Abstract
The two main entities of open-angle glaucoma are primary open-angle glaucoma (POAG) and normal tension glaucoma (NTG). Both diseases may be associated with autoimmune processes. Therefore, IgG and IgM antibodies to phospholipids (APL) and their subspecies cardiolipin (ACL), phosphatidylserine (APS) and β2-glycoprotein (β2GP) were determined in 43 NTG patients, 40 POAG patients and 40 healthy controls in a prospective study. The most prominent observation was the increase in APS concentrations in NTG patients (IgG 20·6 ± 2·7 U/ml, IgM 24·4 ± 3·4 U/ml) compared with POAG patients (IgG 8·8 ± 1·2 U/ml, IgM 11·0 ± 1·7), and controls (IgG 7·7 ± 1·3 U/ml, IgM 12·8 ± 1·5 U/ml). APS may be important due to their binding specificity to phosphatidylserine molecules which become accessible during apoptosis; this in turn may lead to local thrombosis.
Keywords: Glaucoma, antiphosphoplipid antibodies, antiphosphatidylserine antibodies
Introduction
Glaucoma is one of the leading causes of blindness worldwide [1]. Currently glaucoma is defined as a heterogenous group of ocular diseases that cause characteristic progressive loss of retinal ganglion cells, and damage of the optic nerve head followed by visual-field defects. The most common type of glaucoma is open-angle glaucoma which is further divided in two main entities: patients with elevated intraocular pressure (IOP) (primary open-angle glaucoma: POAG) and with normal IOP (normal tension glaucoma: NTG). Although IOP is an important risk factor in POAG it is no longer included in the definition of glaucoma.
In recent years the understanding of the pathogenesis of glaucoma has changed to include risk factors such as disturbances of blood circulation and the immune system, excitotoxicity and neurodegeneration [2–5]. Irrespective of what causes the damage, the result is ganglion-cell death by apoptosis (programmed cell death) [1].
A close association has been recognized between open-angle glaucoma and retinal vein occlusion [6]. Occlusions can be induced by thrombosis which, in combination with antiphospholipid antibodies (APL), satisfy the diagnostic criteria of antiphospholipid syndrome [7]. One of the most commonly reported APL is the antiphosphatidylserine (APS) type. The importance of these antibodies is due to their binding specificity to phosphatidylserine molecules which are shifted from the inner to the outer leaflet of cell membranes during early apoptosis [8]. This event activates the coagulation cascade and thus may cause thrombosis. We were therefore interested in studying concentrations of APL and their subspecies in patients suffering from NTG and POAG, and in healthy controls.
PATIENTS AND CONTROLS
From October 1995 to November 1998, 43 NTG patients, 40 POAG patients and 40 healthy blood donors (controls) were recruited to participate in this prospective study. The clinical and demographic profile of the patients is shown in Table 1. Two of the NTG patients (lupus erythematosus, rheumatoid arthritis) and four POAG patients (rheumatoid arthritis) had autoimmune diseases. These patients were included in this study because they showed only a mild course and received no immunosuppressive agents.
Table 1.
Clinical and demographic profile of patients with normal tension or primary open-angle glaucoma and controls
| NTG* | POAG† | Controls | Significance‡ | |
|---|---|---|---|---|
| Number of individuals | 43 | 40 | 40 | |
| Age in years (mean) | 64 | 69 | 62 | NS§ |
| Age in years (SEM) | 2 | 1 | 1 | |
| Male/female ratio | 19/24 | 21/19 | 30/17 | NS |
| Systemic hypertension | 23 | 15 | 0 | NS |
| Systemic hypotension | 5 | 4 | 0 | NS |
| Thrombosis | 10 | 2 | 0 | 0·027 |
| Autoimmune diseases | 2 | 4 | 0 | NS |
| Diabetes | 6 | 6 | 0 | NS |
| Cerebral infarction | 2 | 3 | 0 | NS |
| Syncopes | 7 | 0 | 0 | 0·012 |
NTG: normal tension glaucoma.
POAG: primary open-angle glaucoma.
NTG versus POAG and controls.
NS: not significant.
