Abstract
The enzyme tissue transglutaminase (tTG) has been recently identified to represent a highly sensitive and specific target of autoantibodies in coeliac disease. To characterize autoantigenic epitopes, we generated novel tTG deletion mutants by polymerase chain reaction, produced radiolabelled fragments by in vitro transcription/translation, immunoprecipitated the mutants using sera from patients with coeliac disease, and related the binding data with putative structural and functional domains of human tTG. We show that tTG antibody positive sera display a heterogeneous autoantibody response covering distinct regions of the molecule. The N-terminal and C-terminal third of tTG, comprising amino acid (aa) 1–281 and aa 473–687, harbour the dominant epitopes (67·4% and 69·4% positive), whereas the catalytic region is of minor antigenicity (22·5% positive). Autoantibodies directed to one, two and three domains were observed in 36·7%, 28·6% and 22·4% of patients, respectively. Comparative analysis revealed the presence of strictly conformational epitopes which were dependent on the N-terminus (aa 1–12) or the intact β-barrel domains in the C-terminus (aa 473–497, aa 649–687). In conclusion, we here demonstrate for the first time that the humoral autoimmunity is directed against distinct functional tTG domains. The spectrum of autoantibodies indicates that the native folded protein may be the target of autoantibodies.
Keywords: tissue transglutaminase, autoantibodies, autoimmunity, coeliac disease
Introduction
Coeliac disease (CD) is characterized by an inflammation of the upper small bowel which is triggered by the ingestion of gliadin, a component of wheat gluten [1]. The enzyme tissue transglutaminase (tTG) was recently identified as a specific target autoantigen in CD [2]. Several studies reported on autoantibodies against tTG (tTG-Ab) in 95–99% of patients with newly diagnosed CD using tTG isolated from guinea pig liver or human recombinant antigen [3–5]. Although these studies conclusively demonstrate that tTG is a major autoantigen in CD, the pathogenic mechanisms how an exogenous antigen such as gliadin can induce a tissue specific autoimmune response are still poorly defined.
CD is thought to be mediated by specific T-lymphocytes and activated intestinal fibroblasts leading to an inflammatory reaction against intestinal mucosa [1,6]. The tTG autoantigen has several interesting features which could contribute to disease development and progression. Gliadin has been shown to act as donor substrates for tTG leading to gliadin–gliadin and gliadin–tTG cross-links and the formation of gliadin neoepitopes by the deamidation of glutamine residues into glutamate [2,7]. These chemically modified peptides strongly bound to HLA-DQ2 and are recognized by gut-derived T cells and CD-specific T cell clones suggesting a key role of tTG in the pathogenesis of CD [8,9]. When the antigen is complex or composed of two physically attached parts, T and B lymphocytes can be triggered to respond to different antigens. Therefore, it can be speculated that gliadin–tTG linkage may also be involved in the cross-activation of a gliadin specific T cell response and the formation of tTG specific autoantibodies [10]. Thus far, only few data are available on the nature of tTG-Ab and the autoantigenic determinants of the tTG protein. Recently, Halttunen and Mäki demonstrated that tTG-Ab may directly contribute to the mucosal lesion by the inhibition of epithelial cell differentiation suggesting that the antibodies block tTG function [11]. A comparison of an enzyme-linked immunosorbent assay (ELISA) with Western blot analysis revealed that some sera may recognize both conformational and linear epitopes [3].
To understand the role of humoral autoimmunity in the pathogenesis of CD it is essential to characterize the autoantigenic domains of tTG. Therefore, we here studied autoantibody binding towards a panel of recombinant tTG fragments covering distinct regions of the molecule. Using this approach we defined immunogenic domains of tTG.
Materials and methods
Patients
Sera from 49 patients (33 females, 16 males; age 2–67 years, median 17 years) with untreated coeliac disease were used to characterize tTG autoantibody epitopes. Diagnosis was based on jejunal biopsy with evidence for the typical mucosal lesion [12]. Fifty sera from healthy individuals (30 females, 20 males, age 16–40 years, median 28 years) served as controls.
Construction of tTG deletion mutants
The tissue transglutaminase cDNA clone encoding full length human tTG (amino acid (aa) 1–687) has been previously described [5]. Deletion mutants were produced by polymerase chain reaction (PCR) using anchored 5′- and 3′-primers corresponding to the published tTG sequences including a Kozak sequence and start codon (GCCGCCACCATG…) and/or a translation termination codon (ACT…) at predetermined sites (start at tTG amino acid: aa 1, aa 46, aa 227, aa 473 and aa 497; stop at tTG aa: aa 227, aa 281, aa 347, aa 473, aa 648 and aa 687). Each PCR products were blunted with Klenow fragment, ligated into the Hinc II site of pGEM 4Z vector (Promega, Madison, WI) and sequenced using an automated sequencing apparatus (ABI, Applied Biosystem, Forster City, CA). Deletion mutants are illustrated in Fig. 1.
Fig. 1.
