Abstract
Antibodies to cytokeratin (CK) are found in some patients with autoimmune hepatitis (AIH). We hypothesized that serum antibodies to CK8, CK18 and CK19 may be formed in patients with AIH. We established an enzyme-linked immunosorbent assay (ELISA) to quantify anti-CK8, anti-CK18 and anti-CK19 antibodies in sera of patients with AIH. In addition, we quantified circulating CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes in patients' sera, by an enzyme-linked immunosorbent assay (ELISA). Furthermore, to evaluate the expression of CK8, CK18 and CK19 in liver tissue, immunohistochemical stainings were performed. Significantly high levels of anti-CK8, anti-CK18 and anti-CK19 antibodies were demonstrated in patients with AIH compared with normal volunteers and patients with chronic active hepatitis C (CH-C). In addition, these antibodies were significantly decreased after steroid treatment. Levels of CK8:anti-CK8 and CK18:anti-CK18 immune complexes in sera of patients with AIH were significantly high compared with those of patients with CH-C and normal volunteers. Immunohistochemically, CK8 or CK18 were absent from some hepatocytes of AIH. CK19 was aberrantly expressed in periportal hepatocytes in patients with AIH, but not CH-C. This is the first study to quantify anti-CK8, anti-CK18, anti-CK19 antibodies and immune complexes in patients with AIH. The clinical significance of anti-CK antibodies and their immune complexes of AIH is also discussed.
Keywords: antibody, autoimmune, hepatitis, cytokeratin, immune complex, immunohistochemistry
Introduction
Autoimmune hepatitis (AIH) is a major autoimmune liver disease characterized by progressive hepatocellular inflammation, which usually responds to treatment with corticosteroids [1–3]. Hepatocytes in AIH seem to be the initial targets of tissue destruction. To diagnose AIH, antibodies to nuclear antigens (ANA) are important diagnostic markers for the differentiation of chronic hepatitis. On the basis of different autoantibody specificity, as many as four types of AIH have been proposed, and antibodies to cytokeratin (CK) can be detected in a subgroup of AIH patients having no other known autoantibodies [4,5].
A recent study revealed that almost 30% of patients with AIH are positive for autoantibodies against CK8 and CK18 [6]. We also found the sera to be positive for antibodies against other proteins in addition to CK8 and CK18 by Western blotting analysis [7].
It has also been reported that mature hepatocytes express hepatocytic phenotypes such as albumin, transferrin, CK8 and CK18 but not biliary markers such as monoclonal antibody BD1, CK7 or CK19 [8–12]. In contrast, bile duct, bile ductules in mechanical obstruction, ductular hepatocytes in massive hepatic necrosis and the ductal plate cells in fetal liver show strong staining for CK19, which characterizes intermediate filaments associated with bile duct epithelial cells [8–12].
Although autoantibodies against CK8 as well as CK18 have been evaluated previously in AIH [4–7], there have been no reports which have quantify anti-CK8 and anti-CK18 antibodies or evaluate anti-CK19 autoantibodies in patients with AIH. Against this background, we quantified anti-CK8, anti-CK18 and anti-CK19 autoantibodies in patients with AIH.
We also hypothesized that circulating CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes may exist in sera of patients with AIH and play a role in persistent hepatocytes destruction in patients with AIH. To prove this, we established a new enzyme‐linked immunosorbant assay (ELISA) and quantified these complexes by ELISA.
Materials and methods
Subjects
The protocols of this study were approved by the institutional review board for human studies and informed written consent was obtained from the subjects. We studied 16 patients with a diagnosis of AIH in Kagawa Medical University Hospital (613 beds) from 1990 to 1998. The median age of the patients was 56 years old ranging from 15 to 74 years (15 female and one male). The diagnoses of AIH were based on clinical, serological and histological data using the descriptive system or scoring system of the International Autoimmune Hepatitis Group [1]. Histological confirmation was carried out in all cases by liver biopsy. None of the patients received immunosuppressive treatment such as corticosteroid or cyclophosphamide at the time of diagnosis. The serum samples were analysed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (T-BIL), γ-globulin, immunoglobulin A (IgA), immunoglobulin M (IgM) and immunoglobulin G (IgG) in AIH patients.
We also studied 12 normal subjects (seven female and five male) who had an average age of 53 years. All subjects had normal liver function and no history of liver diseases or clinical findings suggesting liver disease. As a control for different hepatic diseases, we also studied 19 patients with chronic active hepatitis (CH‐C) caused by hepatitis virus C (six female, 13 male, with an average age of 51 years).
Blood samples
Peripheral venous blood samples were obtained before breakfast. After centrifugation at 1000 g for 10 min at 4°C, the serum was frozen and stored at −70°C until used.
