characterization of murine dendritic cells derived from adherent blood mononuclear cells in vitro. Scand J Immunol. 2000;52:138–47. doi: 10.1046/j.1365-3083.2000.00760.x. [DOI] [PubMed] [Google Scholar]