Glaucoma was diagnosed by standard ophthalmologic examination, visual acuity, IOP, automated perimetry, optic disc and nerve fibre layer photographs. The optic nerve head and its excavation was measured by scanning laser tomography (TopSS, LDT, San Diego, CA). Nerve fibre layer thickness was assessed by scanning laser polarimetry (GDx, LDT).
Materials and methods
Blood specimens
Blood was obtained after venepuncture and serum obtained after centrifugation. Concentrations of autoantibodies and neopterin were measured within one week; APL were determined with serum stored at − 20°C until use. C3, C4, C1 esterase inhibitor, and C reactive protein (CRP) was estimated within two days. The volume of the sera was sometimes too low to perform all measurements indicated below.
Antiphospholipid antibodies
Antibodies to phospholipids (APL) were detected by an enzyme-linked immunosorbent assay (ELISA) using rabbit thromboplastin, a mixture of phospholipids and antihuman sera (Dianova, Hamburg, Germany) specific for immunoglobulin (Ig) G and IgM [9]. A cut-off value for an elevated concentration was defined as >250 optical densities (OD).
IgG and IgM anticardiolipin antibodies (ACL) were measured by ELISA (DPC Biermann, Bad Nauheim, Germany); serum IgM ACL concentrations >7 U/ml were regarded as elevated.
Antiphosphatidylserine antibodies (APS) of IgG and IgM classes were determined by ELISA (Imtec, Berlin, Germany); the normal range was <15 U/ml.
Anti β2-glycoprotein antibodies (β2AGP) were estimated using an ELISA (Imtec); serum concentrations >5 U/ml were regarded as elevated.
Other laboratory tests
In order to confirm additional diagnoses, and to exclude active rheumatic diseases, antibodies to serum nuclear antigens, antibodies to extractable nuclear antigens, antibodies to double-stranded DNA, complement components C3 and C4, C1 esterase inhibitor were determined by standard methods.
Standard methods were also used to identify patients with systemic inflammation by the determination of C reactive protein (CRP, normal range <0·51 mg/dl), neopterin and antineutrophil cytoplasmic antibodies (c and p ANCA).
Statistical analysis
Firstly, the Kruskal–Wallis test was performed to ascertain differences between the unpaired data of the two groups of patients and the controls. If a P-value was below 0·05, Wilcoxon's test was used to identify the different groups. Frequencies of elevated values were compared using chi square or Fisher's Exact test. A correlation test was performed using Spearman's coefficient of correlation; a coefficient of correlation r > 0·5 was regarded as important. The level of significance was designated as ≤0·05 (two-tailed tests). The statistics were performed using the Statistical Analysis System software (SAS, Cary, NC).
Results
Patients and controls
NTG patients had significantly more circulatory disturbancies such as thrombosis and syncopes as compared with POAG patients and controls (Table 1). NTG patients were only slightly (P = 0·0193) older than controls. After exclusion of the six patients with known autoimmune diseases the clinical findings were similar in the patient groups.
Antiphospholipid antibodies
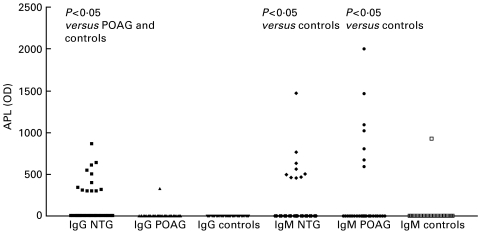
Figures 1 and 3 show that NTG patients had significantly higher concentrations of IgG APL and the subspecies APS than POAG patients and controls. Twenty-three out of 43 NTG patients (i.e. 53%) had IgG APS levels above the normal range, whereas 15% of the POAG patients and 7·5% of the controls showed elevated concentrations (P < 0·002, NTG versus POAG and controls). Tests for age and concentrations of antiphospholipid antibodies revealed no significant correlation.
Fig. 1.
IgG or IgM concentrations of antiphospholipid antibodies of patients with normal tension or primary open-angle glaucoma, and controls. APL, antiphospholipid antibodies; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma.
