Abstract
In this paper we describe the clinical and molecular features of a new case (GOR) of homozygous human TAP2 deficiency, analysing the phenotype and function of NK cells. The patient presented from infancy with recurrent sinopulmonary infections; a selective IgG2 deficiency, negative antibody response to polysaccharide vaccination and low level of cell surface expression of HLA class I antigens were found. The sequence of TAP2 gene identified a single mutation, a C to T substitution changing the CGA arg codon at amino acid 220 into TGA stop codon in exon 3. By using MoAbs for KIRs, CD94, CD94/NKG2A and ILT2 we observed, in agreement with others, that the latter two receptors were overexpressed on TAP2-deficient NK cells. The inhibitory CD94/NKG2A and triggering CD94/NKG2C NK receptors, specific for HLA-E, appeared to be functional in a limited number of NK clones that could be expanded in vitro. Expression of HLA-E was virtually undetectable in GOR B-LCL and very faint in PBMC, further supporting that interactions of class I leader sequence nonamers with HLA-E in the ER depend on a functional TAP complex.
Keywords: immunodeficiency diseases, lung, transporters, MHC, NK cells
Introduction
The previously named Type I Bare lymphocyte syndrome or HLA class I deficiency [1,2] is a rare disease recently associated with mutations in the TAP1/TAP2 peptide transporter complex [3–8]. This complex is a heterodimeric protein constituted by two homologous polypeptides encoded by the TAP1 and TAP2 genes located in the MHC class II region [9]. Peptides generated from newly synthesized proteins are degraded in the cytosol and later transferred into the lumen of the endoplasmic reticulum (ER) by the TAP1/TAP2 complex; the knowledge that MHC class I molecules in the ER can be co-precipitated with TAP by anti-TAP antibodies raised the possibility that TAP could play an active role in the assembly and release of class I molecules; this release is peptide specific and is dependent on the MHC class I allele present [10]. The inhibitory receptors (IR) specific for HLA class I molecules, ILT-2 and CD94/NKG2A, are overexpressed on TAP-deficient NK cells compared to normal NK cells; these results could suggest an adaptive process of resting TAP-deficient NK cells to the low levels of HLA class I expression [11]. Furthermore, HLA-E surface expression, the major ligand for the CD94/NKG2 receptor, was described recently with a variable expression in a TAP1-deficient patient [12], normal in monocytes, slightly decreased in lymphocytes and undetectable in a B-LCL cell line; these data could suggest that TAP is required only by certain cell types to load peptides with leader sequence nonamers, pointing out the possible existence of TAP-dependent and independent pathways for surface expression of HLA-E molecules.
In this paper we report the molecular and clinical characteristics of a new TAP2-deficient patient. Our results confirm that the CD94/NKG2A and ILT2 inhibitory receptors specific for HLA class I-molecules are overexpressed on TAP-deficient NK cells compared to normal NK cells. HLA-E surface expression in PBMC, including monocytes, and B-LCL was defective, supporting that a functional TAP complex is necessary for the interaction of class I leader sequence nonamers with HLA-E. The inhibitory CD94/NKG2A and triggering CD94/NKG2C receptors, specific for HLA-E, appeared to be functionally normal in a very limited number of NK clones that could be expanded in vitro. Functionally, NK cells from our patient present markedly diminished cytotoxicity against class I negative K562 target cells and no detectable autoreactivity. Others have shown that upon short-term in vitro activation with cytokines, polyclonal TAP2 negative NK cells acquired some capacity to kill autologous targets [13]; this reactivity could account for the extremely low cloning efficiency found repeatedly when we attempted the expansion of TAP2-deficient NK clones. A loss of TAP-NK self-tolerance might also occur in the course of an inflammatory response in vivo, contributing in some way to the clinical manifestations.
Materials and methods
Patients
The biological studies in this family include the propositus GOR and his mother. Parents were first cousins; the older patient's brother, who died undiagnosed at 22 years old, had presented since childhood recurrent sinusitis, bronchitis and progressive bilateral bronchiectasis. The propositus has also suffered from recurrent sinopulmonary infections from infancy and he developed progressive inoperable bronchiectasis with severe altered lung function and chronic Pseudomonas aeruginosa infection. In 1994, the patient was diagnosed as a IgG2 subclass deficiency and no specific antibody response was made after polysaccharide vaccination (Pneumovax-23, Merck-Sharp-Dome and Hib-TITER, Lederle); he started substitutive gammaglobulin therapy monthly. In June 1997 the patient was included in a lung transplant programme (Vall d’Hebrón Hospital, Barcelona, Spain); after HLA tissue typing studies the patient was diagnosed with HLA-class I deficiency.
Serological and molecular HLA typing
Serological class I and class II typing were performed according to the standard microlymphocytotoxicity assay. For DNA class I and class II typing, genomic DNA from patient and controls were extracted from peripheral blood using the proteinase K-phenol extraction method. DNA samples were amplified by PCR-SSP procedure (Dynal, Oslo, Norway).