Enzyme-linked immunosorbent assay (ELISA)
To quantify anti-CK8, anti-CK18 or anti-CK19 autoantibodies in human sera, an ELISA was established. Serum was added to wells coated with recombinant human CK8, bovine CK18 or recombinant human CK19 (0·25, 0·5, 1, 2 and 4 µg/ml). After incubation and washing, the solid phase-bound anti-human CK8 autoantibody, anti-human CK18 or anti-human CK19 autoantibodies were further incubated with peroxidase-conjugated goat anti-human IgG antibody (Sigma ImmunoChemicals, lot 094H-4810, St Louis, MO, USA, diluted 1 : 1000). After further washes, TMB Peroxidase EIA Substrate Kit (Bio-RAD Laboratories, Hercules, CA, USA) was used to measure the amount of solid phase-bound antibodies. These assays were calibrated using a standard serum solution of a patient (67-year-old female) who had anti-CK8 antibody, a patient (59-year-old female) who had anti-CK18 antibody and a patient (68-year-old male) who had anti-CK19 antibody, all of which were determined by Western immunoblot. Finally, 1 µg/ml of recombinant CK8, CK18 or CK19 were used to coat plates to measure serum samples. To measure these autoantibodies in patients' sera, diluted sera (at a dilution of 1 : 100) was used. Data are expressed as mean values from duplicate determinations.
ELISA for CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes in human sera
To quantify CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes in human sera, an ELISA was established. Sera diluted 1 : 100 were added to wells coated with monoclonal anti-human CK8 antibody (clone Ks 8·7, Progen Biotechnik GMBH, Heidelberg, Germany, diluted at 1 : 250) or anti-human CK18 antibody (clone 18·27, Progen Biotechnik GMBH, diluted at 1 : 250). After incubation (60 min) and washing (washed four times by PBS-tween), the solid phase-bound CK8:anti-CK8 antibody or CK18:anti-CK18 antibody immune complexes were further incubated with peroxidase-conjugated goat anti-human IgG antibody (Sigma ImmunoChemicals, lot 094H-4810, St Louis, MO, USA, diluted at 1 : 1000). After further washes, the TMB Peroxidase EIA Substrate Kit (Bio-Rad Laboratories, Hercules, CA, USA) was used to measure the amount of solid phase-bound antibodies. The assay was calibrated using a standard solution of the reference patient's serum who had CK8:anti-CK8 antibody or CK18:anti-CK18 antibody immune complexes in a preliminary experiment, and titres were calculated by comparing the control patient's serum that was determined to be 1·0. Data are expressed as mean values from duplicate determinations. In a preliminary experiment, we performed a control ELISA (coated by anti-α1-proteinase inhibitor antibody) to rule out the possibility of non-specific binding. There was no positive reaction in this ELISA. In addition, to evaluate the precision and reproducibility of the ELISA, we also performed repeated experiments and confirmed that the difference between several assays was less than 10%.
Immunohistochemical stainings of liver tissues
To evaluate the expression of CK8, CK18 and CK19 in liver tissues including 12 cases of AIH and 12 cases of CH-C as controls, immunohistochemical stainings by anti-human monoclonal antibody against CK8 (35βH11, Enzo Diagnostics, Inc., New York, NY, USA, 1 : 5000 dilution), CK18 (ScyTek Lab., Logan, UT, USA, each 1 : 30 dilution) or CK19 (ScyTek Lab., 1 : 30 dilution) were performed using each dewaxed section, employing the avidin–biotin peroxidase complex method (Dako LSAB kit-peroxidase, Dako Corp., Kyoto, Japan) according to the kit manual. In order to retrieve and increase the immunoreactivities, autoclave treatment of each section in 0·01 m citrate buffer at 132°C for 12 min was also carried out.
Statistical analysis
Results are expressed as mean values ± standard deviation of the mean. Comparisons of values between groups were analysed with the Mann–Whitney U-test. Correlations were evaluated by the Pearson's correlation coefficient, and Fisher's r to Z method was used to calculate the P-values. A P-value of less than 0·05 was considered significant.
Results
Quantification of anti-CK8, anti-CK18 or anti-CK19 antibodies
It was possible to quantify anti-human CK8, CK18 and CK19 antibodies in sera from patients using the ELISA.
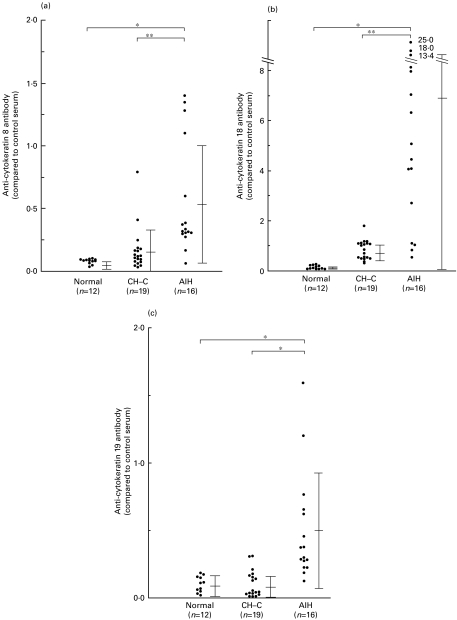
Levels of anti-human CK8 antibodies in sera of 16 patients with AIH (0·553 ± 0·451; mean ± standard deviation, when compared to the control patient's serum which was determined to be 1·0) were significantly high compared with those of normal volunteers (0·080 ± 0·020, P < 0·0001, Mann–Whitney U-test), patients with CH-C (0·166 ± 0·174, P < 0·001) (Fig. 1a).