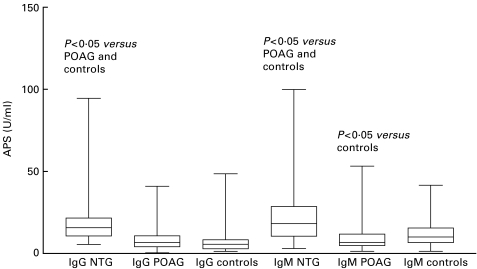
Fig. 3.
IgG or IgM concentrations of antiphosphatidylserine antibodies of patients with normal tension or primary open-angle glaucoma, and controls. APS, antiphosphatidylserine antibodies; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma. The bars extend from the 25th percentile to the 75th percentile with a horizontal line at the median. Whiskers extend to the smallest value and up to the largest.
NTG patients had significantly increased IgM levels of APL (Fig. 1), the subspecies ACL (Fig. 2), and APS (Fig. 3) as compared with controls; IgM ACL and APS concentrations were also significantly increased compared with POAG patients. POAG patients had significantly elevated IgM APL levels compared with controls. IgM APS concentrations above the cut-off value of 15 U/ml were found in 55·8% of the NTG patients, in 20% of the POAG patients, and in 27·5% of the controls (P < 0·01, NTG versus POAG and controls).
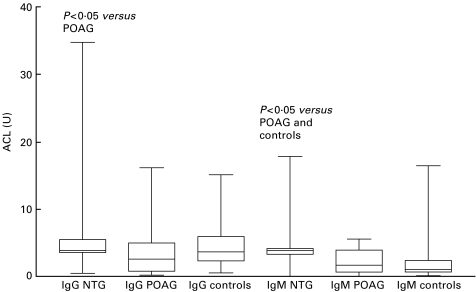
Fig. 2.
IgG or IgM concentrations of anticardiolipin antibodies of patients with normal tension or primary open-angle glaucoma, and controls. ACL, anticardiolipin antibodies; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma. The bars extend from the 25th percentile to the 75th percentile with a horizontal line at the median. Whiskers extend to the smallest value and up to the largest.
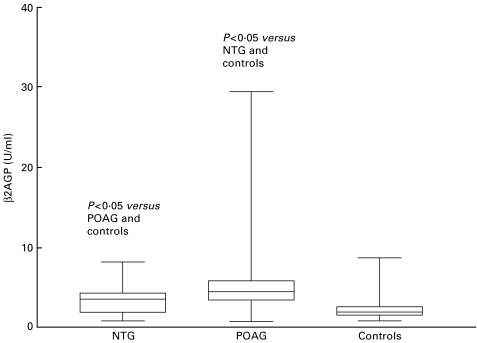
The β2AGP levels increased significantly from the controls to NTG patients and to the POAG patients (Fig. 4). The number of POAG patients with elevated results (32·5%) was significantly increased as compared with NTG patients (9·3%) and controls (5·1%).
Fig. 4.
Concentrations of β2-glycoprotein antibodies of patients with normal tension or primary open-angle glaucoma, and controls. β2AGP: anti β2-glycoprotein antibodies; NTG, normal tension glaucoma; POAG, primary open-angle glaucoma. The bars extend from the 25th percentile to the 75th percentile with a horizontal line at the median. Whiskers extend to the smallest value and up to the largest.
The combination of elevated IgG and IgM APS levels was significantly more common in the NTG patients (34·9%) as compared with the POAG patients (7·5%) or the controls (2·5%).
The only difference seen between the groups after the exclusion of the six patients with autoimmune disease was that concentrations of IgG ACL of NTG patients were significantly increased compared to POAG patients.
Other laboratory tests
None of the additional measurements revealed significant differences between patients and controls.
Discussion
The most prominent observation of this study was the increase in antiphosphatidylserine antibody (APS) concentrations, a subspecies of antiphospholipid antibodies (APL), in patients suffering from normal tension glaucoma (NTG). These autoantibodies bind to phosphatidylserine molecules, which during early apoptosis are shifted from the inner to the outer leaflet of cell membranes [8]. This event activates the coagulation cascade and thus may cause thrombosis. Therefore, increased APS concentrations and a higher frequency of thromboembolism in the NTG patients as compared with POAG patients and controls may be associated.
