Abstract
In susceptible mice, the heavy metal ion mercury is able to induce a strong immune activation, which resembles a T helper 2 (Th2) type of immune response and is characterized by a polyclonal B cell activation, formation of high levels of IgG1 and IgE antibodies, production of autoantibodies of different specificities and development of renal IgG deposits. In the present study, we analysed the in vivo effects of mercury in nonobese diabetic (NOD) mice, which is believed to develop a spontaneous Th1 cell-mediated autoimmune diabetes similar to type 1 diabetes in humans. Three weeks of treatment with mercury induced a strong Th2 like immune/autoimmune response in NOD mice. This response was characterized by an intensive increase in splenic IgG1 antibody secreting cells, a marked elevation in serum IgE levels, a substantial increase in splenic IL-4 mRNA, but a significant decrease in splenic IFN-γ mRNA. Mercury-induced IgG1 antibodies were mainly against ssDNA, TNP and thyroglobulin, but not against nucleolar antigen. Moreover, mercury-injected NOD mice developed high titres of IgG1 deposits in the kidney glomeruli. We further tested if the generated Th2 response could interfere with the development of insulitis and diabetes in NOD mice. We found that three weeks of treatment with mercury was also able to significantly suppress the development of insulitis and postpone the onset of diabetes in these mice. Thus, mercury-induced immune activation can counter-regulate the Th1 cell-mediated autoimmune responses and confer a partial protection against autoimmune diabetes in NOD mice.
Keywords: diabetes, cytokines, Th1/Th2 cells, autoantibodies, in vivo animal models
Introduction
The nonobese diabetic (NOD) mice spontaneously develop an autoimmune diabetes that in most of its immunological features resembles insulin-dependent diabetes mellitus (IDDM) in man (reviewed in [1,2]). In both cases the disease affects the pancreatic islets, i.e. activated inflammatory mononuclear cells infiltrate the islets, which results in the development of insulitis [1,2]. Insulitis leads to the destruction of the insulin-producing beta cells and eventually occurrence of diabetes [1,2]. The mechanisms that lead to the initiation of the autoimmune process are still unknown, but several studies have shown that immunological and genetic factors were involved in this process (reviewed in [3,4]). For instance, it has been demonstrated that T cells play a pivotal role in the development of diabetes as they were the most cell types found in the islet infiltrates and as the disease could be adoptively transferred to nondiabetic NOD recipients by either purified T cells and/or T cell clones obtained from diabetic donors [3]. Further studies have shown that participation of CD4+ T cells is required for fully development of diabetes in NOD mice, i.e. treatment with an anti-CD4 monoclonal antibody and/or cyclosporin was able to prevent the development of diabetes in these mice [3]. Since CD4+ T cells have been subdivided functionally into Th1 and Th2 subsets on the basis of their contrasting and cross-regulating profiles of cytokine production (reviewed in [5]), studies have been performed to apply the Th1/Th2 paradigm in the development of autoimmune diabetes in NOD mice (reviewed in [3] and [6,7]). Results of these studies suggested that Th1 cells, which preferentially secrete interleukin-2 (IL-2) interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), have a pathogenic role, whereas Th2 cells, which mainly produce IL-4, IL-5, IL-10 and IL-13, confer a protective effect on the development of diabetes in these mice [3,6,7].
It is well established that the heavy metal mercury at subtoxic doses can induce a strong immune activation with autoimmune characteristics in various species (reviewed in [8–10]). These characteristics include a CD4+ T cell-dependent polyclonal B cell activation, formation of high levels of IgG1 and IgE antibodies, production of autoantibodies of different specificities and development of renal IgG deposits [11–14]. Although the exact mechanism for mercury-induced immune/autoimmune activation is not well understood, both immunological and genetic factors (like in NOD mice) have been shown to play decisive roles [11–20]. Moreover, like in the NOD model, Th1/Th2 dichotomy has also been proposed to account for susceptibility/resistance to mercury-induced autoimmunity [8,21]. However, in contrast to the NOD model, it is believed that CD4+ cells of Th2 type preferentially mediate the mercury-induced autoimmunity, whereas Th1 cells either down-regulate or confer resistance to immune/autoimmune responses caused by mercury [8,21].
In the context of the Th1/Th2 paradigm and on the bases of the above-mentioned studies, we hypothesized that administration of mercury into NOD mice may result in an immune activation, which thereby can interfere with the spontaneous and destructive Th1-mediated responses in these mice. In this study, we investigated the in vivo effects of mercury on the immune system of NOD mice, by studying B cell activation, antibody/autoantibody production, formation of renal IgG deposits, cytokine synthesis and development of insulitis as well as diabetes in these mice. Our results show that mercury induces a Th2 type immune/autoimmune response in NOD mice, which can interferes with the development of insulitis and partially postpones the occurrence of diabetes.
Materials and methods
Mice
Female NOD/Lt mice were originally purchased from Charles River Sverige AB (Charles River, Uppsala, Sweden). This strain was further bred and kept under a pathogen-free condition in our animal facilities at the Department of Immunology, Stockholm University. Female NOD/Bom mice were purchased from M & B A/S (M & B A/S, Ry, Denmark). The mice were three to five weeks old at the beginning of the experiments.
