Abstract
A previous case report described the formation of a complex between a monoclonal IgA with cryolabile properties and C-reactive protein (CRP). Our study provides the first evidence for the frequent occurrence of CRP in cryoglobulins (Cg) of all three types according to Brouet's classification. We performed a systematic immunochemical analysis of cryoglobulins from 18 patients by Western blotting and in 15 of 18 cryoprecipitates a single band (23 KD), immunoreactive with anti-CRP antibody, was demonstrable irrespective of the clonal composition of the cryoglobulins. This band was detectable in 4/5 of type I, in 6/8 of type II, and in 5/5 of type III cryoprecipitates, classified according to Brouet et al. In addition, the complement proteins C1q and C3 were present in nearly all CRP-containing cryoglobulins, presumably reflecting previous activation of the classical complement pathway at least. All three CRP-negative cryoprecipitates were derived from sera with low cryoglobulin content (1–2 g/l). Longitudinal investigation of 23 cryoprecipitates from seven patients confirmed that successful detection of CRP by Western blotting depends on the protein concentration of the cryoglobulins. Since complexed CRP was previously shown to be an effective activator of complement, via C1q binding, CRP may modulate pathophysiologic effects mediated by cryoglobulins in vivo.
Keywords: C-reactive protein, complement, cryoglobulins
Introduction
Cryoglobulins (Cg) are serum proteins that reversibly precipitate at low temperatures. On the basis of their immunoglobulin composition Cg can be classified into three types, according to Brouet et al. [1]. Type I Cg consists of a monoclonal component alone, type II is a mixture of monoclonal and polyclonal immunoglobulins, and type III consists of a mixture of polyclonal immunoglobulins of different isotypes. In both type II and III Cg polyclonal IgG is bound to another immunoglobulin, which acts as an anti-IgG rheumatoid factor (RF). Cryoglobulinaemia occurs in the presence of immunoproliferative diseases, autoimmune disorders and various infections, especially chronic hepatitis C virus infection (HCV) [2,3]. Cg may lead to immune complex vasculitis due to precipitation of Cg in small vessels, leading to their deposition in the vessel walls and the subsequent infiltration of neutrophils and mononuclear cells. As a result of systemic vasculitis Cg can cause a variety of clinico-pathological symptoms, such as vascular purpura, arthralgias, weakness, neuropathy or glomerulonephritis. The occurrence of vasculitis seems to correlate with the capacity of Cg to activate complement rather than the Cg level [3]. It is likely that some of the pathological consequences of mixed Cg depend on the composition of the immune complexes [4]. Several studies have shown that Cg may also bind proteins other than Ig, e.g. complement (C)-factors [5,6], fibronectin [7], lipoproteins and bacterial or viral antigens [reviewed in 8 and 9]. However, these interactions between the cryolabile immunoglobulins and other components of Cg are not well understood.
C-reactive protein (CRP) is the major acute-phase protein in humans, the serum concentration of which increases dramatically during infections or tissue damage of other causes. This rise in CRP concentrations is the consequence of an increased transcription rate of the CRP gene in hepatocytes after stimulation by proinflammatory cytokines, such as interleukin 6, interleukin 1 and TNFα [10]. Structurally, CRP is a non-glycosylated, macromolecular protein (molecular weight: 115 135 D) which is composed of five identical, non-covalently linked subunits each of a molecular weight of 23 027 D (206 amino acids). The biological function of CRP is still unknown, yet it is generally believed to be related to its ability to recognize foreign pathogens or products of damaged autologous cells via its broad spectrum recognition functions. Due to its reactivity with the complement system, CRP can participate in humoral and cellular inflammatory reactions and host defence [11–13]. As demonstrated originally by Kaplan and Volanakis [14], CRP activates the classical pathway of complement (C) when bound to appropriate ligands. Such ligands include phosphorylcholine-containing substances such as pneumococcus type C polysaccharide (CPS), cholesterol emulsions containing lecithin or sphingomyelin, certain polyanions including deoxyribonucleic acids (DNA) as well as histones, to which CRP binds in a calcium-dependent manner. In addition, a variety of cationic substances interact with CRP in the absence of calcium [11,13]. CRP-mediated C-activation is initiated by binding of one molecule C1q to two adjacent CRP molecules associated with a ligand. The interaction between CRP and C1q results in activation of the classical C-cascade, leading to complement consumption, C-dependent opsonization and clearance reactions as well as to the haemolysis of CPS-coated red blood cells or lysis of liposomal membranes [11–13].
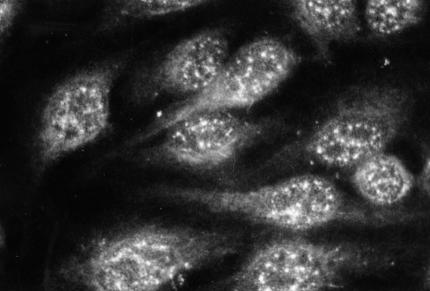
We have reported recently on the usage of HEp-2 cell monolayers to test CRP-mediated C activation in sera [15]. The sequential activation cascade starting with CRP followed by C1q, C1r, C1s, C4 and C3 could be visualized easily on HEp-2 cells by indirect immunofluorescence. It was shown that the binding of the classical C components occurred in the same speckled nuclear pattern, as was demonstrated earlier for CRP [15–17]. When tested in the HEp-2 cell assay some normocomplementemic sera of patients with elevated CRP levels showed incomplete CRP-mediated C activation, ending with C1s. Most of these sera had been obtained from patients with bacterial endocarditis (BE) [15], which are known to contain both circulating immune complexes (CIC) and Cg [18]. In our initial experiments, we made the surprising discovery that isolated Cg from these sera produced a speckled nuclear immunofluorescence pattern on HEp-2 cell monolayers when stained with anti-CRP antibodies (Fig. 1). This observation suggested that CRP may be contained in Cg. In the literature we found one previous case report, describing complex formation between a monoclonal IgA cryoprotein and CRP [19]. Our results reported here present further immunochemical evidence for the presence of CRP in most circulating Cg.
