Abstract
Wegener's granulomatosis initially affects upper respiratory tract organs including the nasal mucosa in more than 90% of patients. The inflammation is typically granulomatous with associated vasculitis. T lymphocytes are usually a prominent component of the leucocyte infiltrate. Previous studies using peripheral blood T cells have implicated IFN-γ rich Th1-type responses. This study addressed the cytokine milieu in nasal mucosa from 10 patients with active Wegener's granulomatosis using immunohistochemistry. Increased levels of CD3+ T cells and eosinophils were present compared with normal and disease controls. There was increased expression of IL-4, down-regulation of IL-2 and no detectable IFN-γ. There was increased expression of the chemokine receptor CCR3 by infiltrating cells, consistent with an IL-4 dominant, Th2-biased response. In contrast, renal biopsy tissue from 10 patients with active Wegener's granulomatosis showed expression of IL-2 and IL-4. The Th2-type environment within nasal mucosa, often the initial site of disease activity in Wegener's, is consistent with a local allergic response in these patients.
Keywords: cytokines, kidney, vasculitis
Introduction
Wegener's granulomatosis is an inflammatory disease affecting the upper and lower respiratory tract. The disease initially affects upper respiratory tract organs, including the nasal mucosa in more than 90% of patients [1]. With disease progression, a necrotizing systemic vasculitis develops and affects small- to medium-sized vessels, i.e. capillaries, venules, arterioles and arteries [2,3] Susceptible capillary beds include those of the lung, causing lung haemorrhage, and glomeruli, causing haematuria, proteinuria and loss of renal function.
Wegener's granulomatosis is associated with the presence of antineutrophil cytoplasm autoantibodies (ANCA), usually with specificity for proteinase 3 (PR3) [4]. In vitro studies have demonstrated that PR3-ANCA can activate neutrophils and monocytes and enhance leucocyte-mediated endothelial cell injury [5,6]. Local activation of neutrophils by ANCA in glomerular capillaries may initiate vascular injury [7]. However, the further development of lesions suggests a major role for effectors of cell-mediated immunity, including memory CD4+ T cells, macrophages, tissue factor and fibrin [8].
Autoreactive T cell responses to PR3 have been demonstrated using peripheral blood from patients with Wegener's granulomatosis [9–11], although cloned lesional cells have not so far demonstrated PR3-specificity [12]. There is some evidence implicating Th1-type responses when peripheral blood T cells from patients with Wegener's granulomatosis are stimulated with mitogens or with antibodies to CD2 and CD28 molecules. Stimulated patient CD4+ T cells produce higher amounts of IFN-γ than do controls [13]. Further, reverse-transcriptase polymerase chain reaction (RT-PCR) of cultured nasal biopsy material from patients with Wegener's granulomatosis detected IFN-γ mRNA, but this was also present in chronic nonallergic rhinitis controls [12]. The cytokine profile of recruited T cells may dictate the pattern of disease activity or resolution. Thus Th1-type T cells, producing interferon-γ and IL-2, are associated with delayed-type hypersensitivity responses [14,15]. Th2-type T cells produce IL-4, IL-5, IL-10 and IL-13. These cytokines are associated with allergic reactions, IgE synthesis, polyclonal B cell activation and eosinophil proliferation and function. We have found evidence for a Th2-type environment within the nasal mucosa, the initial site of disease inflammation in these patients.
Materials and methods
Patients
Patients were studied who fulfilled the Chapel Hill Consensus Conference definition of Wegener's granulomatosis [16]. Nasal biopsies were obtained with consent from 10 patients with Wegener's granulomatosis at first presentation and where the histology confirmed active disease, compatible with a diagnosis of Wegener's. The clinical details of these patients are contained in Table 1. Biopsies were also obtained from 10 patients with nasal polyposis and 10 normal patients while undergoing cosmetic nasal surgical procedures. Seven of the 10 patients with Wegener's had circulating cANCA (PR3-ANCA) at the time of biopsy, two were ANCA negative (one later converted to cANCA), and the ANCA status was not available for one.
Table 1.