HgCl2 treatment
HgCl2 (analytical grade, Merck, Darmstadt, Germany) at a concentration of 0·4 mg/ml in sterile 0·9% NaCl was used. Groups of NOD mice (3–12 mice per group as indicated for each experiment) were injected subcutaneously (s.c.) with 0·1 ml HgCl2 solution (≅1·6 mg/kg body weight). Injections were carried out every third day for different intervals (5, 10 and 21 days). Control groups received either 0·1 ml sterile 0·9% NaCl (saline), s.c. or left uninjected.
Blood and organ collection
At different intervals the mercury- and saline-injected mice were bled by retro orbital puncture under light metophane or ether anaesthesia. Thereafter, the same mice were killed by cervical dislocation and their spleens, pancreata (only on days 10 and 21) and kidneys were removed.
Cell suspension and serum preparation
The spleens (whole spleens collected on day 5 and three-quarters of the spleens collected on days 10 and 21) were carefully dissected and single-cell suspensions were prepared by teasing the spleens with forceps in Earle's balanced salt solution (EBSS). The cell suspensions were washed three times and re-suspended in 5 ml EBSS before carrying out the protein A plaque assay. The blood from each mouse was allowed to clot at room temperature. The serum was separated after centrifugation and stored at −20°C until tested for antibody content.
Protein A plaque assay
Antibody-secreting cells of different Ig classes and subclasses were enumerated in spleen cell suspensions by using a protein A plaque assay as described by Gronowicz et al. [22]. Rabbit antimouse IgM, IgG1, IgG3 (Organon Teknika, Durham, NC, USA) and IgG2b (Nordic Immunological Laboratories, Tillburg, The Netherlands) were used as developing reagents.
ELISA for mouse IgE
Total mouse serum IgE was determined by a sandwich ELISA assay as described previously [23]. We used a rat antimouse IgE MoAb, R35-72 (Pharmingen, San Diago, CA, USA) as the capture antibody and a biotinylated rat antimouse IgE MoAb, R35-92 (Pharmingen) as the detection antibody.
Detection of IgG1 antinucleolar autoantibodies (ANolA)
The presence of IgG1 ANolA in the mouse sera was determined by an indirect immunofluorescence method as described previously [23]. We used rat liver sections as substrate and FITC-conjugated goat antimouse IgG1 (Southern Biotechnology, Birmingham, AL, USA) as the detecting antibody. The initial dilution for the sera was 1/50. The highest serum dilution at which nucleolar fluorescence could be detected was defined as the titre of ANolA.
ELISA for detection of other autoantibodies
Serum IgG1 antibodies against single-stranded DNA (ss-DNA), thyroglobulin and trinitrophenol were measured by ELISA methods as described before [23]. Briefly, 50 μl/well of serially diluted sera (starting with 1/50 dilution) were added to micro-ELISA plates (Corning Costar) precoated with bovine thyroglobulin (Sigma Chemical Co, St Louis, MO, USA), calf thymus DNA (Serva, Heilderberg, Germany) and TNP conjugated to BSA (TNP-BSA). Following an overnight incubation at 4°C, the plates were washed and the unbound sites were blocked with BSA for 2 h at room temperature. Thereafter, the plates were washed three times with PBS-tween and 50 μl alkaline phosphatase-labelled goat antimouse IgG1 (Southern Biotechnology) were added to each well. After a 2-h incubation at 37°C, the plates were washed and 50 μl substrate was added to each well. After 30 min, the absorbance was read at 405 nm.
Detection of renal IgG1 deposits
The presence of glomerular deposits of IgG1 antibodies were detected by direct immunofluorescence as described before [23]. Briefly, 5 µm of the acetone-fixed cryostat sections of the kidneys were incubated with serial dilutions of FITC-conjugated goat antimouse IgG1 antibodies (Southern Biotechnology). The initial dilution for FITC-conjugated antibody was 1/40. When at this dilution no specific green fluorescence was detected, the result was recorded as ‘0’. The highest dilution of the antibody at which a specific fluorescence could be seen was defined as the end-point titre of the glomerular deposits.
RNA extraction and cDNA synthesis
RNA from the splenic cells (obtained from one-quarter of the collected spleens of mercury- and saline-injected mice for 10 and/or 21 days) was extracted with RNAzol B reagent (BioSite, Täby, Sweden). cDNA was synthesized from 5 μg of total RNA extracted from the spleen cells of mercury- and saline-injected mice, with a reaction mixture of 20 μl per tube, 5x reaction buffer (Gibco/BRL, Life Technologies) 4 μl; 10 mm dNTP (Gibco/BRL, Life Technologies) 2 μl; 100 μm DTT (Gibco/BRL, Life Technologies) 1·5 μl; 10 pmol/µl Random hexamer Pd (N6) 1 μl; and reverse transcriptase (Gibco/BRL, Life Technologies) 1 μl. Thereafter, the mixtures were incubated at 42°C for 45 min.
Real-time polymerase chain reaction (RT-PCR) (IL-4 and IFN-γ assay)
We applied real-time PCR [24] to relatively quantify the expression of IL-4 and INF-γ genes in the spleens of mercury- and/or saline-injected mice, which were injected for either 10 and/or 21 days. Real-time PCR was performed on an ABI Prism 7700 Sequense Detector (Perkin-Elmer/Applied Biosystems). Primers and probes were designed to span exon junction to prevent any amplification of possible contaminated genomic DNA (Table 1). For each run, after initial AmpErase UNG enzyme activation (2 min, 50°C), and Ampli Taq Gold® DNA polymerase activation (10 min, 95°C), 40 cycles of a two steps PCR amplification (15 s, 95°C; 1 min 60°C) were carried out. Equal volume of cDNA was used in all assays and PCR conditions were optimized for primers, probes and MgCl2 concentration. All the reactions were performed in MicroAmp Optical 96-well reaction plates with MicroAmp optical caps (Perkin-Elmer/Applied Biosystems).