Monoclonal antibodies
FITC-conjugated anti-CD3, anti-TCR αβ, anti-TCR γδ, anti-CD16, anti-CD14 and anti-CD19 MoAbs and PE conjugated anti-CD4, anti-CD8 and anti-CD56 were purchased from BD (Becton Dickinson, Mountain View, CA, USA). FITC and PE mouse IgG1 conjugated (BD) were used to determine the boundary between positive and negative cells in flow cytometric analysis. W6/32-PE from Dako (Copenhagen, Denmark) was used to detect HLA class I molecules. Anti-CD56 PE and anti-CD3 PerCP (BD) were used in combination with MoAbs specific for the following natural killer receptors (NKRs): anti-NKG2A Z199 (IgG2b), anti-KIR2D EB6 (IgG1) and GL183 (IgG1), kindly provided by A. Moretta (Istituto di Istología. University of Genova, Italy); anti-CD94/NKG2A and CD94/NKG2C P25 (IgG1) was kindly provided by Dr D. Pende (Centro Biotecnologie Avanzate, Genova, Italy); anti-KIR3D DX9 (IgG1), was kindly provided by L.L. Lanier (DNAX, Palo Alto, CA, USA); anti-KIR3D 5·133 (IgG1) was a gift from Dr M. Colonna (Basel Institute for Immunology, Basel, Switzerland). The anti-HLA-E 3D12 (IgG1) was kindly provided by Dr D. Geraghty (Fred Hutchinson Cancer Research Center, Seattle, WA, USA). All others MoAbs used have been described previously [14–20].
Polyclonal antibodies
Rabbit antibodies anti-TAP2 (TAP2A and TAP2B), TAP1, LMP2 and LMP7 have been described previously [3].
Flow cytometric analysis
Whole blood from patient and controls were double or triple labelled with different MoAb combinations and data analysis was performed on a FACScan flow cytometer (BD) using lysis II software (BD). A gate for lymphocyte population was defined by forward and side light scatter characteristics. HLA class I expression was measured as mean fluorescence intensity (MFI).
Western blot analysis of TAP1, TAP2, LMP2 and LMP7
TAP1, TAP2, LMP2 and LMP7 protein expression were studied in EBV transformed cell lines from GOR, his mother and normal class I positive controls; as a negative control the class I negative human mutant B cell line 721·174 was used. Cells were lysed in lysis buffer containing 1% Nonidet P-40, 50 mm TrisHCl pH 7·5, 150 mm NaCl, 1 mm EDTA and the protease inhibitors leupeptin, aprotinin and PMSF. Then, 50 µg of protein per lane from lysates were run on a 10% SDS-polyacrylamide gel, transferred to Hybond-ECL nitrocellulose membranes (Amersham Pharmacia Biotech, Uppsala, Sweden) and blocked in PBS-Tween 20 containing 5% non-fat dry milk. The membranes were incubated with primary rabbit antibodies, anti-TAP1, anti-TAP2 (TAP2A, TAP2B, kindly provided by Prof. J. Trowsdale; Cambridge University, Department of Pathology, Immunology Division, Cambridge, UK), washed twice in PBS-Tween 20 and incubated with horseradish peroxidase-conjugated goat antirabbit IgG. Finally, the membranes were washed in PBS-Tween 20 and resolved with enhanced chemiluminiscence (SuperSignal Substrate, Pierce, Rockford, USA) and exposed to film. Anti-LMP2 and anti-LMP7 polyclonal antibodies were used for LMP protein analysis (kindly supplied by Dr A. Kelly Cambridge University, Department of Pathology, Immunology Division, Cambridge, UK).
TAP2 gene sequencing
TAP2 gene was partially amplified from genomic DNA by using a set of five prime mixtures placed at intron regions. Amplification products included two or three exons covering the 11 exons that form the TAP2 gene. Primer sequences, amplified exons and amplified length were the following: 5′-TCTCGTATCCGTT GACAGAGC-3′ and 5′-AGCAAATGGACCCAGCTGCCC-3′ (exons 1–2, 749 bp); 5′-CCCTCCCCTCTTATTCTCCTA-3′ and 5′-GGGGTTCCCTTAGCACCGC-3′ (exons 3–4, 674 bp); 5′-CTGGTGTTTGCTGGCCCTCTT-3′ and 5′-GGAGAGCCAA GGGGGCCTTGGCTTCTC-3′ (exons 5–6, 546 bp); 5′-TCACTT GTATCTGAGGAAGGG-3′ and 5′-GGGACACGACCTTCAC CACTA-3′ (exons 7–9, 988 bp); 5′-TCACTTGGGTATCTGAG GAAAGGGGG-3′ and 5′-GGGCCCAACCGTATCCCTGGGGG GGCC-3′ (exons 10–11, 744 bp). Amplification products were cloned by using TOPO TA vector (Invitrogene, Carlsbad, CA, USA) and more than eight clones derived from each amplification reaction were sequenced by using Thermo Sequenase, Cy 5-labelled M13 universal primers and cycle amplification protocols (Amersham Pharmacia Biotech, Uppsala, Sweden). Sequencing reactions were analysed in an ALF express automated sequencer.