Fig. 1.
CRP-specific reactivity (speckled nuclear pattern) of a pure monoclonal cryoglobulin (IgM/λ) on HEp-2 cells by indirect immunofluorescence. Staining by rabbit antihuman CRP followed by FITC-conjugated swine antirabbit Ig (see text for details).
Materials and methods
Sera and reagents
Native serum samples were immediately frozen and stored at either −20°C (2 weeks) or at – 70°C (more than 2 weeks). CRP, C3 and C4 concentrations in the sera were routinely determined by laser nephelometry (Behring, Germany). Total haemolytic C activity (CH 50) in the sera was measured routinely according to a previously published method [20]. The complement split product C3d was measured as previously described in fresh EDTA plasma [21].
Purified human CRP (purity >99% in SDS-PAGE) was purchased from Calbiochem, USA (lots 367892, 901733, B09444; protein concentration: 1 mg/ml).
Preparation and classification of cryoglobulins
Twenty ml venous blood was taken at a minimum of 12 h after the last meal and transported at 37°C to the laboratory. After centrifugation serum was collected, incubated at 20°C and 4°C for 1 day each and examined after 2 and 24 h for cryoprecipitation. Cg were washed six times with 0·9% saline to separate the cryoprecipitates from adhering proteins. Contamination of Cg with serum proteins, particularly albumin, was excluded by immunofixation. The immunoglobulin composition of the purified Cg was estimated by immunofixation (Paragon Beckmann) and immunoelectrophoresis using poly- and monospecific antisera [3]. Protein concentration in Cg was estimated by diluting aliquots of Cg in 0·1 m NaOH and reading the absorbance in a spectrophotometer at 280 nm [22]. The values were derived from a standard curve using purified human gamma globulin (Polyglobin NR from Bayer, Germany). Cg were stored at −20°C until use.
Indirect immunofluorescence on HEp-2 cells
Immunofluorescence on HEp-2 cells was performed as described previously [15]. Monolayers of acetone-methanol fixed HEp-2 cells were obtained from Pasteur/Kallestad, USA. Veronal buffered saline, pH 7·2 (VBS) containing Ca2+/Mg2+ (BioMerieuxR, France) was used for dilution of reagents and all washing procedures. Cell slides were incubated with Cg prewarmed at 37°C (1 : 10 dilution in VBS) for 20 min followed by washing in buffer for 15 min. Cells were routinely stained by a two-step reaction with a non-conjugated primary antibody (rabbit antihuman CRP, Dako, Denmark) followed by a species-specific FITC-conjugated secondary antibody (swine antirabbit Ig from Dako). In control experiments the following antibodies were applied: (F(ab′)2)-sheep antihuman CRP (primary antibody) and FITC-conjugated (F(ab′)2)-swine antisheep IgG (secondary antibody), both from the Binding Site Laboratory (Heidelberg, Germany). Primary and secondary antibodies were usually incubated at 1 : 10 dilutions in VBS for 20 min and washed as indicated above. The slides were examined with a fluorescence microscope (Axioskop-20, Zeiss, Germany) under oil immersion (magnification 1000-fold). Photographs were automatically exposed on Kodak 400 Ektachrome films.
Western blotting
Sodium dodecyl sulphate (SDS) electrophoresis and subsequent Western blotting were carried out according to Laemmli [23] and Towbin and Staehelin [24]. Continuous 11% or 12% polyacrylamide running gels were prepared in 0·385 m Tris-HCl (pH 8·9) containing 0·1% SDS. The 4·5% stacking gels were prepared in 0·125 m Tris-HCl (pH 6·7) containing 1% SDS. Washed Cg were disolved in distilled water before measuring the protein concentration. Samples were diluted 1 : 1 in SDS sample buffer (0·0625 m Tris-HCl (pH 6·7), 20% glycerol, 4% SDS, 10% β-mercaptoethanol, 0·004% bromphenol blue) and boiled for 5 min. Proteins were separated at 4°C in electrode buffer (0·05 m Tris, 0·3 m glycine, 0·1% SDS) using a minivertical slab gel apparatus (BioRad Laboratories, USA). To estimate molecular weights, two protein standards were used (prestained marker, Sigma, USA and low molecular weight marker (LMW) stained with Ponceau SS, Sigma, USA). Electrotransfer of proteins to nitrocellulose sheets (Schleicher & Schüll, BA 083, 0·45 µm pore size) was performed at a constant potential of 100 V in transfer buffer (0·025 m Tris, 0·192 m glycine, 20% (v/v) methanol, 0·1% SDS, pH 8,3) using a BioRad trans-blot cell. After transfer the nitrocellulose membrane sheets were blocked with 4% BSA for 2 h, and incubated for 60 min with one of the following antihuman antisera: rabbit anti-CRP, rabbit anti-C1q and rabbit anti-C3c antibodies (all from Dako) diluted 1 : 100 in 4% BSA. The sheets were washed six times in washing buffer (PBS-BSA with 0·03% Tween 20) for 30 min. Peroxidase conjugated antirabbit IgG F(ab′)2 fragment (Dianova, Germany), diluted 1 : 200 in washing buffer (PBS-BSA-Tween), was added for 30 min followed by six washings for 5 min in buffer. Alternatively, direct detection of CRP was performed with peroxidase-conjugated sheep (F(ab′)2) antihuman CRP (The Binding Site) diluted 1 : 50 in 4% BSA and incubated for 45 min. Blots were developed with substrate solution (3-amino-9-ethylcarbachol). The reaction was stopped with 7% acetic acid.