Table 1.
Primer sequences and positions used in real-time PCR assay
| Cytokine | Primer sequence | Amplicon | Position | Reference |
|---|---|---|---|---|
| Il-4 | Sense: 5-ACAGGAGAAGGGACGCCAT-3′ | 100 bp | 208–266 | gb; M25892 |
| Antisense: 5-CCTTGGAAGCCCTACAGACG-3 | 288–307 | |||
| Probe: 5-GTCCTCACAGCAACGAAGAACACCA-3 | 250–274 | |||
| IFN-γ | Sense: 5-GGCATAGATGTGGAAGAAAAG-3′ | 338 bp | 190–210 | gb; K00083 |
| Antisense: 5-CTCCTTTTCCGCTTCCTGA-3 | 509–527 | |||
| Probe: 5-CATCCTTTTGCCAGTTCCTCCAGAT-3 | 226–250 | |||
| GAPDH | Sense: 5-CAGGAGCGAGACCCCACTA-3′ | 309 bp | 272–290 | gb; M32599 |
| Antisense: 5-GGCATGGACTGTGGTCATGA-3 | 561–580 | |||
| Probe: 5-GCATTGCTGACAATCTTGAGTGAGTT-3 | 464–489 |
Using Blue-6-FAM and TMRA-labelled probes, fluorescence signals were generated during each PCR cycle via 5′−3′ endonuclease activity of Ampli Taq Gold® DNA polymerase [24]. For normalization, glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was used as the housekeeping gene.
The level of each target cytokine (IL-4 and IFN-γ) was considered to be 1 for the control mice (saline and/or un-injected mice). The relative quantitative expression (fold increase/decrease) of cytokine genes in the mercury-injected mice was determined using the arithmetic equation 2−ΔΔCT according to Perkin-Elmer instruction manual (User Bulletin #2, December 11, 1997). The calculation of ΔΔCT involves subtraction of Δ Threshold Cycle (ΔCT) in the control mice from the Δ Threshold Cycle (ΔCT) in the mercury-injected mice for each cytokine. ΔCT for the control and/or mercury-injected mice was calculated by subtraction of the endogenous control CT and the target cytokine CT.
Assessment of insulitis
At sacrifice, pancreata were taken out and fixed overnight in 4% formalin in PBS and subsequently transferred to 70% ethanol. Fixed pancreata were embedded in paraffin blocks, a minimum of 12 8-μm sections were cut at least 150 μm apart. The sections were stained with haematoxylin-eosin and evaluated for incidence and severity of insulitis in light microscopy by two independent investigators. A minimum 40 islets from each mouse were observed and the degree of mononuclear cell infiltration was scored using the following ranking: 0, no infiltration; 1, pervascular/periductular infiltrates with leucocytes; 2, leucocyte infiltration of up to 25% of islet mass; 3, leucocyte infiltration of up to 75% of islet mass; and 4, insulitis with more than 75% of the islet infiltrated or only remnant endocrine tissue. Insulitis index (I) for each mouse was calculated according to the following formula:
Assessment of diabetes
In one experiment, groups of 4-week-old, female NOD/Bom mice (9–10 mice per group) were either treated with HgCl2 for 3 weeks or used as controls as described above. From two weeks after the last injection, the mercury-injected and control mice were monitored weekly for the development of glucosuria with BM-Test-GP strips (Boehringer Mannheim Scandinavia AB, Bromma, Sweden). Glucosuric values ≥3 (≥ 300 mg/dl) on more than one occasion were considered diagnostic of diabetes onset.
Statistical analysis
Number of antibody secreting cells of different isotypes, serum IgE levels, titres of glomerular deposits of IgG1 antibodies and the indices for insulitis were shown as the means ± s.e. (standard error). The differences between these parameters in mercury- and control mice (saline-injected and/or un-injected) were analysed with Wilcoxon–Mann–Whitney rank sum test. Multiple comparisons analysis (Tukey's test) was also used to correct the P-values. The difference between mercury and control mice in development of diabetes was analysed with Fisher's exact and/or log-rank tests.
Results
Mercury induces a strong B cell activation and antibody production in young NOD mice
We first evaluated whether the heavy metal ion mercury was able to activate the immune system in NOD mice. Groups of young NOD mice (3–12 mice per group, as indicated in the legend for Fig. 1) were treated with subtoxic doses of HgCl2 for different intervals (5, 10, 21 days). Control mice either received sterile saline or were left uninjected. As parameters for mercury-induced immune activation, we determined the Ig production of different isotypes by using either protein A plaque assay and/or ELISA methods.
Fig. 1.
Mercury induces antibody production of various isotypes in young NOD mice. Groups of young female NOD mice (3–12 mice/group) were repeatedly injected s.c. with mercuric chloride (•). Control mice were either injected with sterile saline or left uninjected (○). At different times after injections, the mice were bled and killed. The spleens were tested for IgM, IgG1, IgG2b and IgG3 antibody secreting cells by using a protein A plaque assay and the sera were tested for IgE by using an ELISA method. Data are shown as the means + 1 s.e. Significant differences between the parameters in mercury- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. Tukey's test was used to correct the P-values. *P < 0·05; **P < 0·01; ***P < 0·001.