Fig. 1.
Levels of anti-human cytokeratin 8 (a), anti-cytokeratin 18 (b) or anti-cytokeratin 19 (c) antibodies in patients' sera and in sera of normal volunteers. The bar represents mean ± standard deviation.
Levels of anti-human CK18 antibodies in sera of 16 patients with AIH (6·857 ± 6·754; mean ± standard deviation, when compared to the control patient's serum which was determined to be 1·0) were significantly high compared with those of normal volunteers (0·147 ± 0·073, P < 0·0001, Mann–Whitney U-test), and patients with CH-C (0·836 ± 0·382, P < 0·001) (Fig. 1b).
Levels of anti-human CK19 antibodies in sera of 16 patients with AIH (0·494 ± 0·399; mean ± standard deviation, when compared to the control patient's serum which was determined to be 1·0) measured by ELISA, were significantly high compared with normal volunteers (0·116 ± 0·075, P < 0·0001, Mann–Whitney U-test), and patients with CH-C (0·106 ± 0·096, P < 0·0001) (Fig. 1C).
Correlation between levels of antibodies to CK and biochemical data in patients with AIH
Biochemical data found in patients with AIH were compared with levels of anti-CK8, anti-CK18 or anti-CK19 antibodies. Levels of anti-CK8, anti-CK18 and anti-CK19 antibodies significantly correlated with levels of IgG as well as γ-globulin (Table 1). There were no significant correlations between levels of anti-CK8 and anti-CK18 antibodies with levels of IgA, IgM, AST, ALT, ALP, γ-GTP, or T-BIL.
Table 1.
Correlations between anti-CK antibodies or immune complexes in serum and other parameters in patients with autoimmune hepatitis (n = 16)
| Anti-CK8 antibody | Anti-CK18 antibody | Anti-CK19 antibody | CK8 immune complex | CK18 immune complex | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | R | P | R | P | R | P | R | P | R | P |
| IgA | 0·269 | 0·3208 | 0·071 | 0·7965 | 0·128 | 0·6434 | −0·112 | 0·6961 | −0·036 | 0·9012 |
| IgM | −0·113 | 0·6825 | 0·15 | 0·5851 | 0·077 | 0·7799 | −0·256 | 0·3467 | −0·274 | 0·3301 |
| IgG | 0·159 | 0·0382* | −0·75 | 0·0004* | 0·79 | 0·0001* | −0·096 | 0·7376 | 0·001 | 0·997 |
| AST | 0·17 | 0·5363 | 0·021 | 0·9398 | 0·289 | 0·2838 | 0·572 | 0·0243* | 0·538 | 0·0374* |
| ALT | −0·132 | 0·6318 | −0·031 | 0·9112 | 0·149 | 0·5883 | 0·073 | 0·8011 | 0·044 | 0·8801 |
| ALP | 0·284 | 0·2926 | 0·314 | 0·2419 | 0·264 | 0·3305 | 0·234 | 0·4097 | 0·193 | 0·4985 |
| γ-GTP | 0·247 | 0·363 | 0·148 | 0·5903 | 0·247 | 0·3633 | 0·189 | 0·5077 | 0·188 | 0·5096 |
| T-BIL | −0·055 | 0·8428 | −0·102 | 0·7123 | 0·005 | 0·9858 | 0·51 | 0·0512 | 0·46 | 0·085 |
| γ-globulin | 0·543 | 0·0282* | 0·622 | 0·0087* | 0·747 | 0·0005* | 0·206 | 0·47 | 0·283 | 0·3133 |
Statistically significant
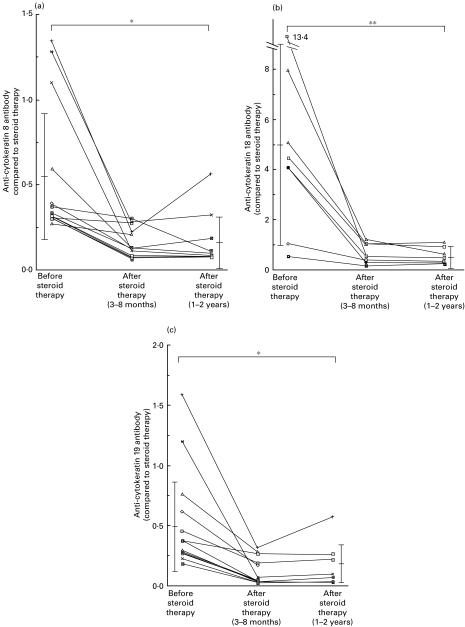
Levels of anti-human CK8, CK18 or CK19 antibodies in AIH patients' sera after treatment
Levels of antibodies to CK8, CK18 and CK19 in patients' sera before and after treatment were shown in Fig. 2a,b and c. Levels of antibodies to CK8, CK18 and CK19 in sera of patients with autoimmune hepatitis were 0·576 ± 0·359 (mean ± standard deviation), 5·072 ± 4·084 and 0·489 ± 0·323, respectively, before treatment. They were significantly decreased 0·175 ± 0·166 (P < 0·005, Mann–Whitney U-test), 0·562 ± 0·330 (P < 0·01) and 0·133 ± 0·175 (P < 0·005), respectively, after treatment.