ARMS-PCR analysis
In order to achieve the homozygosity of the mutation found at nucleotide position 658 of TAP2 gene from GOR patient, an ARMS-PCR typing was carried out [17,18]. Two oligonucleotides were designated in order to distinguish the wild type cytosine from mutated timidine at nucleotide position 658: TAP2WT 5′-GCTTCACCTACACCATGTCTCC-3′ and TAP2 GOR 5′-GCTTCACCTACACCATGTCCTT-3′. Two different amplification reactions by using one of the above described primers, together with a consensus antisense primer placed at intron 4, were carried out for each tested sample. A DNA fragment of 615 bp was obtained and analysed in a 2% agarose gel electrophoresis.
Cells
As described [15], NK clones were grown and characterized by flow cytometry analysis with a panel of receptor-specific MoAbs. The NKL leukaemia cell line was maintained in medium supplemented with 10% (v/v) heat-inactivated human AB serum, and the HLA-class I-deficient EBV-transformed B lymphoblastoid cell line 721·221 was grown as described [15]. 721·221-AEH cells (surface HLA-E+) expressing the AEH chimeric construct, in which the HLA-E leader sequence was replaced by that of HLA-A2, have been reported [14].
Cytotoxicity assays
As described [19], effector cells were tested in a 2-h 51Cr-release assay against either the B-LCL, the murine mastocytoma line P815, the 721·221 cell line (control), or the 721·221 HLA class I transfectants; effector/target ratios ranged from 2/1 to 10/1. The antagonistic effect of MoAbs was tested by preincubating cells for 10 min prior to the assay with saturating concentrations of either receptor-specific reagents (for effectors), or anti-HLA MoAbs (for targets).
Results
Analysis of MHC class I expression
Serological analysis of patient's MHC antigens showed a normal class II but not class I molecule expression; FACS analysis of the patient's PBMC showed a low MFI when labelled with W6/32 MoAb (class I monomorphic region specific) and compared to normal controls; low MFI for class I was confirmed when peripheral T, B and monocytes, identified by lineage specific MoAbs, were studied as well as in GOR EBV-transformed B cells. MFI of class I expression was normal in the mother's cells (Fig. 1). PCR-SSP tissue typing of the patient's PBMC showed homozygosity for MHC class I and class II molecules (HLA-A*01, B*51, Cw*07, DRB1* 1305, -DRB3*0202 or DQB1*03011).
Fig. 1.
Surface expression of HLA class I molecule using W6/32 MoAb. The results are expressed as mean fluorescence intensity (MFI). (a) W6/32 expression on peripheral blood mononuclear cells (PBMC). (b) W6/32 expression on CD3+ lymphocytes. (c) W6/32 expression on CD19+ lymphocytes. (d) W6/32 expression on CD14+ monocytes.
Analysis of TAP complex, LMP2 and LMP7 molecules
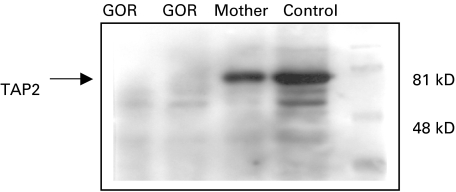
Western blot analysis of TAP1, TAP2, LMP2 and LMP7 (proteasome units) showed clearly that TAP1 molecules were present in 721·221 positive control cell lines, the mother's cells and GOR cells, but not in the 721·174 negative control cell lines. TAP2A were present in the 721·221 cells and the mother's cells but not in GOR or 721·174 cells; TAP2B band were clearly seen in 721·221 and the mother's cells but were not present in GOR cells or 721·174 line (data not shown). Figure 2 shows Western blot analysis of TAP2 from GOR cells, his mother and control cells.
Fig. 2.
Western blot analysis of TAP2 protein from the patient's cells (GOR), his mother and normal control.
TAP2 mutation
TAP2 gene extends around 9Kb organized in 11 exon regions and placed within the HLA Class II region [21]. Complete sequencing of the 11 exons of TAP2 from the patient was carried out; the consensus sequence obtained proved that the patient bears an unique TAP2 sequence with a single point mutation at nucleotide position 658, C to T, placed at exon 3 when compared to the wild-type sequence. This new sequence changes the CGA arginine codon at amino acid 220 to a TGA stop codon (Table 1) GeneBank accession numbers (AF097669AF097679). Therefore, the premature stop of gene translation in the patient would explain the absence of TAP2mature protein. To further confirm sequencing data and the presence of a homozygous point mutation at position 658 of TAP2gene, an ARMS-PCR typing procedure was designed. Amplification by using the wild-type primer combination, failed to amplified the DNA sample from GOR. Therefore, homozygosity for the 658 point mutation in the TAP2gene from the patient was established. TAP2gene shows a limited diversity; eight dimorphic sites placed at exons 1, 5, 6, 7, 9 and 11 have been described. This polymorphism is reflected only in four alleles that have been officially termed: TAP2*0101, *0102, *0103 and *0201 [17]. Comparison with previously described TAP2alleles shows that the TAP2gene from the patient shows a new polymorphism combination; the patient's TAP2gene differs from TAP2*0101 at nucleotide position 489; this mutation is silent.