Clinical features of patients who underwent nasal biopsies or renal biopsies
| Age/sex | URT | Lung | Kidney | Eye | Heart | Skin | Joints | PNS | CNS | GUT | Unwell |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal biopsies | |||||||||||
| 49/M | P | + | – | – | – | – | – | – | – | – | + |
| 71/M | P | + | – | + | – | – | – | – | – | – | + |
| 31/M | P | – | + | P | – | P | P | – | – | – | + |
| 18/F | P | P | – | + | – | – | P | – | – | – | + |
| 47/F | P | + | – | + | – | – | P | – | – | – | + |
| 44/F | + | – | + | + | – | P | + | – | – | P | + |
| 29/F | P | + | – | + | – | + | – | – | – | – | + |
| 24/F | + | P | P | + | – | + | + | + | – | – | + |
| 40/F | P | + | – | – | – | – | – | – | – | – | + |
| 23/F | + | + | – | + | – | P | P | – | – | – | + |
| Renal biopsies | |||||||||||
| 50/M | + | – | P | – | – | – | + | – | – | – | + |
| 41/M | + | – | P | – | – | – | – | – | – | – | + |
| 49/F | – | P | P | – | – | – | P | – | – | – | + |
| 72//F | + | P | P | + | – | P | – | – | – | – | + |
| 70/F | – | P | P | – | – | – | P | – | – | – | + |
| 17/F | – | + | P | – | – | – | + | – | – | – | + |
| 73/F | P | + | P | – | – | + | + | – | – | – | + |
| 67/M | P | P | P | – | – | – | – | – | – | – | + |
| 67/F | + | – | P | – | – | + | + | P | – | – | + |
| 31/M | – | + | P | + | – | + | + | – | – | – | + |
P denotes presenting symptoms, + additional symptoms.
Renal biopsies were taken with consent from 10 patients with generalized systemic Wegener's, different to those providing nasal biopsies (see Table 1). The renal biopsies were obtained at first presentation and prior to treatment. Eight patients had circulating cANCA (PR3-ANCA) and two had pANCA (MPO-ANCA). Control kidney samples were taken from the normal parts of 10 nephrectomy specimens removed for renal carcinoma. Subsequently, peripheral blood was obtained from six cANCA-positive patients with Wegener's when disease was in clinical remission, although cANCA were still detectable in the sera, in order to study T cell responses to PR3, as previously described [11]. All six patients were receiving prednisolone and azathioprine at the time of study. Blood samples from six normal individuals were also obtained.
Immunohistochemistry on nasal and renal biopsy tissue
Tissue was immediately snap frozen and cut into 8 µm cryostat sections. Immunohistochemistry was performed using a streptavidin/horseradish peroxidase based method and visualized using diaminobenzidine and Mayer's haematoxylin.
Endogenous peroxidase activity was blocked with a solution of Tris-buffered saline (TBS) pH 7·4, 30% hydrogen peroxide (H2O2) and 10% sodium azide for 10 min at room temperature followed by washing for 5 min in TBS. To block endogenous biotin, 0·1% avidin was added for 10 min then slides were washed, followed by 0·01% biotin and washing. Non-specific antigenic sites were blocked using 20% rabbit serum and incubated for 30 min. Primary antibody and appropriate isotype controls were added and incubated for 1 h at room temperature, then slides were washed in TBS. Biotinylated rabbit antimouse antibody (Dako Ltd, Cambridge, UK) was added to all sections, incubated for 30 min and followed by a wash. Streptavidin ABC/HRP complex (Dako) was added for 30 min and slides were washed. Diaminobenzidine (DAB) and hydrogen peroxide were added for 10 min and slides were developed and washed with distilled water. If necessary, sections were then counterstained using Mayer's haematoxylin for 1 min and washed for a few minutes under running water to develop. Sections were not counterstained for quantitative analysis.
Mouse monoclonal antibodies were used against the pan T cell marker CD3 (Dako), the cytokines IL-4 and IL-2 (Peprotech EC Ltd, London, UK), IFN-γ (Genzyme Diagnostics, Kent, UK) and eosinophil peroxidase (Serotec, Oxford, UK). The expression of some chemokine receptors known to be up-regulated on activated Th cells [17], including chemokine receptor 3 (CCR3) (Leukosite, Cambridge, MA, USA) on Th2 cells and CCR5 (Pharmingen, Becton-Dickinson UK Ltd, Oxford, UK) and CXCR3 (Leukosite) on Th1 cells, was also studied in five nasal and five renal sections. Appropriate IgG isotype controls were included (Binding Site, Birmingham, UK).
Analysis of immunohistochemistry
Positive staining was measured using an Aequitas IA image analysis system (Dynamic Data Links, Cambridge, UK). The first technically satisfactory microscopic field at ×25 magnification was selected on each specimen by an observer unaware of the antibody used. The threshold of the grey scale was kept constant. The system measured the area of staining above the threshold density, expressed as a percentage of image area. Positive staining cells were counted in 10 × 625 µm2 random fields per biopsy and expressed as mean counts per biopsy.
Peripheral blood T cell proliferation assay
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood using Ficoll-Paque gradients (Amersham-Pharmacia Biotech, St Albans, UK) and resuspended in RPMI 1640 supplemented with 5% human serum. Then, 2 × 105 cells/200 µl were cultured with or without 2 µg/ml PHA (Murex Diagnostics, UK) or 25 µg/ml heat inactivated PR3 (100°C for 15 min) for 5 and 10 days, respectively. Tritiated thymidine (0·15 µCi) was added for the last 18 h of culture and incorporation was measured by means of a liquid scintillation counter. PR3 was prepared from human peripheral blood neutrophils as described previously [11].