Fig. 2.
Levels of anti-human cytokeratin 8 (a), anti-cytokeratin 18 (b), or anti-cytokeratin 19 (c) antibodies in AIH patients' sera before and after treatment. The bar represents mean ± standard deviation. *P < 0.005; **P < 0.01.
Quantification of CK8 or CK18 immune complexes in patients' sera by ELISA
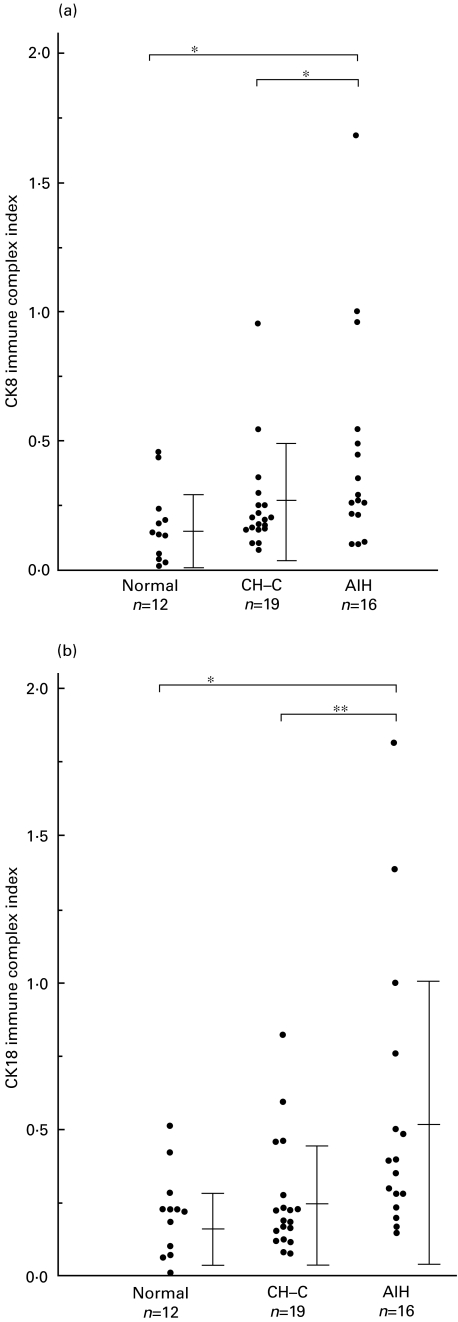
Levels of CK8:anti-CK8 antibody immune complexes in sera of patients with AIH (0·455 ± 0·424, mean ± standard deviation when compared to the control patent's serum which was determined to 1·0.), were significantly high compared to normal volunteers (0·173 ± 0·145, P < 0·05) and patients with CH-C (0·250 ± 0·200, P < 0·05) (Fig. 3a).
Fig. 3.
Levels of cytokeratin 8:anti-cytokeratin 8 antibody immune complexes (a) or cytokeratin 18:anti-cytokeratin 18 antibody immune complexes (b) in AIH patients' sera (n = 16) and in sera of normal volunteers (n = 12) and in sera of patients with CH‐C (n = 19). The bar represents mean ± standard deviation. *P < 0.05; **P < 0.01.
Levels of CK18:anti-CK18 antibody immune complexes in sera of patients with AIH (0·542 ± 0·475, mean ± standard deviation when compared to the control patent's serum which was determined to 1·0) measured by ELISA were significantly high compared with normal volunteers (0·213 ± 0·146, P < 0·05) and patients with CH-C (0·258 ± 0·149, P < 0·01) (Fig. 3b). In patients with AIH, there was a strong correlation between levels of CK8:anti-CK8 antibody immune complexes in sera and CK18:anti-CK18 antibody immune complexes in sera (R = 0·917).
Levels of CK8:anti-CK8 antibody immune complexes as well as CK18:anti-CK18 antibody immune complexes were compared with histochemical and serological data. There were statistically significant correlations between levels of immune complexes and serum levels of AST (P < 0·05) (Table 1).