As shown in Fig. 1a–e, already after 10 days, mercury-injected young NOD mice exhibited a high increase in the number of splenic IgG1 antibody-secreting cells as well as in the serum levels of IgE when compared with the control mice. At this interval, mercury-treatment did not induce significant changes in other Ig isotypes (Fig. 1a–e). After 21 days treatment with mercury, the increased IgG1 and IgE either remained at similar levels (IgG1 antibody-secreting cells) or rose to higher levels (serum IgE) (Fig. 1a–e). At this interval, mercury-injected mice also showed a significant increase in the numbers of slpenic IgM and IgG2b antibody secreting cells as compared to those in the control mice. These results indicate that as a potent stimulator of the immune system, mercury is able to induce a strong B-cell activation in NOD mice with preferential production of IgG1 and IgE.
Induction of antibodies of different specificities in young NOD mice by mercury
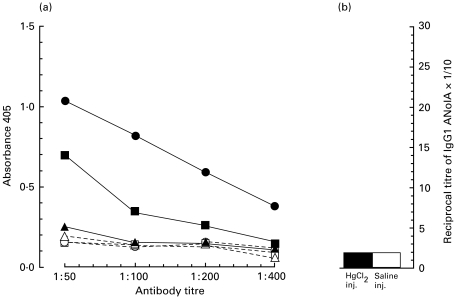
Studies have shown that in susceptible mice, mercury-induced antibodies were of different specificities [12,16,17,23,25]. Therefore, we next performed experiments to test the antigen-specificity of mercury-induced IgG1 antibodies in NOD mice. Sera of control mice and mercury-injected mice for 21 days were analysed for the presence of IgG1 antibodies against one exogenous (TNP) and three endogenous (ss-DNA, thyroglobulin and nucleoli) antigens using ELISA and indirect immunofluorescence techniques. As shown in Fig. 2a, sera of mercury-injected NOD mice exhibited a strong IgG1 antibody reactivity against ss-DNA and TNP antigens and a weak response against thyroglobulin as compared with those of the control mice. Neither mercury-injected sera nor control sera did show any significant IgG1 reactivity against nucleoli (Fig. 2b). These findings suggest that in NOD mice, mercury-induced IgG1 response is at least oligoclonal (if not polyclonal).
Fig. 2.
Mercury induces IgG1 antibodies of different specifities in young NOD mice. The sera from the same (as described for Fig. 1) mercury-injected (solid symbols) or control (open symbols) NOD mice at day 21 interval were tested for the presence of IgG1 antibodies against ss-DNA (•, ○), trinitrophenol (TNP: ▪, □), thyroglobulin (▴, ▵), and nucleolar antigen (ANolA) by using either ELISA (a) or indirect immunofluorescence (b). The data for ELISA are shown as means of absorbance values.
Development of renal IgG1 deposits in mercury-treated NOD mice
To ascertain that mercury was able to induce a full immune/autoimmune response in NOD mice, the development of renal IgG1 deposits was analysed in the same experimental mice. Kidney sections were examined for the accumulation of IgG1 by a direct immunofluorescence technique. As demonstrated in Fig. 3, mercury-injected NOD mice developed a significant increase in the titres of IgG1 deposits in the kidney glomeruli already on day 10, as compared to the control mice. Injection with mercury for a longer time period (21 days) resulted in the development of even higher titres of renal IgG1 deposits in NOD mice (Fig. 3). Thus, NOD mice are also susceptible to mercury-induced renal IgG1 deposition.
Fig. 3.
Induction of renal IgG1 deposits in young NOD mice by mercury. Kidneys from the same (as described for Fig. 1) mercury-injected (•) or control (○) NOD mice were analysed for the presence of glomerular deposits of IgG1 using a direct immunofluorescence method. Data are shown as the means + 1 s.e. Significant differences between the titres of granular IgG1 deposits in mercury- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. Tukey's test was used to correct the P-values. **P < 0·01, ***P < 0·001.
Analysis of mercury-induced splenic IL-4 and IFN-γ gene expression in NOD mice
In several murine experimental systems including mercury-induced autoimmunity, it was well demonstrated that the up-regulation of IgG1 and IgE synthesis is controlled by IL-4, a Th2 type cytokine (reviewed in [8,21,26–28]). This and the finding that mercury induced an intensive antibody production of IgG1 and IgE isotypes in NOD mice, led us to evaluate the cytokine production in NOD mice after treatment with mercury. We directly quantified the mRNA levels of IL-4 and IFN-γ mRNA in the spleens of mercury-injected and control mice at intervals 10 and 21 days by using real-time PCR methods. As shown in Fig. 4a,a′, spleens of NOD mice, which had been injected with mercury for 10 days, exhibited a ≈4·5-fold increase in IL-4 and a ≈6-fold increase in IFN-γ mRNA levels as compared to those of control mice. Interestingly, 21 days injection with mercury induced a marked increase in the splenic IL-4 mRNA levels (≈10-fold increase) (Fig. 4b), but a striking reduction in the splenic IFN-γ mRNA levels (Fig. 4b′). In fact, there was no detectable IFN-γ mRNA in the spleens of mercury-injected NOD mice at this time point (Fig. 4b′). These findings show that in mercury-injected NOD mice, the increase in IgG1 and IgE antibody production correlates with the up-regulation of IL-4 mRNA expression and that mercury predominantly activates Th2 type of responses in this strain.