Despite numerous studies, there is still no clear understanding of how antiphospholipid antibodies cause thrombosis, particularly since some of these antibodies have anticoagulant properties in in-vitro coagulation tests [8], notably ACL [10] and β2AGP [11]. Galli et al. [12] identified an IgG fraction which reacted with phosphatidylserine-bound human prothrombin and showed anticoagulant activity. Yodfat et al. showed in a mouse model for antiphospholipid syndrome that purified IgG APS, but not IgM APS, had strong anticoagulant activity [13]. The IgM APSA, however, was not found to be pathogenic in this experimental model.
Our results support an association between increased APS concentrations and NTG. APS of the IgG and IgM classes were significantly increased as compared with POAG patients and controls; secondly NTG patients showed more significantly elevated APS levels than increased concentrations of APL, ACL and antiβ2GP.
Interestingly, 34·9% of the NTG patients had both elevated IgG and IgM APS antibody levels. This may be indicative of an active (IgM) and persistent (IgG) autoimmune process.
Patients with recurrent spontaneous abortion (RSA) have a high incidence of elevated APS concentrations [14]. The clinical characteristics of women suffering from RSA resemble the patients included in our study in so far as the diagnosis of RSA usually excludes patients with collagen vascular diseases [14]. Indeed, the patients enrolled in this study were not suffering from collagen vascular diseases and signs of systemic inflammation.
POAG patients have significantly increased β2AGP concentrations, and the number of patients with elevated concentrations is significantly increased. The identification of β2AGP as an essential component of the antigenic complex recognized by ACL suggested that this molecule could be a direct target of the antibody response [15]. Our findings contrast with the results of Tsakiris et al. [16] who found comparable β2AGP levels in NTG patients, chronic POAG patients and controls. This may be due to methodological differences because Tsakiris et al. [16] measured β2AGP by Laurell immunoelectrophoresis.
Although IgM ACL concentrations were significantly increased in NTG patients as compared with POAG patients and controls, the number of NTG patients with elevated levels were not significantly different. The latter result corroborates the findings of Tsakiris et al. [16]. ACL molecules may have an important role in thrombosis because they bind apoptotic bodies, resulting in an alteration of their physiological clearance which is associated with an increase in secretion of tumour necrosis factor alpha [15]. This mechanism, however, is not proven in NTG patients so far, although under experimental conditions apoptosis was observed in glaucoma [17,18]. ACL are also able to bind to endothelial cell membranes and induce cell activation [19].
Open-angle glaucoma is divided into two main entities, NTG and POAG. Both diseases show a common autoantibody profile: concentrations of IgM APL, APS and β2AGP were significantly increased as compared with the levels of the controls. In contrast, IgG APL and APS concentrations were only significantly higher in the NTG patients. We cannot exclude the possibility that the death of ganglion cells themselves induces this increase of these IgM autoantibodies but this is unlikely because ganglion cells die in both types of open-angle glaucoma. Clinically, the NTG patients had a very high frequency of circulatory disturbancies (see Table 1), with 10 having a history of thromboembolism. Therefore, apoptotic events outside the eye may occur and induce a systemic autoimmune process. Although retinal vein occlusions are often found in open-angle glaucoma, different autoantibodies may contribute to the pathogenesis of this disease [20] and may define subtypes. It will be useful to correlate autoantibody concentrations with changes in the optic nerve in future independent studies and to establish the relevance of autoantibodies in the pathogenesis of NTG. This may lead to new therapeutic strategies.
Acknowledgments
The authors thank Mrs A. Schmidt and Mrs B. Thiam for their excellent technical assistance.