RNA extraction from T cells
T cell-enriched preparations were isolated from peripheral blood using Ficoll-Pague gradients (Amersham-Pharmacia Biotech) followed by adherence on fibronectin-coated plates for 30 min. These preparations were >85% positive for CD3+ cells by FACS analysis. Then 1 × 106 T cells per ml were stimulated with 2 µg/ml PHA or 25 µg/ml PR3, with equal numbers per time point receiving no stimulation. Cells were pelleted and snap frozen in liquid nitrogen at 72 h post-stimulation and stored at −70°C.
RNA was isolated using a modified NP-40 method. Briefly, 100 µl RNA extraction buffer containing 0·14 m KCl, 1·5 mm MgCl2, 10 mm TrisHCl pH 8·0 and 0·5% NP-40 plus 2·5 µl RNAguard 40 U/µl (Pharmacia, Uppsala, Sweden) were added to each cell pellet. This mix was vortexed for 10 s, incubated on ice for 5 min and spun at 13000 × g at 4°C for 1·5 min. The supernatants were then removed and added to 100 µl Proteinase K buffer containing 0·2 m TrisHCl (pH 8·0), 25 mm EDTA (pH 8·0), 0·3 m NaCl, 2% SDS and 2 µl Proteinase K (20 mg/ml) (Sigma-Aldrich Company Ltd, Dorset, UK) and incubated on a 37°C heating block for 20 min. Then, 200 µl phenol : chloroform (Sigma-Aldrich) was added, vortexed and centrifuged at 13000 × g for 15 min at 4°C. The aqueous top layer was removed and added to 200 µl chloroform (Sigma-Aldrich) and vortexed and spun as above. The upper final layer was transferred to a clean tube containing 10 µg/ml glycogen (20 mg/ml, Boehringer Mannheim, East Sussex, UK) to which 500 µl cold absolute alcohol was added (Sigma-Aldrich). This nucleic acid mix was precipitated over 2 nights at −70°C. Following this period, samples were then treated to remove any contaminating genomic DNA. Samples were pelleted by centrifugation at 13000 × g for 30 min at 4°C, supernatants were then removed and pellets were air-dried at room temperature for about 5 min. The following were added to the dried pellets: 77·5 µl filtered diethyl-pyrocarbonate (DEPC)-treated water, 22·5 µl DNase I reaction mix (2·5 µl DNase I (Amersham-Pharmacia) and 10 µl DNase I 10× reaction buffer: 400 mm TrisHCl pH 7·5, 60 mm MgCl2·6H2O, 10 µl RNase inhibitor (40 U/µl, Amersham-Pharmacia). This mixture was incubated at 37°C for 20 min. RNA was then extracted with phenol : chloroform as described above and precipitated using 5 µg glycogen, 250 µl ethanol and 35 µl sodium acetate overnight for 2 nights.
Reverse transcription
RNA was harvested at 13000 × g for 30 min at 4°C. The supernatant was removed and the pellet dried thoroughly. Next, 10 µl DEPC-treated H2O and 3·5 µl 10 µg/ml oligoDT (Amersham-Pharmacia) was added to each tube, which were briefly vortexed and spun. Samples were incubated at 60°C for 5 min then cooled to 4°C for 3 min. Then, 37·5 µl reaction mix containing 0·5 µl RNAguard (Amersham-Pharmacia), 5 µl of each 10 mm dNTP solution (Amersham-Pharmacia), 10 µl 5× first strand buffer (Gibco BRL Life Technologies, Paisley, UK), 5 µl 0·1 m dithiothreitol (DTT) (Gibco BRL Life Technologies) and 2 µl reverse transcriptase (200 U/µl, Gibco BRL Life Technologies) was added and incubated at 37°C for 40 min. A further 2 µl of reverse transcriptase was added, then incubated at 37°C for 40 min followed by 70°C for 10 min. Samples were spun briefly to remove any condensation; the cDNA was quantified spectrophotometrically at 260 nm and then stored at −20°C.
Polymerase chain reaction
10 µl 10 × reaction buffer, containing 100 mm Tris HCl (pH 8·8), 500 mm KCl, 15 mm MgCl2·6H2O, 1% Triton X-100 (Promega), 6 µl 25 mm MgCl2 (Promega), 2 µl each dNTP, 60·5 µl DEPC-treated H2O and 0·5 µl Taq polymerase (5 U/µl, Promega) and 5 µl of each 20 µm primer stock was added per 100 µl reaction. Primer sequences were as follows: β-ACTIN 5′ CTG GCA TCC ATG AAA CCA CC and 3′ TGT TTT AGA AGC ATT TGC GG; IFN-γ 5′ CCA AGA ATC TGC AGC TAA CT and 3′ TTA GCA ATG CAT TGG AAT GT; IL-4 5′ ATG GGT CTC ACC TCC CAA CTG CT and 3′ CGA ACA CTT TGA ATA TTT CTC TCT CAT.