Fig. 4.
Chronic injection with mercury up-regulates the expression of splenic IL-4 mRNA young NOD mice. Spleen cells obtained from the same (as described for Fig. 1) mercury-injected (▪ and/or  ) and control (□) NOD mice at intervals day 10 (a, a′) and/or day 21 (b, b′) were analysed for expression of IL-4 (a and b) and IFN-γ (a′ and b′) using real-time PCR assay. The relative fold increase/decrease of each cytokine mRNA in the mercury-injected mice was compare to those in control mice where the levels of each target cytokine (IL-4 and IFN-γ) was considered to be 1 (see Materials and methods for further description). Data for mercury-injected mice are shown as the means + 1 s.e.
) and control (□) NOD mice at intervals day 10 (a, a′) and/or day 21 (b, b′) were analysed for expression of IL-4 (a and b) and IFN-γ (a′ and b′) using real-time PCR assay. The relative fold increase/decrease of each cytokine mRNA in the mercury-injected mice was compare to those in control mice where the levels of each target cytokine (IL-4 and IFN-γ) was considered to be 1 (see Materials and methods for further description). Data for mercury-injected mice are shown as the means + 1 s.e.
Effect of mercury on the development of insulitis in NOD mice
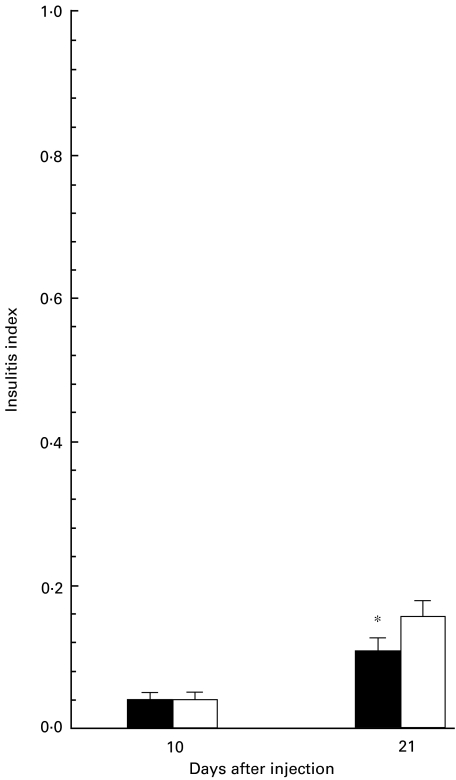
We next asked whether mercury-induced dominant Th2 type of responses could disrupt the Th1-mediated spontaneous development of insulitits in NOD mice. Pancreata sections of mercury-injected and control mice (day 10 and day 21 intervals) were analysed for infiltration of inflammatory mononuclear cells. As shown in Fig. 5, pancreata of NOD mice injected with mercury for 21 days exhibited significantly lower islet infiltration by mononuclear cells as compared with those of control mice. This result implies that chronic treatment with mercury might have a suppressive effect on the development of insulitis in NOD mice.
Fig. 5.
Mercury significantly suppresses the development of insulitis in young NOD mice. Pancreata obtained from the same (as described for Fig. 1) mercury-injected (▪) and control (□) NOD mice at intervals day 10 (4 mice per group) and/or day 21 (11 mice per group) were stained with haematoxylin and eosin. A minimum 40 islets from each mouse were observed and the degree of mononuclear cell infiltration was scored using the following ranking: 0, no infiltration; 1, pervascular/periductular infiltrates with leucocytes; 2, leucocyte infiltration of up to 25% of islet mass; 3, leucocyte infiltration of up to 75% of islet mass; and 4, insulitis with more than 75% of the islet infiltrated or only remnant endocrine tissue. Insulitis index (I) for each mouse was calculated according to the formula described in Materials and methods. Data are shown as the means + 1 s.e. Significant differences between the insulitis indices of mercury- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. Tukey's test was used to correct the P-values. *P = 0·05.
Treatment with mercury delays the onset of diabetes in NOD mice
Finally, we performed a separate experiment to determine whether mercury could also affect the onset of diabetes in NOD mice. A group of prediabetic young NOD/Bom mice (10 mice per group) were continuously treated with mercury for 21 days. Age-matched control mice (9 mice per group) were either treated with saline or left uninjected for an identical time period. The experimental mice were monitored weekly for glucosuria, as a signature for diabetes. As shown in Fig. 6, all (9 of 9) of the control mice became diabetic at 22 weeks of age. In contrast, 60% (6 of 10) of mercury-injected mice developed glucosuria at the same age (P = 0·05 and P = 0·041, Fisher's exact and log-rank test, respectively). In fact, a further delay in the development of diabetes was observed in the mercury-injected mice, i.e. at the age of 28 weeks (when the experiment was terminated) 20% (2 of 10) of these mice were still negative for glucosuria (Fig. 6). This observation indicates that mercury is able to postpone the development of diabetes in NOD mice.
Fig. 6.
Treatment with mercury postpones the onset of diabetes in NOD mice. A group of female NOD/Bom mice (10 mice per group) was repeatedly injected s.c. with mercury (•) for 21 days. The control groups (□) were either injected with sterile saline (5 mice/group) or left un-injected (4 mice/group) for identical time period. From 2 weeks after the last injection, the mercury-injected and control mice were screened weekly for the presence of glucosuria. Diabetes was diagnosed when mice were glucosuric for a consecutive 2 weeks.