Immunohistochemical staining of CK8, CK18 or CK19 in liver tissue obtained from patients with AIH
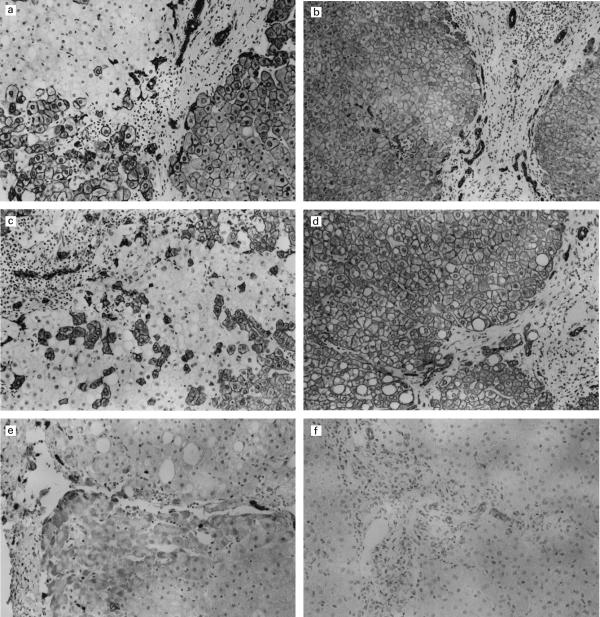
Immunohistochemical staining of liver tissue with anti-CK8 or anti-CK18 monoclonal antibody revealed a strong positive reaction restricted to the cytoplasm of some hepatocytes (Figs 4a,b,c). Interestingly, although the nest of hepatocytes positive for CK8 or CK18 was found, other hepatocytes were not stained by either anti-CK8 or anti-CK18 monoclonal antibodies in 12 patients with AIH (Figs 4a,b,c) featuring a mosaic pattern. Therefore, loss of CK8 or CK18 was found frequently in hepatocytes of patients with AIH. CK8 and CK18 were more strongly stained in plasma membrane than in cytoplasm or perinuclear areas. Single cell positivity was also detected in negative areas, which were usually found in periportal areas consisting of smaller hepatocytes; CK8 and CK18 were found in the same type of hepatocytes. In contrast, the cytoplasm of hepatocytes obtained from 12 patients with CH-C were homogeneously positive for CK8 and CK18 and no mosaic pattern was observed (Fig. 4d).
Fig. 4.
Immunohistochemical staining of the liver with anti-human cytokeratin 8 (a,b), anti-cytokeratin 18 (c,d), or anti-cytokeratin 19 (e,f) monoclonal antibodies in patients with autoimmune hepatitis (AIH). Although some hepatocytes in AIH are stained strongly by anti-cytokeratin 8 monoclonal antibody, other hepatocytes are not stained (a). Hepatocytes positive for cytokeratin 8 seem to reveal a mosaic pattern. In contrast, hepatocytes obtained from patients with chronic active hepatitis are diffusely stained for cytokeratin 8 (b). Although some hepatocytes in AIH are stained strongly by anti-cytokeratin 18 monoclonal antibody, other intermingled hepatocytes are not stained (c). In contrast, hepatocytes obtained from patients with chronic active hepatitis are diffusely stained for cytokeratin 18 (d). Immunohistochemical staining of the liver with anti-human cytokeratin 19 monoclonal antibody in patients with AIH demonstrates the strong positive reaction in the cytoplasm of bile duct cells (e). Some periportal hepatocytes in patients with AIH are also stained positively by anti-CK19 monoclonal antibody (e). In contrast, hepatocytes obtained from 12 patients with chronic active hepatitis are completely negative for cytokeratin 19 (f). In addition, staining intensity of bile duct in patients with AIH is much stronger than that of patients with chronic active hepatitis (compare e and f). Avidin–biotin–peroxidase complex method; Magnification a,b,e,f: ×220, c,d: ×350.
Immunohistochemical staining of liver tissues with anti-CK19 monoclonal antibody revealed a strong positive reaction restricted to the cytoplasm of proliferating bile duct cells (Figs 4e,f). Interestingly, hepatocytes in six of 12 AIH cases examined were also stained by anti-CK19 monoclonal antibody (Fig. 4e). In contrast, hepatocytes obtained from 12 patients with CH-C were completely negative for CK19 (Fig. 4f). In addition, the staining intensity of bile duct cells in patients with AIH was much stronger than in patients with CH-C (compare Fig. 4e,f).
Discussion
In our present study we have demonstrated that serum anti-CK8, anti-CK18 and anti-CK19 autoantibodies were found in patients with AIH. Quantification of these antibodies by ELISA demonstrated that levels of these antibodies in sera from patients with AIH were significantly increased compared with those of normal volunteers and paitents with other liver disease. In addition, we have demonstrated that levels of anti-CK8, anti-CK18 and anti-CK19 autoantibodies decreased significantly after steroid treatment. Furthermore, levels of CK8:anti-CK8 and CK18:antiCK18 immune complexes in sera from patients with AIH were significantly increased compared with normal volunteers. Immunohistochemically, we have demonstrated that hepatocytes in AIH were heterogeneously stained by the monoclonal anti-CK8 and CK18 antibodies, compared to homogeneous staining in CH-C. Interestingly, hepatocytes in AIH were stained by monoclonal anti-CK19 antibody as well as bile duct cells.