Quantitative measurement of CRP in Cg
To estimate the CRP concentration in Cg, an enzyme-linked immunoassay (ELISA) and an immuno-turbidimetric method were applied. The ELISA assays followed the procedures previously described by Highton and Hessian [25] with minor modifications Ninety-six-well plastic microtitre plates (Greiner immunoplate F) were coated with rabbit antihuman CRP (protein concentration 8·3 mg/ml; Dako) or sheep (F(ab′)2) antihuman CRP (protein concentration 5 mg/ml; The Binding Site) for 90 min at 37°C in bicarbonate buffer pH 9·6. Antisera were added as 200 µl aliquots, diluted 1/300 or 1/500 for F(ab′)2 fragments and 1/1000 for the whole antisera, respectively. The wells were then treated for 60 min at 37°C with 200 µl of VBS containing 1% bovine serum albumin (VBS-BSA, ICN, Germany). Thereafter, the plates were washed three times in VBS-BSA containing 0·05% Tween 20 (VBS-BSA-T). Cg were solubilized at 37°C for 30 min, diluted 1 : 10 in VBS-BSA and added as 200 µl aliquots in duplicate wells. The plates containing diluted Cg, CRP standard and buffer controls were incubated for 90 min at 37°C, followed by three washing steps. Peroxidase conjugated antisera (HRP-sheep F(ab′)2 antihuman CRP; protein concentration 1 mg/ml; The Binding Site Laboratory) were diluted 1/300 in VBS-BSA and 200 µl were added to each well. Following another 90-min incubation at 37°C the plates were washed in VBS-BSA-T and 100 µl of substrate o-phenylendiamine (OPD; Dako) were added. The substrate was dissolved in 0·04 m Tris-HCl and 0·15 m NaCl, pH 7·6. The enzyme reaction was terminated after 30 min by addition of 50 µl of 3 m HCl. Absorbance at 490 nm was read in a Dynatech ELISA reader. Standardization of the assay was achieved using a purified CRP standard solution (Calbiochem) containing a CRP concentration of 1 mg/ml. Duplicate standard dilutions from 1 µg/ml to 0·001 µg/ml were employed to establish standard curves (linearity from approximately 0·1 µg/100 ml−2 µg/100 ml).
CRP concentrations of Cg were also estimated with an immuno-turbidimetric assay (LaboTest, C-Reaktives Protein, Super Sensitiv, LaboMed, Waldkirch, Germany) with a sensitivity down to 5 µg CRP/100 ml. For this approach Cg were washed six times with 0·9% saline or with veronal buffered saline (VBS), pH 7·2 containing 10 mm EDTA [20].
Results
Clinical data of cryoglobulinaemic patients
The clinical characteristics of the 18 cryoglobulinaemic patients under study are presented in Table 1. Four of the five patients with cryoglobulinaemia type I had immunocytomas (NHL) producing monoclonal immunoglobulins of the IgM class; the fifth patient (No. 1) had a monoclonal gammopathy of undetermined origin. Eight patients had mixed cryoglobulinaemia type II and five patients type III. Cg of type II consisted of a mixture of polyclonal IgG with varying monoclonal constituents, predominantly of the IgM isotype (n = 6). Cg of type III consisted of polyclonal IgG and IgM. Associated diseases in mixed cryoglobulinaemia were malignant lymphomas (n = 4), hepatitis C virus infection (n = 5), chronic hepatitis B virus infection (n = 2), streptococcal endocarditis (n = 2) and septic skin abscesses (n = 1). Patient no. 7 had liver cirrhosis and hepatocellular carcinoma due to chronic hepatitis B virus infection. In only one patient (no. 18) could no underlying cause for the cryoglobulinaemia be detected. Clinically the majority of patients presented either with cutaneous vasculitis, glomerulonephritis or polyneuropathy. Two patients (nos. 14, 15) were asymptomatic.
Table 1.