Table 1.
Patient’s TAP2 gene sequence
| S | L | S | A | G | C | R | G | G | C | F | T | Y | T | M | 218 | |
| c | tca | ctg | tct | gca | ggc | tgc | cga | gga | ggc | tgc | ttc | acc | tac | acc | atg | |
| S | Stp | I | N | L | R | I | R | E | Q | L | F | S | S | L | L | |
| tct | tga | atc | aac | ttg | cgg | atc | cgg | gag | cag | ctt | ttc | tcc | tcc | ctg | ctg | |
| R | Q | D | L | G | F | F | Q | E | T | K | T | 246 | ||||
| cgc | cag | gac | ctc | ggt | ttc | ttc | cag | gag | act | aag | aca | g |
Phenotype of peripheral blood lymphocyte subsets and NK cells; NK cytotoxic activity
Patient CD3+ CD4+ and CD3+ CD8+ αβ T cell percentages were normal, 37% and 30% (normal ranges: 28–57% and 10–39%), respectively. Absolute numbers of CD3+ CD4+ and CD3+ CD8+ subpopulations were low, as patient total lymphocyte count was repeatedly below 800 cells/mm3. An increase of TCR γ/δ + cells were observed in the patient, 24% (normal range 2–10%), of which 10% were CD8+.
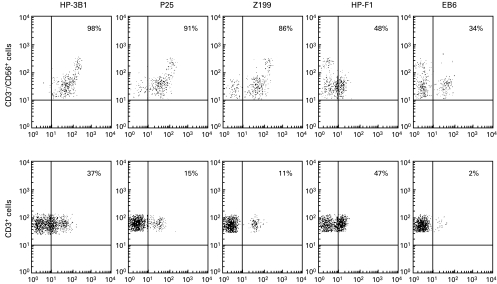
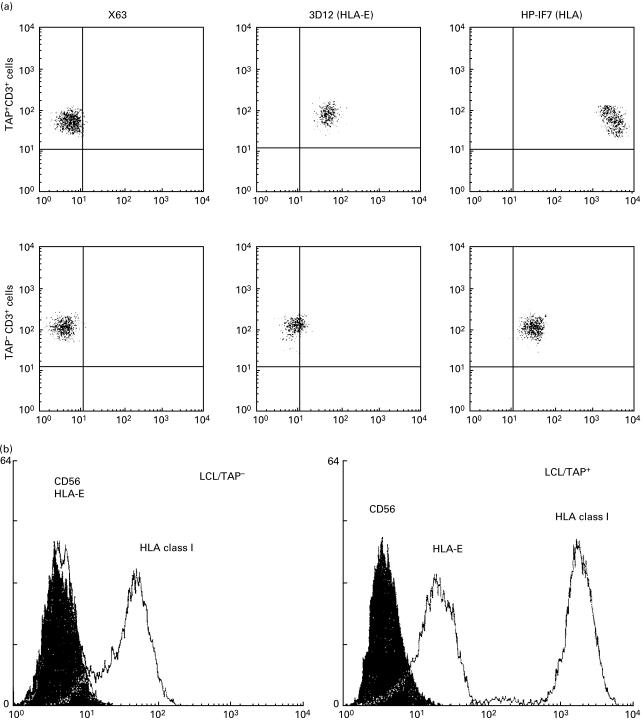
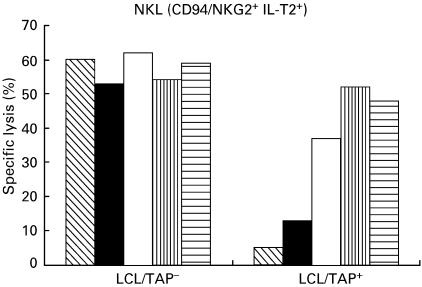
In the three-colour immunofluorescence analysis with NKRs specific MoAbs, most KIR-specific MoAbs stained variable proportions of NK and T cells (34% EB6+ CD56+ CD3−; 2% EB6+ CD3+; 30% GL183+ CD56+ CD3−; 62% 5·133+ CD56+ CD3−; 8% 5·133+ CD3+). Similarly, the HP-F1 MoAb reacted with about 50% of NK and T cells (Fig. 3). CD94 was detected virtually on all NK cells and on a high proportion of T lymphocytes. Among the latter, two subsets (CD94 bright and CD94 dull) could be discriminated. It is of note that the NKG2-specific Z199 and P25 MoAbs also stained most CD56+ CD3− cells, but reacted only with a minor T cell subpopulation of CD94+ cells, thus indicating that the latter do not express CD94/NKG2A nor CD94/NKG2C. CD94 dull T cells might bear CD94 homodimers or, alternatively, a different CD94/NKG2 receptor not recognized by P25 MoAb. In agreement with another study, the Z199+ P25+ CD94/NKG2A inhibitory heterodimer was predominant and moreover, the CD94, CD94/NKG2A and ILT2 MFI are clearly augmented in NK cells from the patient when compared to normal controls (Table 2). The ligand for CD94/NKG2 dimers is the HLA-E class Ib molecule, which is stabilized upon binding to nonamers derived from the leader sequences of other HLA class I molecules; remarkably, this process has been proposed to require TAP and tapasin. Thus, we comparatively assessed HLA-E expression on PBMC and B-LCL derived from the TAP2-deficient patient (GOR) and his mother (R); cells were stained with an HLA-E specific MoAb (3D12) or with anti-class I reagents (HP-1F7, W6/32). Expression of HLA-E was virtually undetectable in B-LCL from GOR and PBMC were very faintly stained (Fig. 4a,b). Furthermore, an ILT2+ CD94/NKG2A+ NK cell line