In order to look at β-actin and IFN-γ expression, a 1-min precycle was carried out at 94°C followed by 30 and 40 cycles, respectively, of denaturation at 94°C for 1 min, annealing at 50°C for 40 s and extension at 72°C for 1 min. Cycling parameters for IL-4 were a precycle at 95°C for 5 min followed by 50 cycles of 95°C for 45 s, 50°C for 30 s and 72°C for 1 min. PCR products were visualized on 2% agarose gels using ethidium bromide and analysed by densitometry using E.A.S.Y. UVP software (Ultra Violet Products, Cambridge, UK), with results expressed as a ratio of cytokine to β-actin mRNA levels.
Statistical methods
All data were entered into an Excel spreadsheet and either analysed using the statistical tests contained in the programme or exported into Minitab for Windows (v13). P-values of 0·05 were considered as significant.
Image analysis
Data were analysed using two-tailed, nonparametric Mann–Whitney tests. These tests were chosen as normal distributions could not be assumed, and the fact that we were detecting both increases and decreases in expression defined the use of two-tailed values. The comparisons were between patient groups, as opposed to paired samples, therefore unpaired tests were carried out. Quantitative analysis was performed for CD3+ T cells, eosinophil peroxidase, IL-4 and IL-2. Results were analysed as the median and range for the group of biopsies. Multiple comparisons were made but the validity of the results is shown by the grouping of the significant results which contained values of P = 0·01 (Table 2). Image analysis tends to minimize differences as demonstrated in the eosinophil peroxidase value of P = 0·067 in the polyps patients in Table 2. Table 3 contained only three comparisons.
Table 2.
Medians (ranges) of positive staining density (arbitrary units) as measured by image analysis in nasal biopsy material
| Nasal tissue | CD3 | IL-4 | Eosinophil peroxidase | IL-2 |
|---|---|---|---|---|
| Normal | 0·72 (0·35–22·09) | 0·37 (0·31–0·76) | 1·01 (0·35–2·43) | 1·26 (0·37–4·38) |
| WG | 4·65 (3·01–16·2) *P = 0·010 | 3·80 (0·34–7·71) *P = 0·015 | 2·80 (0·33–12·25) *P = 0·046 | 0·53 (0·31–2·27) *P = 0·049 |
| Polyp | 1·90 (0·42–5·74) P = 0·308 | 0·44 (0·3–6·22) P = 0·191 | 2·16 (0·42–6·44) P = 0·067 | 0·41 (0·3–1·65) *P = 0·029 |
Denotes statistically significant value compared to that of normal control tissue.
Table 3.
Medians (ranges) of positive staining density (arbitrary units) as measured by image analysis in renal biopsy material
| Renal tissue | CD3 | IL-4 | IL-2 |
|---|---|---|---|
| Normal | 18·6 (10·6–39·8) | 28·8 (6·6–46·0) | 38·0 (20·6–63·0) |
| WG | 67·0 (30·8–83·7) *P = 0·038 | 40·4 (8·6–99·4) P = 0·224 | 26·6 (1·6–72·6) P = 0·806 |
Denotes statistically significant value compared to that of normal control tissue.
Peripheral T-cell assay
Results were recorded as mean counts per minute (cpm) ± standard errors of means (s.e.) and are presented as stimulation indices (SI) ± s.e., where SI = (cpm of cells proliferating in the presence of antigen)/(cpm of cells proliferating in the absence of antigen). A Mann–Whitney test was used to compare proliferation to PR3 between normal controls and vasculitis patients.
Polymerase chain reaction
Regression analysis was performed using Spearman rank correlation, a non-parametric test. The results were presented as values between +1 and −1.