Clinical features and associated diseases in 18 cryoglobulinaemic patients
| Type of cryoglobulin | Clinical manifestations | Underlying diseases | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Sex | Age | Ig 1 | Ig2 | Skin | Kidney | PNP | CNS | Arthr. | Raynaud | NHL | HBs-Ag | HCV-antibody | HCVRNA | Other Therapy |
| 1 | f | 59 | Gκ | − | + | (+) | − | − | − | − | − | − | − | − | none |
| 2 | f | 67 | Mλ | − | + | − | + | − | + | − | + | − | − | − | P |
| 3 | f | 61 | Mκ | − | − | − | − | + | − | + | + | − | − | − | C(o) + P |
| 4 | m | 42 | Mκ | − | + | − | − | + | − | − | + | − | − | − | COP |
| 5 | f | 74 | Mλ | − | + | (+) | − | − | − | − | + | − | − | − | C(o) + P |
| 6 | m | 36 | Aκ | polyG | + | − | − | − | + | − | − | + | − | − | Aza |
| 7 | f | 83 | Gκ | polyG | − | + | − | − | + | − | − | + | − | − | C(b) + P |
| 8 | m | 38 | Mκ | polyG | − | + | − | − | − | − | Nt | − | + | − | None |
| 9 | f | 39 | Mκ | polyG | + | + | + | − | + | − | + | − | + | − | C(b) + P, PP |
| 10 | f | 66 | Mκ | polyG | − | − | − | − | + | + | + | − | − | − | C(o) + P |
| 11 | m | 73 | Mκ | polyG | + | − | + | − | − | − | + | − | + | + | P |
| 12 | f | 81 | Mκ | polyG | + | (+) | + | + | + | + | Nt | − | + | + | C(o) + P |
| 13 | f | 65 | Mλ | polyG | − | − | − | + | + | − | + | − | − | − | C(o) + P |
| 14 | m | 51 | polyG | polyM | − | − | − | − | − | − | Nt | − | − | − | BE Ab |
| 15 | f | 37 | polyG | polyM | − | − | − | − | − | − | Nt | − | − | − | BA Ab |
| 16 | m | 60 | polyG | polyM | + * | − | − | − | − | − | Nt | − | − | − | BE Ab |
| 17 | f | 49 | polyG | polyM | + | + | − | − | − | − | Nt | − | + | + | PP |
| 18 | f | 80 | polyG | polyM | − | − | + | − | − | + | − | − | − | − | P |
Skin, skin involvement, e.g. purpura; Kidney: (+, acute or chronic renal failure; (+), microhaematuria or proteinuria <2 g/d, no renal failure); PNP, polyneuropathy; CNS, central nervous system (cerebral symptoms due to hyperviscosity (nos. 3, 4 & 13), apoplexy (no. 12); Arthr., arthralgia, arthritis; Raynaud, Raynaud's phenomenon; NHL, non-Hodgkin's lymphoma: +, bone marrow biopsy positive for NHL; –, biopsy negative for NHL; Nt, biopsy not performed; HBs-Ag, hepatitis B surface antigen; HCV antibody, antibody against hepatitis C; C, cyclophosphamide (o, oral; b, bolus); P, prednisone; Aza, azathioprine; COP, cyclophosphamide, vincristin, prednisone; PP, plasmapheresis; Ab, antibiotics; BE, bacterial endocarditis; BA, bacterial skin abscesses
Purpura fulminans. Cryoglobulins are listed in the table according to their type in Brouet's classification [1].
Detection of CRP in a monoclonal cryoglobulin by indirect immunofluorescence on HEp-2 cells
A cryoprecipitate from a 74-year-old female patient (S.L.), presenting with cutaneous vasculitis, was isolated from serum by cold precipitation and extensive washing, as described under Methods. Macroglobulinaemia Waldenström (IgM/l) was diagnosed and the isolated Cg, containing exclusively the same monoclonal IgM/λ as found in the serum, was classified as type I according to Brouet et al. [1]. After incubation of the prewarmed, resolubilized Cg on HEp-2 cell monolayers, the characteristic speckled pattern of immunofluorescence was obtained when stained for CRP by indirect immunofluorescence (Fig. 1). This reactivity was also observed with Cg from several patients with type II and type III Cg. False positive binding of antibodies by rheumatoid factor activity in the IgM Cg was excluded by using F(ab′)2-preparations of primary and secondary antibodies.
Analysis of CRP and complement factors in cryoglobulins by Western blotting
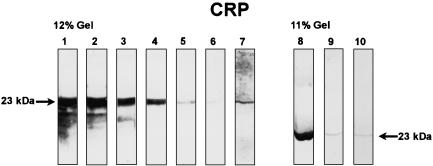
When purified CRP was tested by Western blotting, a single band (23 kD) corresponding to the subunit of CRP was seen [26]. Bands of the same molecular weight were obtained with Cg after staining with CRP-specific antibodies (Fig. 2). Staining for C1q in Cg by Western blotting produced two bands: a stronger one at 26·5 kD and a weaker one at 24 kD (data not shown). As was previously demonstrated [27], the 26·5 kD band represented a combination of the A and B chains and the 24 kD band corresponds to the C chain of the C1q molecule, respectively. Staining of blotted Cg for C3 always revealed several bands under the experimental conditions used. The simultaneous occurrence of three bands with molecular weights of 75 kD (β chain of C3), 43 kD and 40 kD (fragments of the α chain of C3), respectively, was regularly seen in positive cases, whereas the presence of an additional, smaller band (e.g. 27 kD) was variable (data not shown). These bands have previously been identified as characteristic split products of the C3 molecule [28].
Fig. 2.
Western blot analysis of CRP in cryoglobulins. Strips 1–6: purified CRP in different concentrations (1 = 100 µg/ml, 2 = 50 µg/ml, 3 = 10 µg/ml, 4 = 5 µg/ml, 5 = 1 µg/ml, 6 = 0·5 µg/ml). Strip 7 = cryoglobulin S.E., strip 8 = cryoglobulin G.A., strip 9 = cryoglobulin S.A., strip 10 = cryoglobulin S.L. (F(ab′)2-anti-CRP).
Search for CRP, C1q and C3 in cryoglobulins
Isolated Cg from the 18 patients were analysed for the presence of CRP and the complement factors C1q and C3 by Western blotting. A summary of the results is presented in Table 2. CRP was detected in 15 of 18 Cg, irrespective of immunoglobulin composition or Brouet's classification type. Two of the three CRP-negative Cg belonged to type II and one to type I Cg, respectively. Serum CRP levels of the patients with CRP-negative Cg were lower (mean: 0·73 mg/100 ml) when compared with the CRP-positive Cg group (mean: 3·0 mg/100 ml). Obviously, however, CRP levels as low as 0·5 mg/100 ml could be found in CRP-positive cryoglobulinaemic sera (e.g. Patient nos. 9, 12 & 18). Therefore a simple correlation between underlying diseases and the CRP content of Cg was not apparent.