Tissue transglutaminase (tTG) constructs used for the analysis of humoral epitopes. Numbers represent amino acid position in tTG. A summary of reactivity patterns of 49 sera from patients with confirmed CD are indicated on the right margin.
Characterization of B cell epitopes
For the mapping of tTG epitopes 1 µg purified plasmid cDNA encoding deletion mutants of full length tTG was in vitro transcribed and translated in the presence of [35S]-methionine (Amersham Pharmacia, Braunschweig, Germany) using a rabbit reticulocyte lysate system (Promega, Madison, WI) as described previously [5]. Briefly, aliquots of radiolabelled polypeptides (15 000 cpm for each construct) were incubated with 5 µl serum diluted in 100 µl Tris-buffer (20 mm Tris, pH 7·4, 150 mm NaCl, 2 mm EDTA, 5 mm benzamidine, 5 mm methionine, 0·5% Triton X100) at 4°C for 12 h. After addition of 20 µl antihuman IgA-Agarose (Sigma) for 2 h, absorbed immunocomplexes were washed extensively, followed by measurement of bound radioactivity in a β-counter (Top Count, Canberra Packard, Groningen, The Netherlands). To test the influence of Ca2+ on the preservation of tTG epitopes some experiments were performed in which Tris-buffer was supplemented with 5 mm CaCl2 (without the addition of EDTA). In each experiment the same positive and negative serum was used as internal control. All measurements were performed in duplicates. Values above the 99th percentile of normal controls were considered antibody positive.
To confirm correct expression of deletion mutants and the specificity of autoantibody binding, sera from 5 patients were also analysed by immunoprecipitation and fluorography. 15 000 cpm of each polypeptide was incubated with 20 µl serum diluted in 100 µl Tris-buffer. After 12 h at 4°C 100 µl antihuman IgA-Agarose was added for 2 h. Then immunoprecipitates were washed five times in Tris-buffer, eluted by boiling for 3 min in sample buffer, and separated by SDS-PAGE using the buffer system of Laemmli [13]. Gels were fixed, rinsed for 15 min in Amplify (Amersham, Braunschweig, Germany), dried under vacuum and exposed to XAR-5 films (Kodak, Rochester, NY) for 10 days.
Results
The major tTG epitopes are located in N- and C-terminal domains
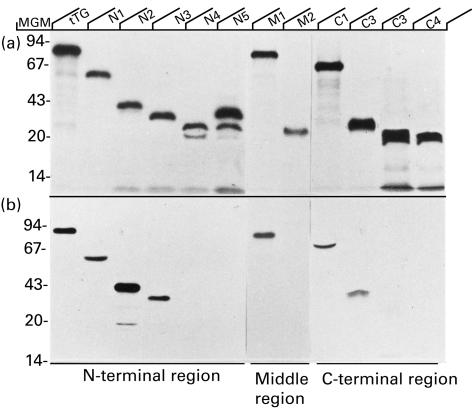
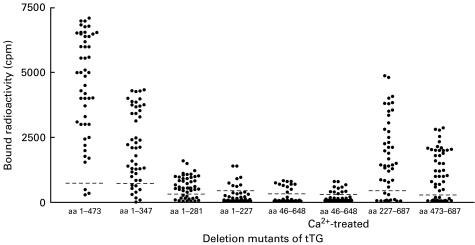
All deletion mutants were successfully expressed by in vitro transcription and translation using the reticulocyte lysate system. Autoradiography revealed that the polypeptides migrated on SDS-PAGE with the predicted molecular weights (Fig. 2). In some cases, additional bands were present suggesting low level protein degradation. These radiolabelled polypeptides were used to define autoantigenic regions recognized by 49 sera from patients with CD who were found positive for autoantibodies to full length tTG in comparison to 50 sera from normal controls. None of the control sera did react with any of the truncated proteins. Antibody binding was first analysed against overlapping polypeptides comprising large fragments of the N-terminus (aa 1–473, frag. N1), the middle region (aa 46–648, frag. M1) or the C-terminus (aa 227–687, frag. C1). 46 of 49 (93·4%) tTG antibody positive sera recognized fragment N1 and 34 (69·4%) patients had antibodies directed to mutant C1. A dominant reactivity towards the middle domain was excluded by the finding that only 22·5% of sera recognized fragment M1 comprising aa 46–648 and none of the sera immunoprecipitated residues aa 227–473 (frag. M2) (Fig. 1). The use of Tris-buffer with 5 mm CaCl2 in the incubation and washing step has no influence on the percentage of positive antibodies or the antibody levels against fragment M1 (Fig. 3). These findings suggest the presence of at least one epitope depending on the intact N-terminus (aa 1–45) and another epitope in the C-terminus which requires aa 649–687. A representative immunoprecipitation with a serum from patients with CD is shown in Fig. 2.
Fig. 2.
Reactivity of sera from patients with CD against tTG deletion mutants expressed by in vitro transcription and translation. Crude lysate of [35S]methionine labelled tTG deletion mutants (a). Immunoprecipitation of mutants identical to that shown in (a) using a serum from a patient with CD (b). Molecular weight markers are indicated at the left margin.