was tested against both B-LCL in the presence of NKR- or HLA-specific MoAbs. As shown in (Fig. 5), HLA class I+ B-LCL (R) cells were partially protected from NK lysis involving both receptor-ligand systems; this was supported by the observation that a combination of anti-CD94 and ILT2 MoAbs fully restored cytotoxicity, exerting an antagonistic effect comparable to that reached by the anti class I MoAb. In contrast, the patient's B-LCL (GOR) cells were not protected from lysis, confirming that expression of class I molecules plays a central role in preventing susceptibility to NK-mediated cytotoxicity, a function that was not apparently compensated by other inhibitory NKR system under our experimental conditions.
Fig. 3.
Expression of NK cell receptors in peripheral blood NK and T lymphocytes of the TAP2-deficient patient. Peripheral blood lymphocytes were stained by indirect immunofluorescence with different NK receptors-specific MoAbs including HP-3B1 (anti-CD94), P25 (anti-CD94/NKG2A/C), Z199 (anti-CD94/NKG2A), HP-F1 (anti-ILT2) and EB6 (anti-KIR2DL1/S1), in combination with PerCP-labelled anti-CD3 and phycoerythrin-labelled anti-CD56 MoAbs.
Table 2.
Expression of different NK receptors on peripheral blood NK and T lymphocytes of normal controls and TAP-deficient patient
| CD3+ | CD3−CD56+ | |||
|---|---|---|---|---|
| % | MFI | % | MFI | |
| CD94 | ||||
| Controls | 10 | 100 | 93 | 80 |
| (n = 6) | ||||
| Patient | 19 | 66 | 83 | 232 |
| CD94/NKG2A | ||||
| Controls (n = 6) | 7 | 145 | 44 | 97 |
| (n = 6) | ||||
| Patient | 8 | 118 | 69 | 267 |
| ILT2 | ||||
| Controls | 15 | 35 | 19 | 36 |
| (n = 6) | ||||
| Patient | 31 | 36 | 48 | 106 |
The results are expressed as mean fluorescence intensity (MFI) and percentage of positive cells.
Fig. 4.
Deficient HLA-E expression in TAP2-PBMC and B-LCL. (a) PBMC from the TAP-deficient patient and normal controls were stained by indirect immunofluorescence with anti-HLA class I (HP-1F7) or anti-HLA-E (3D12) MoAbs in combination with PerCP-labelled anti-CD3 MoAb. (b) B-LCLs from the TAP-deficient patient and his TAP+ mother were stained by indirect immunofluorescence with anti-HLA class I (HP-1F7) or anti-HLA-E (3D12) MoAb and analysed by flow cytometry. Anti-CD56 (C218) MoAbb was used as negative control.
Fig. 5.
Susceptibility to NK lysis of TAP2-cells. The CD94/NKG2A+ ILT2+ NKL cell line was tested in 51Cr-release assays against LCL/TAP− and LCL/TAP+ and the effects of anti-CD94, anti-ILT2 and anti-HLA class I MoAbs were assessed in parallel; anti-CD56 was used as a control. The poor expression of HLA class I molecules on the LCL/TAP− was in correlation with its increased susceptibility to lysis by NKL compared to LCL/TAP+ 0.  , CD56; ▪, CD4; □, ILT2;
, CD56; ▪, CD4; □, ILT2;  , CD94/ILT2;
, CD94/ILT2;  , HLA class I.
, HLA class I.