Discussion
In the present study, we investigated the in vivo effects of the heavy metal mercury on the immune system of young and prediabetic NOD mice. We found that in these mice, mercury elicited a strong immune activation, which was characterized by the formation of high numbers of IgG1 splenic antibody secreting cells, increased levels of IgE in the serum, production of IgG1 antibodies of different specificities and by deposition of high titres of IgG1 in the kidney. Appearance of these characteristics in the NOD mice, categorizes this strain among the highly mercury-susceptible strains such as A.SW (H-2s) and SJL (H-2s) mice [15–20]. However, in contrast to A.SW and SJL mice, NOD mice do not produce ANolA after treatment with mercury. Synthesis of ANolA is possibly the most specific characteristic of mercury-induced immune activation [11,12,15–20]. Expression of this phenotype is strictly controlled by the H-2 genes, i.e. only mouse strains of H-2s, H-2q and H-2f produce high levels of IgG ANolA after treatment with mercury [11,15,16,20]. In fact, by using intra-H-2 recombinant mouse strains, susceptibility to mercury-induced ANolA production could be mapped to the I–A loci of H-2 class II genes [16]. NOD mice carry a special H-2 genotype (H-2g7) in which the I–E complex is absent because of a deletion in the α chain gene [29] and the I–A chain has a unique sequence [30]. Interestingly, sequence analysis of I–A α and β chains from NOD mice has revealed that the I–A molecule in this strain differs from that of H-2s, H-2q and H-2f genotypes (susceptible to mercury-induced ANolA), but exhibits broad similarities with H-2d genotype [30], which is carried by highly mercury-resistant strains such as DBA/2 and BALB/c mice [11,15,16,20]. Thus, the presence of high degrees of homology between I–Ag7 (NOD mice) and I–Ad (mercury-resistant genotype for ANolA) genotype might explain why NOD mice do not develop ANolA after treatment with mercury.
One of the striking characteristics of mercury-induced immune activation in young NOD mice was the formation of high titres of renal IgG1 deposits. The genetic make up of the host, as well as the specificity of the mercury-induced antibodies play important roles for development of renal IgG deposits [16,20,31,32]. This and the observation that injection with mercury resulted in the production of antibodies of different specificities in NOD mice suggest that these mice carry the susceptible gene(s) for mercury-induced renal IgG1 deposition and that mercury-induced IgG1 antibodies/autoantibodies are able to accumulate in the kidney.
Preferential production of IgE, IgG1 and IgG2b, enhanced expression of IL-4 mRNA and decrease in IFN-γ mRNA expression indicate that chronic exposure to mercury leads to a dominant activation of Th2 cells in NOD mice. Although mercury-induced dominant Th2 responses have already been well described [8–10,21,28], several studies have shown that Th1 effector functions (as measured by corresponding cytokines, or Ig isotypes) were either coincidentally activated with [25,33] or required for the development of mercury-induced Th2 type of responses [34]. In this study, we observed an increase in the expression of IFN-γ (a typical Th1 cytokine) mRNA, which coincided with the up-regulation of IL-4 mRNA expression only at the initial, but not at the chronic phase of mercury-induced immune activation. This suggests that in NOD mice, while infrequent injection with mercury may lead to development of Th1 as well as Th2 type of immune responses, chronic treatment with mercury would induce a dominant Th2 response.
It is known that IFN-γ mainly controls the synthesis of IgG2a in mice [27]. Therefore, it was informative to determine if mercury-induced down-regulation of IFN-γ would affect the production of IgG2c, which is the corresponding isotype for IgG2a in NOD mice [35]. Unfortunately, due to unavailability of a proper antibody against IgG2c, we were not able to measure the levels of this isotype in NOD mice. Thus, the issue whether down-regulation of IFN-γ would also alter the serum levels of IgG2c remains unanswered.
The final observation in this study was that mercury could confer beneficial effects on NOD mice, i.e. it interfered with the development of insulitis and postponed the onset of diabetes in this strain. In fact, beneficial effects of mercury have also been demonstrated in several other experimental autoimmune models. For instance, chronic injection with mercury could protect the Lewis rats against Heymann's nephritis [36] and experimental autoimmune encephalomyelitis (EAE) [37]. Treatment with mercury could also prevent the experimental autoimmune uveoretinitis (EAU) and pinealitis (EAP) in (Lewis × Brown-Norway) F1 rats [38]. Furthermore, multiple administrations of subtoxic doses of mercury were able to down-regulate the development of diabetes in streptozotocin-induced diabetes in DA rats [39]. Concerning these experimentally induced autoimmune diseases, it has been suggested that mercury confers it beneficial effects by activating regulatory cells in the form of either suppressor CD8+ T cells (in the case of Heymann's nephritis) [36] or TGF-β producing helper T cells (in the case of EAE) [40], or regulatory Th2 cells (in the cases of streptozotocin-induced diabetes and EAU) [39,41]. This suggestion and our finding that mercury induced a typical Th2 type of immune response in NOD mice led us to propose the following mechanism for mercury-induced partial protection against diabetes in these mice. We conceive that mercury either directly and/or via induction of IL-4 synthesis would first activate a subset of regulatory T cells, which are predominantly (if not completely) Th2 cells. This subset of regulatory Th2 cells may be quiescent in NOD mice, possibly due to the absence of either IL-4 synthesis or a proper milieu required for clonal expansion and effector function [42,43]. Mercury-induced activation of regulatory Th2-type cells would thereby counter-regulate the Th1-mediated autoimmune responses in NOD mice and confer protection against diabetes. Why mercury-induced protection against diabetes was not complete may have two explanations. First, NOD mice produce low levels of IL-4 and administration of exogenous IL-4 in vivo prevents the development of insulitis and diabetes in these mice [42,44]. In fact, to observe the preventive effects of IL-4, this cytokine has to be either continuously administered into the mice or introduced as a transgene [42,44,45]. Therefore, it is possible that 3 weeks of treatment with mercury would induce an increase in IL-4 production, which is inadequate for long-term activation of the regulatory Th2 cells. In such a condition, IL-4 dependent regulatory Th2 cells may again return to the hypo-responsive state, which might lead to an incomplete prevention of diabetes in NOD mice. Second, as a polyclonal T-cell activator, mercury may activate not only regulatory, but also other Th2 cells, which are incapable of regulating the Th1 cell-mediated autoimmune responses in NOD mice. Expansion of these cells would probably interfere with the clonal expansion and effector function of regulatory Th2 cells in such a way that the later cells either lose or reduce their ability to confer complete protective effects on the development of diabetes. Presently, we are performing experiments to evaluate the accuracy of these explanations and to characterize the mercury-induced regulatory Th2 cells in NOD mice.