References
- 1.Coleman AL. Glaucoma. Lancet. 1999;354:1803–10. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 2.Flammer J, Orgül S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retinal Eye Res. 1998;17:267–89. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 3.Dreyer EB, Lipton SA. New perspectives on glaucoma. JAMA. 1999;281:306–8. doi: 10.1001/jama.281.4.306. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz M, Yoles E. Self-destructive and self-protective processes in the damaged optic nerve: implications for glaucoma. Invest Ophthalmol Vis Sci. 2000;41:349–51. [PubMed] [Google Scholar]
- 5.Tomita G. The optic nerve head in normal-tension glaucoma. Curr Opin Ophthalmol. 2000;11:116–20. doi: 10.1097/00055735-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Lindblom B. Open angle glaucoma and non-central retinal vein occlusion – the chicken or the egg? Acta Ophthalmol Scand. 1998;76:329–33. doi: 10.1034/j.1600-0420.1998.760315.x. [DOI] [PubMed] [Google Scholar]
- 7.Kutteh WH, Rote NS, Silver R. Antiphospholipid antibodies and reproduction: the antiphospholipid antibody syndrome. Am J Reprod Immunol. 1999;41:133–52. doi: 10.1111/j.1600-0897.1999.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 8.Bevers EM, Confurius P, Dekkers DWC, Harmsma M, Zwaal RFA. Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus. 1998;7(Suppl):S126–S131. doi: 10.1177/096120339800700228. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Berg PA. High incidence of antibodies to 5-hydroxytryptamine, gangliosides and phospholipids in patients with chronic fatigue and fibromyalgia syndrome and their relatives: evidence for a clinical entity of both disorders. Eur J Med Res. 1995;1:21–6. [PubMed] [Google Scholar]
- 10.Rauch J, Meng QH, Tannenbaum H. Lupus antiplatelet properties of human hybridoma autoantobodies. J Immunol. 1987;139:2598–604. [PubMed] [Google Scholar]
- 11.Galli M, Barbui T. Prothrombin as cofactor for antiphospholipids. Lupus. 1998;7(Suppl):S37–40. doi: 10.1177/096120339800700209. [DOI] [PubMed] [Google Scholar]
- 12.Galli M, Beretta G, Daldossi M, Bevers EM, Barbui T. Different anticoagulant and immunological properties of anti-prothrombin antibodies in patients with antiphospholipid antibodies. Thromb Haemost. 1997;77:486–91. [PubMed] [Google Scholar]
- 13.Yodfat O, Blank M, Krause I, Shoenfeld Y. The pathogenic role of anti-phosphatidylserine antibodies: active immunization with the antibodies leads to the induction of antiphospholipid syndrome. Clin Immunol Immunopathol. 1996;78:14–20. doi: 10.1006/clin.1996.0003. [DOI] [PubMed] [Google Scholar]
- 14.Branch DW, Hatasaka HH. Antiphospholipid antibodies and infertility: fact or fallacy. Lupus. 1998;7(Suppl):S90–S94. doi: 10.1177/096120339800700220. [DOI] [PubMed] [Google Scholar]
- 15.Tincani A, Spatola L, Cinquini M, Cattaneo R, Meroni P, Balestri G. Anti β2-glycoprotein I antibodies: clinical significance. Lupus. 1998;7(Suppl):S107–S109. doi: 10.1177/096120339800700224. [DOI] [PubMed] [Google Scholar]
- 16.Tsakiris DA, Osusky R, Kaiser HJ, Mueri R, Flammer J, Marbet GA. Lupus anticoagulants/anticardiolipin antibodies in patients with normal tension glaucoma. Blood Coagulation Fibrinolysis. 1992;3:541–5. doi: 10.1097/00001721-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Valenzuela S, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 18.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Visual Sci. 1995;36:774–86. [PubMed] [Google Scholar]
- 19.Meroni PL, Del Papa N, Raschi E, et al. β2-glycoprotein I as a ‘cofactor’ for anti-phopholipid reactivity with endothelial cells. Lupus. 1998;7(Suppl):S44–S47. doi: 10.1177/096120339800700211. [DOI] [PubMed] [Google Scholar]
- 20.Tezel G, Edward DP, Wax MB. Serum autoantibodies to optic nerve head glycosaminoglycans in patients with glaucoma. Arch Ophthalmol. 1999;117:917–24. doi: 10.1001/archopht.117.7.917. [DOI] [PubMed] [Google Scholar]