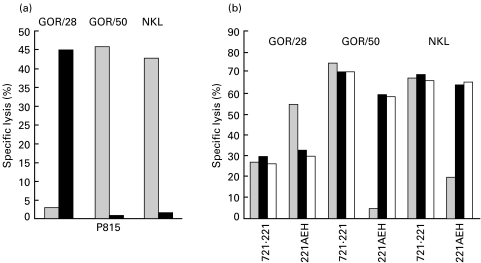
To further extend these studies we attempted the generation of TAP2-deficient NK clones by applying a conventional limiting dilution procedure; after several unsuccessful attempts only three NK microcultures could be expanded enough to be phenotypically and functionally characterized; this extremely poor yield was in marked contrast with results obtained culturing in parallel CD3-CD56+ cells from normal donors. Two TAP2-deficient NK clones (GOR/50 (CD94/NKG2A+) and GOR/28) CD94/NKG2A−/C+) were analysed in more detail. The lectin-like receptors appeared functional when tested in a rADCC system (Fig. 6a), where anti-CD94 MoAbs inhibited cytotoxicity of the NKG2A+ GOR/50 clone and, conversely, triggered lysis of the NKG2A−GOR/28 cells against the P815 targets. More importantly, when tested against the surface HLA-E+ 721·221-AEH cells, cytotoxicity of clone GOR/50 was specifically inhibited whereas that of clone GOR/28 was significantly enhanced, compared to lysis of wild-type HLA-defective 721·221 cells (Fig. 6b). These effects were antagonized by anti-CD94 MoAbs supporting the specific involvement of the CD94/NKG2 heterodimers that recognize HLA-E. The ability to expand in culture TAP-negative cytolytic NK cells indicates that at least some NK cell subsets may not become self-reactive even upon in vitro activation.
Fig. 6.
Inhibitory and triggering CD94/NKG2 receptors inhibitors and triggering are functional on TAP-negative NK clones. (a) The TAP–NK clones GOR/28 (CD94/NKG2A−/C+) and GOR/50 (CD94/NKG2A+) and the TAP+ NKL cell line (CD94/NKG2A+) were analysed in redirected killing assay against P815 cells in the presence of anti-CD94 and anti-CD56 MoAbs. ▪, CD56; ▪, CD94. (b) Effector cells were evaluated in a cytotoxic assay against the HLA-deficient 721·221 cell line and HLA-E+ transfectant 221-AEH; experiments were carried out in the presence of anti-CD94, anti-HLA class I or anti-CD56 (control) MoAbs. ▪, CD56; ▪, CD94; □, HLA class I.
Discussion
No more than 10 cases of human HLA class I deficiency associated to TAP1 or TAP2 deficiency have been described to date [3,5,7,8,22,23]. This new patient (GOR) has presented since childhood recurrent sinopulmonary infections and bilateral progressive inoperable bronchiectasis; he associated an IgG2 deficiency and has been on substitutive gammaglobulin therapy for the last 7 years. GOR molecular studies demonstrated a single point mutation that provokes a premature stop codon and would explain the absence of TAP2 mature protein. Previous TAP2 gene mutation was described by de la Salle et al. [3] and recently by Moins-Teisserenc [23], in two different families displaying HLA class I deficiency. As in GOR, both mutations caused a premature stop codon; therefore, these three TAP2 deficiencies would be provoked by a similar molecular mechanism. GOR peripheral blood lymphocyte counts were repetitively low; with normal CD3+ CD8 αβ to CD3 CD4+ αβ ratio, these results suggest that despite the diminished expression of class I HLA molecules, there may be enough to allow the positive selection of CD8+ T cells. As Donato et al. hypothesized [4], a more extensive exposure to pathogens in the older patients, as in our 40-year-old patient, induces the development of more CD8 αβ T cells than in younger cases. More studies should be undertaken to explore the functional characteristics and development of CD8+ T cells in TAP deficiencies. The increased numbers of γδ T cells, 24% (2–10), 14% CD8+ and 14% CD8CD4 double negatives observed in GOR was reported previously by de la Salle et al. [3]; they related this increase to the chronic lung infection. Indeed, our patient was chronically infected by P. aeruginosa. Phenotypical studies of GOR γδ T cells demonstrated that Vδ2-positive cells, the γδ T cells subset related to the P. aeruginosa infection, were in the normal range [24]; in contrast, the δ1 positive subpopulation, which is specifically stimulated by TAP-independent MHC-class I-related molecules MICA and MICB, were elevated [24,25]. A functional study of infiltrating cells in the lesion areas might help to better explain some clinical manifestations of TAP deficiency [23].