In summary, the data presented in this paper demonstrate that mercury induces a strong Th2 type immune activation, capable of interfering with development of spontaneous autoimmune diabetes in NOD mice. Since the disease in these mice shares several similarities with IDDM in humans, it would be of interest to evaluate whether, mercury could possibly have beneficial effects on development of IDDM in man. We believe that epidemiological studies on the prevalence of IDDM in populations living in mercury-polluted areas would be useful and would help us to understand better the regulatory mechanisms that control IDDM in humans.
Acknowledgments
We thank Professor Klavs Berzins for critically reading the manuscript, Mrs Lena Israelsson for her excellent technical assistance and Mrs Margareta Rodensjo for preparing the pancreatic slides. This study was supported by grants from the Swedish Medical Research Council and the Swedish Foundation for Health Care Sciences and Allergy Research.
References
- 1.Castano L, Eisenbarth GS. Type-I Diabetes: a chronic autoimmune disease of human, mouse and rat. Annu Rev Immunol. 1990;8:647–79. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- 2.Kikutani H, Smakino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–323. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 3.Toyoda H, Formby B. Contribution of T cells to the development of autoimmune diabetes in the NOD mouse model. Bioessays. 1998;20:750–7. doi: 10.1002/(SICI)1521-1878(199809)20:9<750::AID-BIES8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Todd JA. From genome to aetiology in a multifactorial disease, type 1 diabetes. Bioessays. 1999;21:161–74. doi: 10.1002/(SICI)1521-1878(199902)21:2<164::AID-BIES10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 6.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–8. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 7.Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol. 1995;7:793–8. doi: 10.1016/0952-7915(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M, Druet P, Gleichmann E. Th2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 9.Griem P, Gleichmann E. Metal ion induced autoimmunity. Curr Opin Immunol. 1995;7:831–8. doi: 10.1016/0952-7915(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 10.Eneström S, Hultman P. Does amalgam affect the immune system? A controversial issue. Int Arch Allergy Immunol. 1995;106:180–203. doi: 10.1159/000236843. [DOI] [PubMed] [Google Scholar]
- 11.Goter Robinson CJ, Abraham AA, Balazs T. Induction of anti-nuclear antibodies by mercuric chloride in mice. Clin Exp Immunol. 1984;58:300–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Hultman P, Eneström S. Mercury induced B-cell activation and antinuclear antibodies in mice. Clin Lab Immunol. 1989;28:143–50. [PubMed] [Google Scholar]
- 13.Hultman P, Eneström S. Murine mercury-induced immune-complex disease: effect of cyclophosphamide treatment and importance of T cells. Br J Exp Path. 1989;70:227–36. [PMC free article] [PubMed] [Google Scholar]
- 14.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity: the role of T-helper cells. J Autoimmun. 1995;8:809–23. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 15.Mirtcheva J, Pfeiffer C, De Bruijn JA, et al. Immunological alterations inducible by mercury compounds. III. H-2A acts as an immune response and H-2E as an immune ‘suppression’ locus for HgCl2-induced antinucleolar autoantibodies. Eur J Immunol. 1989;19:2257–61. doi: 10.1002/eji.1830191212. [DOI] [PubMed] [Google Scholar]
- 16.Hultman P, Bell LJ, Eneström S, et al. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992;65:98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- 17.Hultman P, Bell LJ, Eneström S, et al. Murine susceptibility to mercury. II. Autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin Immunol Immunopathol. 1993;68:9–20. doi: 10.1006/clin.1993.1088. [DOI] [PubMed] [Google Scholar]
- 18.Hultman P, Turley SJ, Eneström S, et al. Murine genotype influences the specificity, magnitude and persistence of murine mercury-induced autoimmunity. J Autoimmun. 1996;9:139–49. doi: 10.1006/jaut.1996.0017. [DOI] [PubMed] [Google Scholar]
- 19.Hanley GA, Schiffenbauer J, Sobel ES. Class II haplotype differentially regulates immune response in HgCl2-treated mice. Clin Immunol Immunopathol. 1997;84:328–37. doi: 10.1006/clin.1997.4405. [DOI] [PubMed] [Google Scholar]
- 20.Abedi-Valugerdi M, Möller G. Contribution of H-2 and non-H-2 genes in the control of mercury-induced autoimmunity. Int Immunol. 2000;12:1425–30. doi: 10.1093/intimm/12.10.1425. [DOI] [PubMed] [Google Scholar]