Table 2.
Summary of the Western blot results and serological data in 18 cryoglobulinaemic patients
| Type of cryoglobulin | Cryoglobulin CRP | Serum | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Ig1 | Ig2 | Protein content of cryoglobulin g/l | CRP | C1q | C3 | CRP mg/dl | CH 50 U/ml | C3 g/l | C4 g/l | C3d mg/l | RF |
| 1 | Gκ | – | 1 | – | + | + | 0·7 | 23 | 0·74 | 0·37 | 33 | – |
| 2 | Mλ | – | Nt | + | + | + | 0·8 | 19 | Nt | Nt | 24·2 | – |
| 3 | Mκ | – | 35 | + | + | Nt | 2·1 | Nt | Nt | Nt | Nt | Nt |
| 4 | Mκ | – | 19 | + | + | + | 0·6 | Nt | Nt | Nt | Nt | Nt |
| 5 | Mλ | – | 2 | + | + | + | 2·1 | 21 | 0·7 | 0·34 | Nt | + |
| 6 | Aκ | G | 2 | – | + | + | 1·0 | Nt | 0·82 | 0·42 | Nt | + |
| 7 | Gκ | G | 4 | + | + | + | 4·7 | 10 | 0·49 | 0·17 | 13·5 | + |
| 8 | Mκ | G | 8 | + | + | + | 1·6 | Nt | 0·46 | 0·06 | 39 | + |
| 9 | Mκ | G | 4 | + | + | Nt | 0·5 | 21 | 0·71 | 0·08 | Nt | + |
| 10 | Mκ | G | 25 | + | Nt | Nt | 1·9 | Nt | Nt | Nt | Nt | Nt |
| 11 | Mκ | G | 1 | – | + | + | 0·5 | 10 | 0·7 | < 0·06 | 42·7 | + |
| 12 | Mκ | G | 12 | + | + | + | 0·5 | 16 | Nt | Nt | 29·7 | + |
| 13 | Mλ | G | 22 | + | Nt | + | 10·3 | 10 | Nt | Nt | 41·4 | – |
| 14 | G | M | 9 | + | + | + | 3·2 | 44 | Nt | Nt | Nt | – |
| 15 | G | M | 3 | + | + | + | 7·5 | 20 | Nt | Nt | < 10 | + |
| 16 | G | M | 8 | + | + | + | 4 | 30 | Nt | Nt | < 10 | + |
| 17 | G | M | 7 | + | + | + | 4·8 | 7 | 0·25 | < 0·06 | 37·6 | + |
| 18 | G | M | 3 | + | + | + | 0·5 | Nt | 0·82 | 0·42 | Nt | Nt |
The Western blot results of cryoglobulins are marked with + in the presence of characteristic bands for CRP, C1q or C3, respectively, or as – in their absence. Nt = not tested. Normal values: CRP: < 0·5 mg/dL; CH50 = 20–50 U/ml; C3: 0·5–1·2 g/l; C4: 0·2–0·5 g/l; C3d: < 10 mg/l. Cryoglobulins are listed in the table according to their type in Brouet's classification [1].
With regard to the presence of complement factors in Cg, both C1q and C3 were found in all cases tested (Table 2). In only three patients (nos. 1, 6 & 11) were C1q and C3 detectable in Cg lacking CRP. In the corresponding sera the CH50 values were depressed in six of 12 patients. Low C3 or C4 levels were found in three and five of nine sera, respectively. C3d levels were elevated in eight of 10 sera.
The protein concentration of Cg in the three CRP-negative cases (nos. 1, 6 & 11) was lower (mean 1·33 g/l; range 1–2 g/l) compared to the CRP-positive cases (mean 11·5 g/l; range 2–35 g/l). To demonstrate the correlation between the occurrence of CRP and the protein concentration of cryoprecipitates, we analysed a total of 23 cryoprecipitates from seven patients by Western blotting (Table 3). Two patients were negative for CRP in the initial study (nos. 1 & 11). All CRP-negative cases showed a low concentration of Cg (1 g/l). In contrast, Western blot analysis of samples with high concentrations of Cg from the same patients, then revealed strong CRP-specific bands. Interestingly, CRP, C1q and C3 were not detectable in a cryoprecipitate from patient B.P. obtained 2 days after the patient had undergone plasmapheresis treatment (date of sample 10/88). A correlation between the treatment and a low protein concentration of cryoprecipitates was also found in patients no. 9 (plasmapheresis and pulse cyclophosphamide treatment in 7/87) and no. 11 (pulse cyclophosphamide treatment 8/89–1/90).
Table 3.
Association between the protein content of cryoprecipitates and the detection of CRP in 23 cryoglobulins from seven patients
| Cryoglobulin | |||
|---|---|---|---|
| Patient no. | Date of blood sample | Protein content (g/l) | CRP (Western blot) |
| 1 | 11/93 | 1 | − |
| 3/95 | 12 | + + | |
| 4/95 | 5 | + + | |
| 10/95 | 42 | + | |
| 6/99 | 13 | + + | |
| 10/99 | 12 | + + | |
| 11 | 11/87 | 15 | + + |
| 7/89 | 47 | + + | |
| 5/90 | 1 | − | |
| 2/96 | 93 | + + + | |
| 9/96 | 103 | + + + | |
| 1/98 | 33 | + | |
| 8 | 11/86 | 8 | + + + |
| 12/86 | 1 | − | |
| 11/89 | 23 | + + + | |
| 9 | 5/87 | 28 | + + + |
| 7/87 | 4 | + | |
| 7 | 6/94 | 4 | + |
| 7/94 | 2 | + | |
| 17 | 4/93 | 7 | + + + |
| 5/93 | 5 | + + + | |
| 12 | 10/88 | 1 | − |
| 10/90 | 12 | + + + | |
The Western blot results of cryoglobulins are marked with +, ++ and ++ + in the presence of weak, moderate and strong characteristic bands for CRP, respectively, or - in their absence.