Results
Immunohistochemical analyses on nasal tissue
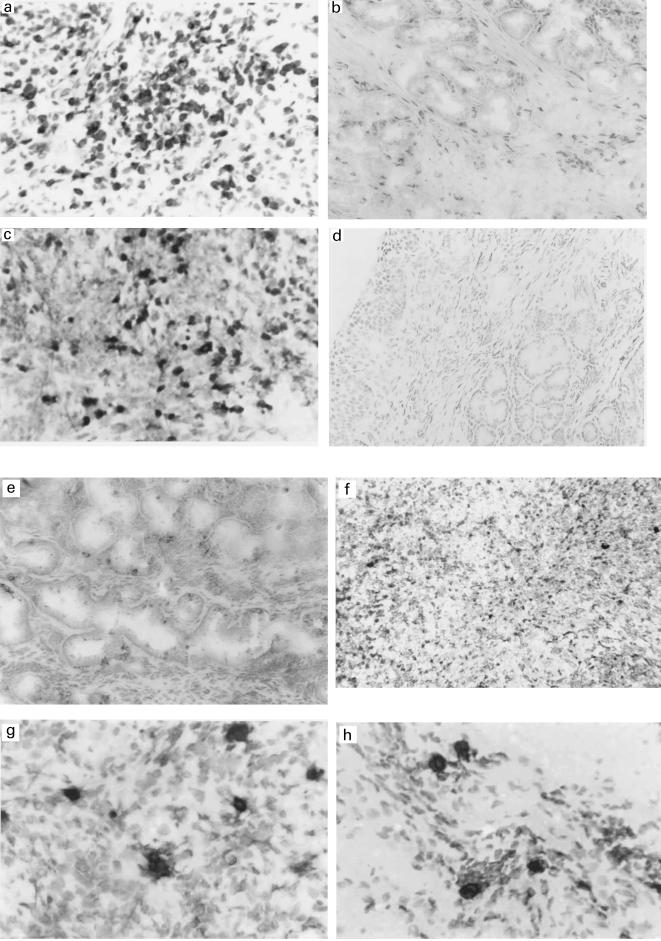
The immunohistochemical analyses demonstrated increased numbers of CD3+ T cells in the nasal mucosa of biopsies from Wegener's patients compared to normal tissue (P = 0·010); Table 2 and Fig. 1a,b. There was a non-significant increase in T cells in polyp tissue compared to normal tissue. Given the marked increase in infiltrating T cells in Wegener's granulomatosis, an analysis of expressed cytokines, IL-2, IL-4 and IFN-γ, was performed. In the Wegener biopsies, IL-4 was markedly and significantly increased over normal tissue (Table 2 and Fig. 1c,d) while IL-2 was reduced (Table 2). There was no detectable cellular staining with the antibody to IFN-γ, although the antibody bound along the basement membrane of glandular walls in both disease and normal tissue, possibly detecting IFN-γ that was bound to glycosaminoglycans (Fig. 1e) [18]. The anti-IFN-γ antibody demonstrated cellular staining of infiltrating T cells in acute liver allograft rejection (Fig. 1f). Polyp tissue showed no difference in IL-4 levels compared to normal tissue although the levels of IL-2 were increased. Thus in Wegener nasal mucosa, lack of detectable cellular IFN-γ expression, together with the increased expression of IL-4 and reduced expression of IL-2 compared to normal and polyp tissues, suggested that the cytokine milieu favoured a Th2-type response.
Fig. 1.
(a)CD3+ T cells in nasal biopsy tissue from a patient with Wegener's granulomatosis (same as in 1c and 1e) (× 100). (b) CD3+ T cells in nasal biopsy tissue from a normal control (×50). (c) IL-4+ T cells in nasal biopsy tissue from a patient with Wegener's granulomatosis (× 100). (d) IL-4+ T cells are few in nasal biopsy tissue from a normal control (×50). (e) No detectable cellular staining of IFN-γ in nasal biopsy tissue from a patient with Wegener's granulomatosis (× 50). (f) Positive cellular staining of IFN-γ in liver biopsy tissue from a patient with acute liver allograft rejection (× 100). (g) CCR3+ cells are present focally in nasal biopsy tissue from a patient with Wegener's granulomatosis (× 100). (h) Degranulating eosinophils present in nasal biopsy tissue from a patient with Wegener's granulomatosis (× 100).
Th2-type cells have enhanced surface expression of the chemokine receptor CCR3 (whose ligands are eotaxin, eotaxin-2, RANTES, MCP-2,-3,-4), while Th1-type cells have enhanced surface expression of CCR5 (binding MIP-1α,-1β, RANTES) and CXCR3 (binding IP-10, Mig, I-Tac) [17]. CCR3-expressing cells were present in Wegener nasal tissue (Fig. 1g). As CCR3-expressing cells were not present in normal control tissue, quantitative analysis was not performed. Cells expressing CCR5 and CXCR3 were scattered in low numbers throughout normal and Wegener tissue without any apparent difference in quantity; image analysis quantification was not applied to these markers. Since CCR3 is expressed on a subset of T cells (particularly Th2 cells) but also by eosinophils, presence of eosinophil peroxidase was sought in tissues. There was a significant increase in presence of eosinophil peroxidase in Wegener tissue (Table 2 and Fig. 1h) compared to normal tissue, which was compatible with a Th2-type environment.
These results demonstrate increased levels of T cells and eosinophils in patients with Wegener's granulomatosis compared to normal controls. The cytokine profiles suggest there is an ‘allergic’ Th2-type bias at the nasal site, with increased production of IL-4 expression accompanied by a down-regulation of IL-2 and no detectable change in IFN-γ. In addition, there are greater numbers of infiltrating cells expressing CCR3 (likely to be T cells, particularly Th2 cells, and eosinophils).