- 21.Mathieson PW. Mercury: god of Th2 cells. Clin Exp Immunol. 1995;102:229–30. doi: 10.1111/j.1365-2249.1995.tb03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gronowicz E, Coutinho A, Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976;6:588–90. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- 23.Al-Balaghi S, Möller E, Möller G, et al. Mercury induces polyclonal B cell activation, autoantibody production and renal immune complex deposits in young (NZB × NZW) F1 hybrids. Eur J Immunol. 1996;26:1519–26. doi: 10.1002/eji.1830260717. [DOI] [PubMed] [Google Scholar]
- 24.Heid CA, Stevens J, Livak KJ, et al. Real time quantitative PCR. Genome Res. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 25.Hu H, Möller G, Abedi-Valugerdi G. Mechanism of mercury-induced autoimmunity: both T helper 1- and T helper 2-type responses are involved. Immunol. 1999;96:348–57. doi: 10.1046/j.1365-2567.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snapper CM, Finkelman FD, Paul WE. Regulation of IgG1 and IgE production by interleukin 4. Immunol Rev. 1988;102:51–77. doi: 10.1111/j.1600-065x.1988.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 27.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–35. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 28.Ochel M, Vohr H-W, Pfeiffer C, et al. IL-4 is required for the IgE and IgG1 increase and IgG1 autoantibody formation in mice treated with mercuric chloride. J Immunol. 1991;146:3006–11. [PubMed] [Google Scholar]
- 29.Hattori M, Buse JB, Jackson RA, et al. The NOD mouse: recessive diabetogenic gene in the major histocompatibility complex. Science. 1986;231:733–5. doi: 10.1126/science.3003909. [DOI] [PubMed] [Google Scholar]
- 30.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II, I–A b chain is unique. Proc Natl Acad Sci. 1987;84:2435–2439. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hultman P, Eneström S. Mercury induced antinuclear antibodies in mice: characterization and correlation with renal immune complex deposits. Clin Exp Immunol. 1988;71:269–74. [PMC free article] [PubMed] [Google Scholar]
- 32.Abedi-Valugerdi M, Hu H, Möller G. Mercury-induced renal immune complex deposits in young (NZB × NZW) F1 mice: characterization of antibodies/autoantibodies. Clin Exp Immunol. 1997;110:86–91. doi: 10.1046/j.1365-2249.1997.4901392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillespie KM, Saoudi A, Kuhn J, et al. Th1/Th2 cytokine gene expression after mercury chloride in susceptible and resistant rat strains. Eur J Immunol. 1996;26:2388–92. doi: 10.1002/eji.1830261018. [DOI] [PubMed] [Google Scholar]
- 34.Kono DH, Balomenos D, Pearson DI, et al. The prototype Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th/Th2 imbalance. J Immunol. 1998;161:234–40. [PubMed] [Google Scholar]
- 35.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Meth. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier L, Galceran M, Pasquier R, et al. Down regulation of Hemann's nephritis by mercuric chloride. Kidney Int. 1987;32:227–32. doi: 10.1038/ki.1987.196. [DOI] [PubMed] [Google Scholar]
- 37.Pelletier L, Rossert J, Pasquier R, et al. Effect of HgCl2 on experimental allergic encephalomyelitis in Lewis rats. HCl2-induced down-regulation of the disease. Eur J Immunol. 1988;18:243–7. doi: 10.1002/eji.1830180210. [DOI] [PubMed] [Google Scholar]
- 38.Saoudi A, Bellon B, De Kozak Y, et al. Prevention of experimental autoimmune uveoretinitis and experimental autoimmune pinealitis in (Lewis × Brown-Norway) F1 rats by HgCl2 injections. Immunol. 1991;74:348–54. [PMC free article] [PubMed] [Google Scholar]
- 39.Stosic-Grujicic S, Mijatovic S, Ejdus L, et al. Enhancement of Th2-type activity downregulated diabetes induction. Transplant Proc. 1996;28:3257. [PubMed] [Google Scholar]
- 40.Bridoux F, Badou A, Saoudi A, et al. Transforming growth factor β (TGF-β)-dependent inhibition of T Helper cell 2 (Th2) -induced autoimmunity by self-major histocompatibility complex (MHC) class II-specific, regulatory CD4+ T cell lines. J Exp Med. 1997;185:1769–75. doi: 10.1084/jem.185.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saoudi A, Kuhn J, Huygen K, et al. TH2 activated cells prevent experimental autoimmune uveoretinitis, a TH1-dependent autoimmune disease. Eur J Immunol. 1993;23:3096–103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]
- 42.Cameron MJ, Arreaza GA, Zucker P, et al. 1997. IL-4 prevents insulitis and insulin-dependent diabetes mellitus in non-obese diabetic mice by potentiation of regulatory T helper-2 cell function. J Immunol. 1997;159:4686–92. [PubMed] [Google Scholar]
- 43.Jaramillo A, Gill BM, Deloyitch TL. Insulin dependent diabete mellitus in the non-obese diabetic mouse: a disease mediated by T cell anergy? Life Sci. 1994;55:1163–77. doi: 10.1016/0024-3205(94)00655-5. [DOI] [PubMed] [Google Scholar]
- 44.Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–9. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]