Interactions of specific receptors for peptide complexes associated to HLA class I antigens, as the killer inhibitory receptors, play a role in the control of some immune responses. The deficiency of HLA class I antigens in TAP-deficient patients could induce an altered expression of these receptors and, in some instances, an alteration in the responses mediated by these interactions. Our results confirm previous studies indicating that Ig-SF and lectin-like NKR specific for HLA class I molecules are found in NK cells from TAP-deficient individuals, thus supporting the notion that stochastic expression occurs despite low levels of MHC class I ligands, as shown originally for the Ly49 receptors in TAP-negative and β2-microglobulin-deficient mice. In such experimental systems, low levels of H-2 molecules were associated with a high expression of Ly-49, similar to that observed for the CD94/NKG2 receptor in TAP-deficient patients [26,27]. Surface Ly49 was rapidly down-modulated to normal levels when NK cells were introduced in a class I-positive environment [11]; these data strongly suggest that overexpression of class I specific NKR is secondary to a reduced ligand-induced receptor turnover. The presence of autoreactive TAP-negative NK cells is not a general finding in these patients. Recently, Moins-Tesserenc et al. [23] obtained several autoreactive NK and γδ clones and demonstrated the presence of autoreactive NK and γδ T cells in peripheral blood samples from TAP1- and TAP2-deficient patients, who characteristically associated a syndrome resembling Wegener's granulomatosis. It has been reported previously [13] that upon short-term in vitro activation with cytokines, polyclonal TAP2-negative NK cells acquire some ability to kill autologous targets; these results might explain why NK cloning efficiency was found to be extremely low in our hands. In this regard, de novo expression of triggering receptors on activated NK cells has been demonstrated [26], thus suggesting that in the course of an inflammatory response, a loss of TAP-NK self-tolerance might also occur in vivo; whether this mechanism could contribute to the development of the chronic lung disease found recurrently in these patients deserves attention. The defective HLA-E expression in TAP2-deficient PBMC and B-LCL is in line with original observations indicating that the interaction of class I leader sequence nonamers with HLA-E in the ER depends on functional TAP and tapasin [28,29]. Strikingly, our results are in contrast to the data obtained in a TAP1-deficient individual where HLA-E expression, analysed with the 3D12 MoAb, was reported to be detectable at normal levels in monocytes, slightly decreased in lymphocytes, but undetectable in a B-LCL cell line [12].The reason for this discrepancy is uncertain and additional studies in other TAP-deficient cases are required to solve the issue [30]. In contrast with other TAP-deficient patients, the clinical manifestation in our patient is limited to a chronic lung inflammation; neither vasculitis nor granulomas were present. It is uncertain whether the beneficial impact of gammaglobulin therapy in the lung disease progression in GOR comes from its effect as substitutive therapy for the IgG2 deficiency, or is also related to the reported anti-inflammatory activity of human gammaglobulins [31].
Acknowledgments
The authors thank Prof. John Trowsdale for providing us with TAP1 and TAP2 antibodies, Dr Adrian Kelly to confirm TAP2 deficiency and Dra Isabel Mir for patient care.
References
- 1.Touraine JL, Betuel H, Souillet T, Jeune M. Combined immunodeficiency disease associated with absence of cell surface HLA-A and B antigens. J Pediatr. 1978;93:47–51. doi: 10.1016/s0022-3476(78)80598-8. [DOI] [PubMed] [Google Scholar]
- 2.Payne R, Brodsky FM, Peterlin BM, Young LM. Bare lymphocytes without immunodeficiency. Hum Immunol. 1983;6:219–27. doi: 10.1016/0198-8859(83)90095-2. [DOI] [PubMed] [Google Scholar]
- 3.De la Salle H, Hanau D, Fricker D, et al. Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science. 1994;265:237–41. doi: 10.1126/science.7517574. [DOI] [PubMed] [Google Scholar]
- 4.Donato L, de la Salle H, Hanau D, et al. Association of HLA class I antigen deficiency related to TAP2 gene mutation with familial bronchiectasis. J Pediatr. 1995;127:895–900. doi: 10.1016/s0022-3476(95)70024-2. [DOI] [PubMed] [Google Scholar]
- 5.Matamoros N, Milá J, Pons J, Crespí C, Iglesias J, Martinez P. Clinical and molecular studies of a new case of TAP peptide transporter deficiency. Abstracts Issue ESID'98. Mol Immunol. 1998;35:715. [Google Scholar]
- 6.Pons J, Milà J, Balas A, et al. A new mutation in a TAP2 gene associated with defective expression of HLA Class I molecules and severe bronchiectasis in a Spanish patient. Abstracts of the 13th European Histocompatibility Conference. Human Immunol. 1999;1(Suppl):62:S33. [Google Scholar]