In an attempt to quantify the CRP content of Cg, resolubilized Cg were analysed with an immuno-turbidimetric assay and an ELISA, respectively. Six Cg from the patients of this study were available for testing. Using the ELISA, a mean CRP concentration of 10·45 ± 8·07 ng per mg cryoprotein (range 0·3–15·0 ng/mg) was obtained with these samples. Another set of Cg (n = 9) was assayed with a sensitive immuno-turbidimetric method. Using this assay, a mean CRP concentration of 11·3 ± 14·9 ng per mg cryoprotein (range 0·27–45 ng/mg) was detected. Overall, there was no strict correlation between the concentration of CRP and that of the respective cryoprotein of these 15 Cg. However, Cg with the highest CRP concentrations (127–300 ng/ml) were found in cases with the highest concentrations of cryoprotein (25–35 mg/ml).
In order to investigate whether the removal of Ca2+ would effect the detectability of CRP, Cg of three patients were analysed using the immuno-turbidimetric assay after six washing steps either with 0·9% saline or with VBS containing 10 mm EDTA. Depletion of Ca2+ by EDTA did not alter the amount of CRP in isolated Cg (Cg 1: 98·7 ng/ml; Cg 2: 6·2 ng/ml; Cg 3: 1·2 ng/ml) compared with 0·9% saline (Cg 1: 76·1 ng/ml; Cg 2: 7·8 ng/ml; Cg 3: 0·4 ng/ml).
Discussion
Our study provides evidence for the frequent occurrence of CRP in Cg of all three types in Brouet's classification. In intra-individual follow-up studies it was demonstrated that the detectability of CRP by Western blotting correlated positively with the concentration of the cryoprotein (Fig. 3); therefore, the apparent absence of CRP in a few Cg samples may reflect the sensitivity of the method rather than real variations in the composition of individual Cg. Our aims at quantitative measurements of CRP in Cg were pursued both by ELISA and immuno-turbidimetric assay. Using these methods, very similar CRP values in the nanogram range (per mg of cryoprotein) were obtained with two different sets of Cg samples. Our measurements of high CRP concentrations (up to 300 ng/ml) in some Cg match with their detectability by indirect immunofluorescence on HEp-2 cells (Fig. 1). As reported previously [15], the detection threshold of CRP in the HEp-2 cell assay was found to lie between 10 and 100 ng CRP per ml.
The chemical nature of the association between CRP and the other constituents of Cg is currently unknown. Because the cryoproteins were extracted from sera, it seems likely that CRP is bound Ca2+-dependently to one of its specific ligands on microbial antigens or on nuclear or cell membrane-derived material [16,17,29] in the Cg complex. However, in our studies CRP was quantitatively detected in isolated Cg after extensive washing with Ca2+-free saline. Very similar amounts of CRP were found when isolated Cg were washed six times with VBS containing 10 mm EDTA, which argues against a strictly Ca2+-dependent association of CRP with Cg. These controversial issues have still to be resolved by detailed analyses of the binding of CRP to cryolabile components. Recently, we obtained experimental evidence that a series of resolubilized Cg (mainly of type II) could be separated into three peaks of different molecular weight, when chromatographed by FPLC at 37°C under non-denaturing conditions. While IgM and fibronectin, when present, were found in the high molecular weight fractions (first peak) by Western blotting, the strongest bands for CRP were detected in the second (IgG-containing) peaks [30]. Experiments are ongoing in order to analyse the molecular composition of the CRP-containing fraction. These findings actually suggest that cryoglobulins consist of different immunoglobulin and nonimmunoglobulin compounds (e.g. CRP) which aggregate into a macromolecular complex only when the temperature is lowered. Depending upon the temperature amplitude of Cg, such aggregating conditions may occur in the peripheral circulation [31].
In the few reports published on the occurrence of CRP in non-cryolabile immune complexes (CIC), the mechanism of binding of CRP to CIC was unfortunately not addressed [32–34]. However, most in vitro studies have shown that direct binding between native CRP and mono- or multimeric IgG does not occur [32,34–37]. In contrast to native CRP, a modified form of CRP (mCRP), which is generated under denaturing conditions, has high binding activity for complexed IgG in vitro [35,36]. Our ex vivo studies do not support the presence of potential mCRP-Ig complexes, since we could not trace mCRP epitopes in a series of CRP-containing Cg with a mCRP-specific ELISA [38].
There are a few case reports where CRP was found in association with monoclonal immunoglobulins in macroglobulinaemic sera. After the first description of a monoclonal IgM/λ antibody reacting with purified CRP in vitro [39], complex formations between CRP and monoclonal IgM and IgA were demonstrated in two further cases, respectively, by immunofixation techniques [19,40]. In conclusion, except for the report of Korngold [39] demonstrating true antibody reactivity of a monoclonal immunoglobulin against CRP, there is no experimental evidence suggesting direct binding between CRP and immunoglobulins.