The function of CKs has been clarified recently [11,13]. It has been reported that nickel causes a selective rearrangement of CK filaments in a liver epithelial cell line (T51B) [14,15]. CK filaments are directly connected with the plasma membrane [16,17], the nucleus and the cell organelles including endoplasmic reticulum and cytoplasmic vesicles [18,19]. Also, CK filaments are accumulated around bile canaliculi to form the pericanalicular sheath [20]. After nickel treatment, the CK filaments become detached from the cell periphery and the bile canaliculi disappears [13]. The effect of nickel treatment are similar to that caused by micro-injection of antibodies to intermediate filaments into well-established tissue culture cells [21–25], where antibodies cause the CK filaments to aggregate in the juxtanuclear cytoplasm.
It has also been reported that the disruption of CK8 or CK18 filaments results in increased hepatocyte fragility with liver perfusion [26]. Therefore, an intact CK8 and CK18 filament network appears to be important for protection from other stresses such as exposure to toxins [11,27]. Interestingly, we demonstrate a loss of CK8 or CK18 in some hepatocytes in AIH but not CH-C, suggesting that some hepatocytes in AIH lost the function of CK8 and CK18.
There are several reports which have evaluated anti-CK antibodies in liver diseases. It has been reported that several patients with AIH are positive for anti-SLA antibodies [4,5,28]. In addition, it has been reported that anti-SLA antibodies are directed primarily against CK8 as well as CK18 [28]. Antibodies to CK filaments have also been demonstrated in patients with alcoholic liver disease [29] and primary biliary cirrhosis [30].
The mechanism of antibodies to CK8 and CK18 formation should be discussed. CK8 and CK18 have been shown to undergo dramatic reorganization during apoptosis [31,32]. It has been reported that CK8 and CK18 contain the caspase cleavage site [32]. Therefore, it is possible that fragments of CK8 and CK18 are released to the blood vessels after liver injury and these fragments may play a role in the formation of circulating autoantibodies. In addition, the resulting antigen–antibody interaction with immune complex formation could have a significant role in the perpetuation of the disease process, either by direct injury of hepatocytes or via local macrophage activation.
We also demonstrate that hepatocytes in AIH were heterogeneously stained by the monoclonal anti-CK8 and CK18 antibodies, compared to homogeneous staining in CH-C. These mosaic patterns usually appeared in periportal areas. Hepatocytes positive for CK8 as well as CK18 seem to reflect mature hepatocytes, and intermingled hepatocytes negative for CK8 or CK18 may reflect regenerative hepatocytes. The changes of distribution in CK8 and 18 in hepatocytes seemed to be characteristic in the liver from patients. The mechanism for these mosaic patterns and detailed explanations for these different hepatocytes should be investigated in future studies in relation to pathological roles.
We demonstrate that CK19 was expressed in some hepatocytes in patients with AIH but not in CH-C. In addition, Bombardieri et al. have demonstrated that serum levels of the CK19 fragment increase in some patients with hepatitis [33]. Due to the aberrant CK19 expression found in hepatocytes in patients with AIH, CK19 or its soluble fragments may have played a role in producing anti-CK19 autoantibodies. Importantly, we also demonstrated that levels of anti-CK8, anti-CK18 and anti-CK19 antibodies significantly decreased after steroid treatment, suggesting that anti-CK19 antibody played a role in the pathogenesis of AIH.
However, we could not rule out the possibility that the existence of anti-CK autoantibodies was a non-specific consequence of liver injury. Therefore, the significance of anti-CK antibodies administered in animal experiments should be investigated in future studies. In addition, antigenic epitopes of cytokeratins in which patients' sera reacted should be examined in future studies to clarify the mechanism of antibody formation.
This is the first report to demonstrate the existence of circulating CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes in AIH patients' sera. Therefore, the significance of circulating CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes in the pathogenesis of AIH should be discussed. In the liver, CK8 as well as CK18 are expressed mainly in hepatocytes [34]. CK8 as well as CK18 may have been released from hepatocytes following cell injury. The resulting antigen–antibody interaction with immune complex formation may play a significant role in the perpetuation of the disease process, either by direct injury of hepatocytes or via local macrophage activation as they are cleared by phagocytosis [35].
In the present study, we used sera diluted to 1 : 100. Therefore, the possibility of false positive results caused by the ‘inflammatory’ nature of AIH patients' sera should be considered. To avoid this problem, we compared values between sera diluted to 1 : 100 and sera diluted to 1 : 1000 in selected patients, and obtained a good correlation. Therefore, we believe that our results did not show a non-specific reaction.