- 7.Teisserenc H, Schmitt W, Blake N, et al. A case of primary immunodeficiency due to a defect of the major histocompatibility gene complex class I processing and presentation pathway. Immunol Lett. 1997;57:183–7. doi: 10.1016/s0165-2478(97)00072-2. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa HT, Yabe K, Watanabe R, et al. Tolerance of NK and LAK activity for HLA class I-deficient targets in a TAP1-deficient patient (Bare lymphocyte syndrome Type I) Human Immunol. 1999;60:32–40. doi: 10.1016/s0198-8859(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 9.Joly E, Butcher GW. Why are two TAPs? Immunol Today. 1998;19:580–1. doi: 10.1016/s0167-5699(98)01348-6. [DOI] [PubMed] [Google Scholar]
- 10.Elliott T. How does TAP associate with MHC class I molecules? Immunol Today. 1997;8:375–9. doi: 10.1016/s0167-5699(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 11.Dorfman JR, Zerrahn J, Coles MC, Raulet DH. The basis for self-tolerance of natural killer cells in β2-microglobulin− and TAP1− mice. Eur J Immunol. 1998;228:370–8. [PubMed] [Google Scholar]
- 12.Furukawa H, Yabe T, Akaza T, et al. Cell surface expression of HLA-E molecules on PBMC from a TAP1-deficient patient. Tissue Antigens. 1999;53:292–5. doi: 10.1034/j.1399-0039.1999.530310.x. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer JL, Donato D, Hanau JP, et al. Inefficient protection of human TAP-deficient fibroblast from autologous NK cell-mediated lysis by cytokines inducing HLA class I expression. Eur J Immunol. 1999;29:1286–91. doi: 10.1002/(SICI)1521-4141(199904)29:04<1286::AID-IMMU1286>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Lee NDR, Goodlett A, Ishitani H, Marquardt A, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998;160:4951–60. [PubMed] [Google Scholar]
- 15.Perez Villar JJ, Melero I, Navarro F, et al. The CD94/NKG2A inhibitory receptor complex is involved in natural killer cell-mediated recognition of cells expressing HLA-G1. J Immunol. 1997;158:5736–43. [PubMed] [Google Scholar]
- 16.Bellón T, Heredia AB, Llano M, Minguela A, Rodriguez A. Triggering of effector functions on a CD8+ T cell clone upon the aggregation of an activatory CD94/kp39 heterodimer. J Immunol. 1999;162:3996–4042. [PubMed] [Google Scholar]
- 17.Colonna M, Bresnahan M, Bahram S, Strominger JL, Spies T. Allelic variants of the human putative transporter involved in antigen processing. Proc Natl Acad Sci USA. 1992;89:3932–6. doi: 10.1073/pnas.89.9.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullen M, Erlich H, Klitz W, Carrington M. Molecular mapping of a recombination hotspot located in the second intron of the human TAP2 locus. Am J Hum Genet. 1995;56:1350–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Llano M, Lee N, Navarro F, et al. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol. 1998;28:28854–63. doi: 10.1002/(SICI)1521-4141(199809)28:09<2854::AID-IMMU2854>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.Colonna M, Navarro F, Bellón T, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–18. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powis SH, Mockridge I, Kelly A, et al. Polymorphism in a second ABC transporter gene located within the class II region of the human region of the human major histocompatibility complex. Proc Natl Acad Sci USA. 1992;89:1363–7. doi: 10.1073/pnas.89.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De la Salle H, Zimmer J, Fricker D, et al. HLA class I deficiencies due to mutations in subunit 1 of the peptide transporter TAP1. J Clin Invest. 1999;103:R9–R13. doi: 10.1172/JCI5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moins-Teisserenc HT, Gadola SD, Cella M, et al. Association syndrome resembling Wegener's granulomatosis with low surface expression of HLA class I molecules. Lancet. 1999;354:1598–603. doi: 10.1016/s0140-6736(99)04206-3. [DOI] [PubMed] [Google Scholar]
- 24.Juliá MR, Serra P, Matamoros N, Raga S, Martinez P. Small cytoplasmic antigens from Pseudomonas aeruginosa stimulate γδ T lymphocytes. Scand J Immunol. 1998;48:672–8. doi: 10.1046/j.1365-3083.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 25.Groh V, Steinle A, Bauer S, Sories TH. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 26.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Human NK-cell receptors. Immunol Today. 2000;21:420–2. doi: 10.1016/s0167-5699(00)01673-x. [DOI] [PubMed] [Google Scholar]
- 27.Zimmer J, Donato L, Hanau D, et al. Activity and phenotype of natural killer cells in peptide transporter (TAP)-deficient patients (Type 1 bare lymphocyte syndrome) J Exp Med. 1998;187:117–22. doi: 10.1084/jem.187.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braud VM, Allan DS, Wilson D, McMichael AJ. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr Biol. 1998;8:1–10. doi: 10.1016/s0960-9822(98)70014-4. [DOI] [PubMed] [Google Scholar]
- 30.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998;160:4951–60. [PubMed] [Google Scholar]
- 31.Gadola SD, Moins-Teisserenc HT, Trowsdale J, Gross WL, Cerundolo V. TAP deficiency syndrome. Clin Exp Immunol. 2000;121:173–8. doi: 10.1046/j.1365-2249.2000.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]