Our demonstration of the complement components C1q and C3 in all CRP-containing Cg, and the elevated levels of the complement split product C3d in the corresponding sera, confirm previous reports on activation and binding of classical complement proteins by these Cg [5,6]. Since complexed CRP was reported to be as effective in activating the classical C pathway [41,42] as aggregated IgG, the deposition of C1q and C3 on Cg may have resulted from CRP-mediated C activation [43]. Additional activation of the alternative pathway, which was shown previously to occur with some Cg [6,31], resulting in C3 binding, cannot be excluded by our experiments. Interestingly, CRP was shown to inhibit alternative pathway C activation, by increasing binding of the complement regulatory factor H to C3b [44,45].
In conclusion, our study presents evidence for a frequent occurrence of CRP along with the complement proteins C1q and C3 in isolated cryoglobulins. Further experiments are necessary to determine the mechanisms of the association between immunoglobulin and non-immunoglobulin compounds of Cg. It is not clear whether the CRP detected in Cg-complexes ex vivo modulates the pathogenic activity of immune complexes in cryoglobulinaemic diseases.
Acknowledgments
The results presented here are part of a medical dissertation (University of Freiburg, 1995) by V. Prasauskas who was supported by grants from the Deutsche Forschungsgemeinschaft (LIT 436) and the Hans-Hench-Stiftung, Lörrach. We are indebted to PD Dr Bisse (Clinical Chemistry, University Hospital Freiburg) for performing the immune-turbidimetric assays of CRP.
References
- 1.Brouet JC, Clauvel P-C, Danon F, Klein M, Seligman M. Biological and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–88. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 2.Abel G, Zhang Q, Agnello V. Hepatitis C virus infection in type II mixed cryoglobulinemia. Arthritis Rheum. 1993;36:1341–9. doi: 10.1002/art.1780361003. [DOI] [PubMed] [Google Scholar]
- 3.Weiner SM, Berg T, Berthold H, et al. A clinical and virological study of hepatitis C virus-related cryoglobulinemia in Germany. J Hepatol. 1998;29:375–84. doi: 10.1016/s0168-8278(98)80054-8. [DOI] [PubMed] [Google Scholar]
- 4.Miescher PA, Huang YP, Izui S. Type II cryoglobulinemia. Semin Hematol. 1995;32:80–5. [PubMed] [Google Scholar]
- 5.Rother U, Rother K, Flad HD, Miescher PA. Bithermic complement activation in cryoglobulinemic serum. Eur J Clin Invest. 1972;2:59–65. doi: 10.1111/j.1365-2362.1972.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MR, Arroyave CM, Miles L, Tan EM. Immune reactants in cryoproteins. Relationship to complement activation. Ann Rheum Dis. 1977;36:540–8. doi: 10.1136/ard.36.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morel F, Cholley LC, Dianoux AC, et al. Modulation of human neutrophil function by fibronectin degradation products isolated from cryoglobulins. Inflammation. 1992;16:325–41. doi: 10.1007/BF00917625. [DOI] [PubMed] [Google Scholar]
- 8.Fontana A, Grob PJ, Frick P. Die Kryoglobulinämien. Erg Inn Med Kinderheilk. 1988;56:1–31. [PubMed] [Google Scholar]
- 9.Weiner SM, Röther E, Weber S, Schlesier M, Berthold H, Peter HH. Kältelabile Serum- und Plasmaproteine: klinische und diagnostische Wertigkeit von Kryoglobulinen, Kryofibrinogen und Kälteagglutininen. Immun Infekt. 1994;22:169–76. [PubMed] [Google Scholar]
- 10.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 11.Gewurz H, Mold C, Siegel J, Fiedel B. C-reactive protein and the acute phase response. Adv Int Med. 1982;27:345–72. [PubMed] [Google Scholar]
- 12.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick JM, Volanakis JE. Molecular genetics, structure, and function of C-reactive protein. Immunol Res. 1991;10:43–53. doi: 10.1007/BF02918166. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 15.Vaith P, Prasauskas V, Potempa L, Peter HH. Complement activation by C-reactive protein on the HEp-2 cell substrate. Int Arch Allergy Immunol. 1996;111:107–17. doi: 10.1159/000237354. [DOI] [PubMed] [Google Scholar]
- 16.Pepys MB, Booth SE, Tennent GA, Butler PJG, Williams DG. Binding of pentraxins to different nuclear structures: C-reactive protein binds to small nuclear ribonucleoprotein particles, serum amyloid P component binds to chromatin and nucleoli. Clin Exp Immunol. 1994;97:152–7. doi: 10.1111/j.1365-2249.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Clos TW. C-reactive protein reacts with the small nuclear ribonucleoprotein. J Immunol. 1989;143:2553–9. [PubMed] [Google Scholar]
- 18.Hurwitz D, Quismorio FP, Friou GJ. Cryoglobulinaemia in patients with infectious endocarditis. Clin Exp Immunol. 1975;15:131–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Grützmeier S, von Schenck H. C-reactive protein-immunoglobulin complexes in two patients with macroglobulinemia. Scand J Clin Lab Invest. 1987;47:819–22. doi: 10.1080/00365518709168951. [DOI] [PubMed] [Google Scholar]