Immunohistochemical analyses on renal tissue
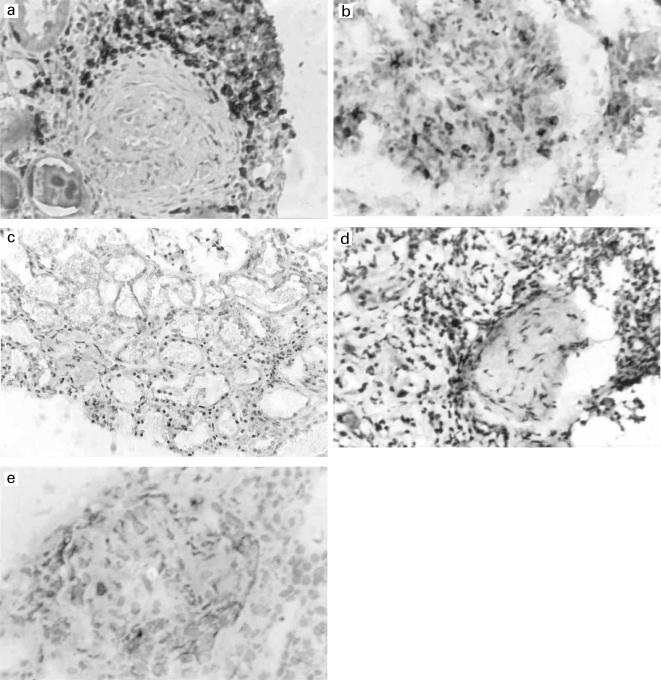
To determine whether a similar cytokine milieu might be present at other affected tissue sites, renal biopsies from patients with active Wegener's granulomatosis and renal involvement were studied. Significantly higher numbers of CD3+ T cells (P = 0·038) (Table 3) were detectable at predominantly interstitial sites (Fig. 2a), compared to control renal tissue (Fig. 2b). IL-4 positive cells were present in vasculitic (Fig. 2c) and control biopsies and no significant difference was observed between them. The IL-4 positive cells were seen mainly around tubules but also within glomeruli. Widespread IL-2 staining was observed in both vasculitic (Fig. 2d) and control kidney in interstitial tissues and within glomeruli in Wegener's renal tissue, but no significant differences were observed (Table 3). IFN-γ antibody binding, as in the nasal tissue, appeared to be located on the basement membrane of vessel walls as opposed to inflammatory cells, in both vasculitic and control kidney.
Fig. 2.
(a) CD3+ T cells in renal biopsy tissue from a patient with Wegener's granulomatosis (× 100). (b) A lower number of CD3+ T cells were present in control renal biopsy tissue (× 100). (c) Widespread IL-4 staining in interstitial tissue in a renal biopsy from a patient with Wegener's granulomatosis but overall, there was no difference between Wegener and normal renal tissue staining for IL-4 (× 50). (d) IL-2+ cells were presents throughout renal biopsy tissue from a patient with Wegener's granulomatosis but overall, there was no difference between Wegener and normal renal tissue staining for IL-4 (× 100). (e) IL-2 regulates the expression of CCR5, detectable in renal tissue from patients with Wegener's (× 100).
The chemokine receptors CCR5 (a representative example is shown in Fig. 2e) and CCR3 were expressed at both peritubular and periglomerular sites in Wegener renal tissue. As there was no CCR5 or CCR3 expression within control renal tissue, quantitative studies were not performed with these markers.
These results suggest that there is no Th2 bias at the renal site and that both Th1-and Th2-type cells are present. Thus, in addition to T cells and macrophages, IL-2 and IL-4 are both detectable as well as Th1-associated CCR5 and Th2-associated CCR3 expressing cells.
Th1 and Th2 cytokine mRNA production in cells responding to proteinase 3
Since T cells localized to nasal tissue but not kidney in acute Wegener's granulomatosis appeared to be Th2-biased, and since evidence points to peripheral blood T cells exhibiting Th1-type responses, we studied the cytokine profile of peripheral blood cells. Although we had been unable to focus on antigen-specific T cells in tissues, this was possible using peripheral blood. We have previously shown autoreactive T cell responses to PR3 using peripheral blood T cells from patients with Wegener's granulomatosis [11]. The six patients studied here showed enhanced stimulation indices for proliferation in the presence of PR3 at 10 days (found to be optimal in earlier studies) compared with normal controls (5·7 ± 1·3 versus 2·2 ± 1·3; mean ± standard error of the mean; P = 0·045).
Cytokine mRNA expression in unstimulated T cells or in T cells stimulated with PHA or PR3 was studied after 72 h. Cytokine mRNA levels were correlated with proliferative T cell responses after 5 days culture with PHA or 10 days culture with PR3.