Since low levels of CK8:anti-CK8 antibody as well as CK18:anti-CK18 antibody immune complexes were also detected in patients with CH-C, the detection of serum immune complexes did not necessarily indicate pathological changes of AIH. In addition, the correlation with AST levels suggests that these immune complexes might simply have been a physiological response to increased CK8 and CK18 in the circulation caused by hepatocyte necrosis. However, there is a possibility that impaired clearance of immune complexes evoke an autoimmune response and cause persistent tissue destruction [36]. To confirm the role of these immune complexes, additional experiments to prove increased complement turnover or deposition of activated complement components in patients with AIH are necessary.
References
- 1.Johnson PJ, McFarlane IG. Meeting report. Int Autoimmune Hepatitis Group Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 2.Czaja AJ. Autoimmune hepatitis: evolving concepts and treatment stategies. Dig Dis Sci. 1995;40:435–56. doi: 10.1007/BF02065434. [DOI] [PubMed] [Google Scholar]
- 3.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 1996;334:897–903. doi: 10.1056/NEJM199604043341406. [DOI] [PubMed] [Google Scholar]
- 4.Manns M, Gerken G, Kyriatsoulis A, Staritz M, Meyer zum Buschenfelde KH. Characterization of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet. 1987;1:292–4. doi: 10.1016/s0140-6736(87)92024-1. [DOI] [PubMed] [Google Scholar]
- 5.Manns M. Autoantibodies and antigens in liver disease — updated. J Hepatol. 1989;9:272–80. doi: 10.1016/0168-8278(89)90063-9. [DOI] [PubMed] [Google Scholar]
- 6.Morshed SA, Parveen S, Nishioka M. Antibodies to cytoskeleton antigens in autoimmune liver diseases. In: Krawitt EL, Wiesner RH, Nishioka M, editors. Autoimmune liver diseases. 2. Netherlands: Elsevier Science B. V.; 1998. pp. 217–56. [Google Scholar]
- 7.Matsuoka H, Chen M, Ando T, et al. Frequency of antibody to soluble liver antigen in patients chronic liver diseases and their characteristics. Kanzo. 1997;3:152–8. [Google Scholar]
- 8.Bartek J, Bartkova J, Taylor-Papadimitriou J, et al. Differential expression of keratin 19 in normal human epithelial tissues revealed by monospecific monoclonal antibodies. Histochem J. 1986;18:565–75. doi: 10.1007/BF01675198. [DOI] [PubMed] [Google Scholar]
- 9.Kasper M, Stosiek P, Typlt H, Karsten U. Histological evaluation of three new monoclonal anti-cytokeratin antibodies. 1. Normal tissues. Eur J Cancer Clin Oncol. 1987;23:137–47. doi: 10.1016/0277-5379(87)90007-1. [DOI] [PubMed] [Google Scholar]
- 10.Osborn M, Van Lessen G, Weber K, Kloppel G, Altmannsberger M. Methods in laboratory investigation. Differential diagnosis of gastrointestinal carcinomas by using monoclonal antibodies specific for individual keratin polypeptides. Lab Invest. 1986;55:497–504. [PubMed] [Google Scholar]
- 11.Ramaekers F, Huysmans A, Schaart G, Moesker O, Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987;170:235–49. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- 12.Van Eyken P, Sciot R, Desmet VJ. A cytokeratin immunohistochemical study of cholestatic liver diseases: evidence that hepatocytes can express ‘bile duct-type’ cytokeratins. Histopathology. 1989;15:125–35. doi: 10.1111/j.1365-2559.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara H, Cadrin M, Perry G, et al. Role of cytokeratin intermediate filaments in transhepatic transport and canalicular secretion. Hepatology. 1990;11:435–48. doi: 10.1002/hep.1840110315. [DOI] [PubMed] [Google Scholar]
- 14.Katsuma Y, Swierenga SH, Marceau N, French SW. Selective rearrangement of cytokeratin filaments in cultured liver epithelial cells induced by nickel. J Hepatol. 1987;5:344–54. doi: 10.1016/s0168-8278(87)80041-7. [DOI] [PubMed] [Google Scholar]
- 15.Swierenga SHH, MaLean JR. Further insights into mechanism of nickel-induced DNA damage: studies with cultured rat liver cells. In: Brown SS, Sunderman Jr FW, editors. Progress in nickel toxicity. Oxford: Blackwell Scientific Publications; 1985. pp. 101–4. [Google Scholar]
- 16.Oda M, Price VM, Fisher MM, Phillips PJ. Ultrastructure of bile canaliculi with special references to the surface coat and the pericanalicular web. Lab Invest. 1974;31:314–23. [PubMed] [Google Scholar]
- 17.Jahn W. Visualization of a filamentous network in cryo sections of liver tissue. Eur J Cell Biol. 1980;20:301–4. [PubMed] [Google Scholar]