- 20.Mayer MM. Complement and complement fixation. In: Kabat EA, Mayer MM, editors. Experimental immunochemistry. 2. Springfield, IL: Charles C. Thomas; 1961. pp. 133–240. [Google Scholar]
- 21.Roether E, Lang B, Coldewey R, Hartung K, Peter HH. Complement split product C3d as an indicator of disease activity in systemic lupus erythematosus. Clin Rheumatol. 1993;12:31–5. doi: 10.1007/BF02231555. [DOI] [PubMed] [Google Scholar]
- 22.Musset L, Diemert MC, Taibi F, et al. Characterization of cryoglobulins by immunoblotting. Clin Chem. 1992;38:798–802. [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Highton J, Hessian P. A solid-phase enzyme immunoassay for C-reactive protein: clinical value and the effect of rheumatoid factor. J Immunol Meth. 1984;68:185–92. doi: 10.1016/0022-1759(84)90149-2. [DOI] [PubMed] [Google Scholar]
- 26.Potempa LA, Siegel J, Gewurz H. Binding reactivity of C-reactive protein for polycations. II. Modulatory effects of calcium and phosphocholine. J Immunol. 1981;127:1509–14. [PubMed] [Google Scholar]
- 27.Ziccardi RJ. Spontaneous activation of the first complement component of human complement (C1) by an intramolecular autocatalytic mechanism. J Immunol. 1982;128:2500–4. [PubMed] [Google Scholar]
- 28.Cheung AK, Parker CJ, Janatova J. Analysis of the complement C3 fragments associated with hemodialysis membranes. Kidney Int. 1989;35:576–88. doi: 10.1038/ki.1989.26. [DOI] [PubMed] [Google Scholar]
- 29.Gorevic PD, Kassab HJ, Levo Y, et al. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980;69:287–308. doi: 10.1016/0002-9343(80)90390-3. [DOI] [PubMed] [Google Scholar]
- 30.Weiner SM, Lebrecht D, Peter HH, Vaith P. Immunochemical analysis of cryoglobulins. Immunol Lett. 2000;73:196. [Google Scholar]
- 31.Müller S, Rother U, Westerhausen M. Complement activation by cryoglobulin. Evidence for a pathogenic role of an IgG (kappa) cryoglobulin in cutaneous vasculitis. Clin Exp Immunol. 1976;23:233–41. [Google Scholar]
- 32.Maire MA, Barnet M, Carpentier N, Miescher PA, Lambert PH. Identification of components of IC purified from human sera. I. Immune complexes purified from sera of patients with SLE. Clin Exp Immunol. 1983;51:215–24. [PMC free article] [PubMed] [Google Scholar]
- 33.Croce MV, Segal-Eiras A. Identification of acute-phase proteins (APP) in circulating immune complexes (CIC) in esophageal cancer patients' sera. Cancer Invest. 1996;14:421–6. doi: 10.3109/07357909609018899. [DOI] [PubMed] [Google Scholar]
- 34.Smith AJ, Kyle V, Cawston TE, Hazleman BL. Isolation and analysis of immune complexes from sera of patients with polymyalgia rheumatica and giant cell arteritis. Ann Rheum Dis. 1987;46:468–74. doi: 10.1136/ard.46.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motie M, Brockmeier S, Potempa LA. Binding of model soluble immune complexes to modified C-reactive protein. J Immunol. 1996;156:4435–41. [PubMed] [Google Scholar]
- 36.Köttgen E, Hell B, Kage A, Tauber R. Lectin specificity and binding characteristics of human C-reactive protein. J Immunol. 1992;149:445–53. [PubMed] [Google Scholar]
- 37.Ballou SP, Macintyre SS. Absence of a binding reactivity of human C-reactive protein for immunoglobulin or immune complexes. J Lab Clin Med. 1990;115:332–8. [PubMed] [Google Scholar]
- 38.Prasauskas V. Komplementaktivierung durch C-reaktives Protein (in vitro). Immunologische Analysen zur Rolle von neo-CRP und Kryoglobulinen. Doctoral Thesis University Freiburg, Germany. 1995 [Google Scholar]
- 39.Korngold L. A human monoclonal IgM with binding activity for C-reactive protein (CRP) Clin Immunol Immunopathol. 1974;3:236–42. doi: 10.1016/0090-1229(74)90009-9. [DOI] [PubMed] [Google Scholar]
- 40.Ponge TD, Le Carrer DL, Sagniez MM, Pontet M, Cottin SL. False rise in C-reactive protein in a patient with monoclonal IgM immunoglobulin. Clin Chim Acta. 1993;220:101–6. doi: 10.1016/0009-8981(93)90010-2. [DOI] [PubMed] [Google Scholar]
- 41.Mold C, Gurule C, Otero D, Du Clos TW. Complement-dependent binding of C-reactive protein complexes to human erythrocyte CR1. Immunol Immunopathol. 1996;2:153–60. doi: 10.1006/clin.1996.0171. [DOI] [PubMed] [Google Scholar]
- 42.Romero IR, Morris C, Rodriguez M, Du Clos TW. Inflammatory potential of C-reactive protein complexes compared to immune complexes. Clin Immunol Immunopathol. 1998;87:155–62. doi: 10.1006/clin.1997.4516. [DOI] [PubMed] [Google Scholar]
- 43.Wolbink GJ, Brouwer MC, Buysmann S, ten Berge IJM, Hack CE. CRP-mediated activation of complement in vivo. Assessment by measuring circulating complement-C-reactive protein complexes. J Immunol. 1996;157:473–9. [PubMed] [Google Scholar]
- 44.Mold C, Kingzette M, Gewurz H. C-reactive protein inhibits pneumococcal activation of the alternative pathway by increasing the interaction between factor H and C3b. J Immunol. 1984;133:882–5. [PubMed] [Google Scholar]
- 45.Jarva H, Jokiranta TS, Hellwage J, Zipfel PF, Meri S. Regulation of complement activation by C-reactive protein: targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. J Immunol. 1999;163:3957–62. [PubMed] [Google Scholar]