Proliferation (measured at 10 days) and IFN-γ mRNA expression (measured at 72 h) of T cells from six patients with Wegener's granulomatosis (five tested twice) that had been stimulated with PR3 showed a correlation (rs = 0·36). This was not seen in the normal control group. The patient T cells were not spontaneously producing IFN-γ mRNA in quantities above basal levels as there was no correlation between IFN-γ mRNA expression in cells that had not been stimulated with PR3 and the 10-day T cell-proliferative responses to PR3 (rs = − 0·18). Additionally there was no relationship between T cell proliferation to PR3 after 10 days and IFN-γ mRNA production in non-specifically PHA-activated T cells. No association was found between IFN-γ mRNA levels in PHA-stimulated patient T cells (or control T cells) and proliferative responses to PHA at day 5. These data suggest that IFN-γ is important during autoreactive T cells responses to PR3 and not PHA.
IL-4 mRNA levels were not detected in control or patient T cells that had been stimulated with PR3. IL-4 mRNA was only detectable following PHA-stimulation. There was a negative correlation between IL-4 mRNA levels in PHA-stimulated patient T cells and day 5 proliferative responses to PHA which was not observed with controls.
Discussion
This study demonstrates a marked Th2-type environment within nasal tissue from patients with active Wegener's granulomatosis compared to normal and polyp tissues. The initial trigger for Wegener's granulomatosis remains to be defined, although the upper respiratory tract mucosa may be the initial site of disease sensitization [1]. Nasal infections have been proposed to play a role in relapse of Wegener's. Stegeman et al. found that 63% of Wegener patients were chronic carriers of Staphylococcus aureus, of whom 67% relapsed [19]. More recently, T cells from peripheral blood that respond to S. aureus have been cloned from Wegener patients [20]. These particular clones showed a Th2 type of cytokine secretion in culture, while seven of the 27 S. aureus-reactive clones responded to PR3 in culture. These studies suggest links between T cell recognition of S. aureus and PR3 that occurs in a Th2-dependent manner, although the reasons for this remain unclear. Our findings support a Th2 bias for nasal mucosa-derived T cells in Wegener's granulomatosis.
Csernok et al. performed RT-PCR on cultured nasal biopsy material from patients with Wegener's granulomatosis [12]. They detected IFN-γ mRNA in the Wegener tissue and in all chronic non-allergic rhinitis controls, indicating that expression of the Th1 cytokine IFN-γ is not a particularly Wegener-specific phenomenon in the nose. We were able to detect binding with the monoclonal antibody to IFN-γ in both nasal Wegener and polyposis tissue, in addition to normal control tissue. The staining was predominantly membrane-associated and did not seem to be related to inflammatory infiltrating cells. This suggests that it is not playing a specific role in directing leucocyte behaviour in Wegener's. Interestingly, Csernok et al. detected IL-4 mRNA at low levels in nasal tissue from two of five Wegener patients and the chronic rhinitis controls, and this seemed to relate to the presence of eosinophils in these samples [12]. We have found significantly increased staining for IL-4 and eosinophil-specific peroxidase in Wegener's, when compared to normal and polyp control nasal tissue. Csernok et al. found that T cell clones derived from Wegener nasal biopsy specimens that did not have any defined antigen specificity, secreted high levels of IFN-γ; however, this is not surprising as the cells had been cultured in vitro with IL-2, which probably introduced a predilection towards a Th1 cytokine profile. Further, since the derived clones were not antigen specific it is not possible to directly correlate the cytokines produced with disease pathogenesis.
A predominant Th2-type environment was not apparent within renal tissue. While we have not obtained nasal and renal biopsy material from the same patients for logistical reasons, the data suggest that Wegener's granulomatosis is not associated with a Th2-type environment at renal sites. This is consistent with other studies in ANCA-associated vasculitic nephritis which show prominence of mediators of delayed type hypersensitivity responses within glomeruli [8]. Further, peripheral blood T cells from patients with Wegener's in remission that were capable of responding to PR3 as we have shown previously [11], also demonstrated IFN-γ but not IL-4 production. While we cannot exclude that peripheral blood T cells from patients with acute Wegener's might have a more marked Th2-bias, our findings are consistent with those of Csernok et al. [12]. These investigators found that peripheral blood mononuclear cells from patients with Wegener's that were stimulated with PMA and anti-CD3, produced more IFN-γ than did normal controls. Further, Ludvikkson et al., stimulating CD4+ T cells from patients with active Wegener's with PMA and ionomycin, also found enhanced IFN-γ production compared with control CD4+ T cells [13]. Whether these T cell responses overlap with or are independent of those observed with T cells responsive to S. aureus remains to be seen.