- 18.French SW, Kondo I, Irie T, Ihrig TJ, Benson N, Munn R. Morphologic study of intermediate filaments in rat hepatocytes. Hepatology. 1982;2:29–38. doi: 10.1002/hep.1840020106. [DOI] [PubMed] [Google Scholar]
- 19.Okanoue T, Ohta M, Fushiki S, et al. Scanning electron microscopy of the liver cell cytoskeleton. Hepatology. 1985;5:1–6. doi: 10.1002/hep.1840050102. [DOI] [PubMed] [Google Scholar]
- 20.Katsuma Y, Marceau N, Ohta M, French SW. Cytokeratin intermediate filaments of rat hepatocytes: different cytoskeletal domains and their three-dimensional structure. Hepatology. 1988;8:559–68. doi: 10.1002/hep.1840080321. [DOI] [PubMed] [Google Scholar]
- 21.Tolle HG, Weber K, Osborn M. Microinjection of monoclonal antibodies specific for one intermediate filament protein in cells containing multiple keratins allows insight into the composition of particular 10 nm filaments. Eur J Cell Biol. 1985;38:234–44. [PubMed] [Google Scholar]
- 22.Tolle HG, Weber K, Osborn M. Microinjection of monoclonal antibodies to vimentin, desmin, and GFA in cells which contain more than one IF type. Exp Cell Res. 1986;162:462–74. doi: 10.1016/0014-4827(86)90350-2. [DOI] [PubMed] [Google Scholar]
- 23.Gawlitta W, Osborn M, Weber K. Coiling of intermediate filaments induced by microinjection of a vimentin-specific antibody does not interfere with locomotion and mitosis. Eur J Cell Biol. 1981;26:83–90. [PubMed] [Google Scholar]
- 24.Klymkowsky MW. Intermediate filaments in 3T3 cells collapse after intracellular injection of a monoclonal anti-intermediate filament antibody. Nature. 1981;291:249–51. doi: 10.1038/291249a0. [DOI] [PubMed] [Google Scholar]
- 25.Klymkowsky MW, Miller RH, Lane EB. Morphology, behaviour and interaction of cultured epithelial cell after the antibody-induced disruption of keratin filament organization. J Cell Biol. 1983;96:494–509. doi: 10.1083/jcb.96.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku NO, Michie S, Oshima RG, Omary MB. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol. 1995;131:1303–14. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku NO, Michie SA, Soetikno RM, et al. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J Clin Invest. 1996;98:1034–46. doi: 10.1172/JCI118864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wachter B, Kyriatsoulis A, Lohse AW, Gerken G, Meyer zum Buschenfelde KH, Manns M. Characterization of liver cytokeratins as a major target antigen of anti-SLA antibodies. J Hepatol. 1990;11:232–9. doi: 10.1016/0168-8278(90)90119-c. [DOI] [PubMed] [Google Scholar]
- 29.Kurki P, Virtanen I, Lehto VP, Alfthan O, Salaspuro M. Antibodies to cytokeratin filaments in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1984;8:212–5. doi: 10.1111/j.1530-0277.1984.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 30.Oda M, Azuma T, Komatsu H, Kaneko K, Nishizaki Y, Tsuchiya M. Formation of anti-cytokeratin antibody in primary biliary cirrhosis – its origin and pathologic significance. Hepatology. 1988;8:1416A. [Google Scholar]
- 31.Prasad S, Soldatenkov VA, Srinivasarao G, Dritschilo A. Identification of keratins 18, 19 and heat-shock protein 90β as candidate substrates of proteolysis during ionizing radiation-induced apoptosis of estrogen-receptor negative breast tumor cells. Int J Oncol. 1998;13:757–64. doi: 10.3892/ijo.13.4.757. [DOI] [PubMed] [Google Scholar]
- 32.Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–94. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bombardieri E, Seregni E, Bogni A, et al. Evaluation of CK19 serum fragments (CYFRA 21-1) in patients with lung cancer: results of a multicenter trial. Int J Biol Markers. 1994;9:89–95. doi: 10.1177/172460089400900205. [DOI] [PubMed] [Google Scholar]
- 34.Omary MB, Ku NO. Intermediate filament proteins of the liver: emerging disease association and functions. Hepatology. 1997;25:1043–8. doi: 10.1002/hep.510250537. [DOI] [PubMed] [Google Scholar]
- 35.Kim JW, Wierda WG, Kim YB. Immobilized IgG immune complex induces secretion of tumor necrosis factor-α by porcine alveolar macrophages. Am J Respir Cell Mol Biol. 1991;5:249–55. doi: 10.1165/ajrcmb/5.3.249. [DOI] [PubMed] [Google Scholar]
- 36.Davies KA, Peters AM, Beynon HL, Walport MJ. Immune complex processing in patients with systemic lupus erythematosus. In vivo imaging and clearance studies. J Clin Invest. 1992;90:2075–83. doi: 10.1172/JCI116090. [DOI] [PMC free article] [PubMed] [Google Scholar]