In conclusion, there is enhanced recruitment/retention of CD3+ lymphocytes within nasal mucosa in patients with active Wegener's granulomatosis involving this site. The enhanced expression of IL-4 and presence of CCR3 positive cells compared with control tissues, favours a Th2-type response within nasal mucosa. Interestingly, this bias appears to be lost both at renal sites and by the bulk of circulating T cells that can respond to PR3. Whether a Th2 response within the nasals mucosa reflects T cell responses to local antigens such as S. aureus, remains to be determined.
Acknowledgments
C.E.J.B. was funded by a PhD studentship from the Medical Research Council, London, UK. This work was presented at the American Society of Nephrology, Miami, November 1999.
References
- 1.Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener's granulomatosis. Arthritis Rheum. 2000;43:1021–32. doi: 10.1002/1529-0131(200005)43:5<1021::AID-ANR10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Matsubara O, Yoshimura N, Doi Y, Tamura A, Mark EJ. Nasal biopsy in the diagnosis of early Wegener's (pathergic) granulomatosis. Virchows Arch. 1996;428:13–9. doi: 10.1007/BF00192922. [DOI] [PubMed] [Google Scholar]
- 3.Godman GC, Churg J. Wegener's granulomatosis pathology and review of the literature. Arch Pathol. 1954;58:533–51. [PubMed] [Google Scholar]
- 4.Goldschmeding R, van der Schoot CE, ten Bokkel Huinink D, et al. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorphosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989;84:1577–87. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–83. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- 6.Savage COS, Pottinger BE, Gaskin G, Pusey CD, Pearson JD. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity towards cultured endothelial cells. Am J Pathol. 1992;141:335–42. [PMC free article] [PubMed] [Google Scholar]
- 7.Cockwell P, Brooks CJ, Adu D, Savage COS. Interleukin-8: a pathogenic role in antineutrophil cytoplasmic autoantibody (ANCA)-associated glomerulonephritis. Kidney Int. 1999;55:852–63. doi: 10.1046/j.1523-1755.1999.055003852.x. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham MA, Huang XR, Dowling JP, Tipping PG, Holdsworth SR. Prominence of cell-mediated immunity effectors in ‘pauci-immune’ glomerulonephritis. J Am Soc Nephrol. 1999;10:499–506. doi: 10.1681/ASN.V103499. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer E, Stegeman CA, Huitema G, Limburg PC, Kallenberg CGM. T cell reactivity to proteinase 3 and myeloperoxidase in patients with Wegener's granulomatosis (WG) Clin Exp Immunol. 1994;98:448–53. doi: 10.1111/j.1365-2249.1994.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith ME, Coulthart A, Pusey CD. T cell responses to myeloperoxidase (MPO) and proteinase 3 (PR3) in patients with systemic vasculitis. Clin Exp Immunol. 1996;103:253–8. doi: 10.1046/j.1365-2249.1996.d01-629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King WJ, Brooks CJ, Holder R, Hughes P, Adu D, Savage C. T lymphocyte responses to ANCA antigens are present in patients with ANCA-associated systemic vasculitis and persist during disease remission. Clin Exp Immunol. 1998;112:539–46. doi: 10.1046/j.1365-2249.1998.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csernok E, Trabandt A, Muller A, et al. Cytokine profiles in Wegener's granulomatosis: Predominance of type 1 (Th1) in the granulomatous inflammation. Arthritis Rheum. 1999;42:742–50. doi: 10.1002/1529-0131(199904)42:4<742::AID-ANR18>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Ludviksson BR, Sneller MC, Chua KS, et al. Active Wegener's granulomatosis is associated with HLA-DR+ CD4+ T cells exhibiting an unbalanced Th1-type T cell cytokine pattern: reversal with IL-10. J Immunol. 1998;160:3602–9. [PubMed] [Google Scholar]
- 14.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–4. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 15.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 16.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: the proposal of an International Consensus Conference. Arth Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 17.Cockwell P, Savage COS. Glycosaminoglycans contribute to multiple finctions of vascular endothelial cells. Clin Exp Immunol. 1996;104:1–3. doi: 10.1046/j.1365-2249.1996.d01-638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cockwell P, Adams DH, Savage COS. Glycosaminoglycans contribute to multiple functions of vascular endothelial cells. Clin Exp Immunol. 1996;104:1–3. doi: 10.1046/j.1365-2249.1996.d01-638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegeman C, Cohen Tervaert J, de Jong PE, Kallenberg CGM. Trimethoprim-sulfamethoxazole for the prevention of relapses of Wegener's granulomatosis. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 20.Mayet W-J, Marker-Hermann E, Schlaak J, Zum Buschenfelde K-H. Irregular cytokine pattern of CD4+ T lymphocytes in response to Staphylococcus aureus in patients with Wegener's granulomatosis. Scand J Immunol. 1999;49:585–94. doi: 10.1046/j.1365-3083.1999.00544.x. [DOI] [PubMed] [Google Scholar]