Fig. 3.
Distribution of antibody levels against several tTG deletion mutants. The dotted lines represent the cut-offs at levels defined by the 99th percentiles of healthy controls.
The main N-terminal epitope requires the presence of the first 13 residues
To further localize epitopes progressive deletion of fragment N1 was performed. The majority of sera displayed a positive reaction with mutant N3 comprising aa 1–281 (67·4%). Deletion of aa 228–281 from N3 (frag. N4) resulted in a loss of reactivity in 75·6% of N3 positive sera. Reactivity against fragment N1 (81·6%) indicated the presence of further epitopes within aa 1–347 of tTG. Interestingly, the truncation of the first 13 residues (frag. N5) completely abolished antibody binding against all epitopes within the N-terminal part of the molecule. This indicates that the major epitope residing in the N-terminal third of tTG is strongly dependent on the intact N-terminus (aa 1–13). Our data suggest that residues 1–347 harbour at least three distinct epitopes. The first is located within aa 1–227 (16·3% positive), the second epitopes requires aa 1–281 (at least 51% positive) and the third is dependent on the presence of aa 1–347 (at least 14·3% positive).
Characterization of the C-terminal autoantigenic region
The dominant epitopes located at the C-terminus of tTG were mapped by progressive deletion of the N- and C-terminal part of frag. C1. Among 49 tTG antibody positive sera 34 (69·4%) recognized mutant C2 comprising aa 473–687 including all sera which displayed a positive reaction towards frag. C1. The binding to these major epitopes were completely lost after deletion of residues aa 473–497 or aa 649–687 (Figs. 1 and 2). Both parts of the molecule may therefore be of crucial importance for the formation of the dominant C-terminal epitope. In addition, the comparison of reactivity patterns against polypeptides C2 and C4 with N5 suggest that autoantigenic regions distinct from the N- and C-terminal epitopes are located within aa 46–648 recognized by 22·5% of the sera.
Autoantibody tTG epitopes are conformation-dependent
The analysis of immunoreactivity of individual sera towards overlapping fragments N2, N4-5, M1-2, C1 and C4 clearly demonstrates that the major tTG epitopes are not directed against linear epitopes. The requirement of several not neighboured residues suggests that the autoantibodies may target conformational epitopes. Therefore, aa 1–13, aa 473–497 and 648–687 are either directly involved in the formation of conformational epitopes or induce a major conformational change that affects binding to the truncated polypeptides.
Epitope recognition patterns in patients with CD
Among 49 sera from patients with newly diagnosed CD, four major patterns of autoantibody reactivity were observed using fragments covering distinct autoantigenic determinants (Fig. 4). Pattern I reactivity, detected in 10 of 49 (20·4%) sera, was defined by the presence of autoantibodies to multiple epitopes within the N-terminal domain, the middle region and the C-terminus of tTG (Table 1). About one third (28·6%) of sera had autoantibodies that were directed to the N- and C-terminal part of tTG (pattern II). Reactivity restricted to only one region either the N-terminal domain (pattern III) or the C-terminus (pattern IV) was observed in 36·7% of the sera. There was no significant difference between the epitope patterns and age, gender, or the level of antibodies to full length tTG.
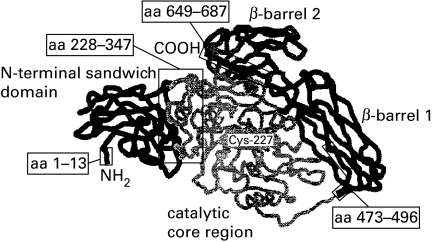
Fig. 4.
Cartoon showing the secondary structure of tissue transglutaminase generated by homology alignment with the known three-dimensional structure of plasma transglutaminase (factor XIII).The image was built using the programs SWISS Model and RasMol [14]. The four domains of tTG are illustrated in different grey patterns. Critical amino acid residues involved in the preservation of dominant tTG epitopes are boxed.
Table 1.
Immunoreactivity patterns in 49 patients with coeliac disease
| Recognition of epitopes | ||||
|---|---|---|---|---|
| Pattern | N-terminus (aa 1–281) | Middle region (aa 46–648) | C-terminus (aa 473–687) | Positive |
| I | + | + | + | 10 (20·4%) |
| II | + | − | + | 14 (28·6%) |
| III | + | − | − | 9 (18·4%) |
| IV | − | − | + | 9 (18·4%) |
| V | − | + | − | 0 |
| VI | + | + | − | 0 |
| VII | − | + | + | 1 (2·0%) |
Six patients had autoantibodies only against fragment N1 (aa 1–473) and fragment N2 (aa 1–347), respectively.
Discussion
The enzyme tTG is a highly sensitive and specific target of autoantibodies in CD. In this study we present the first data on the characterization of autoantigenic regions in tTG. The analysis of antibody binding patterns against a panel of tTG deletion mutants revealed a heterogeneous antibody response directed to strongly conformation-dependent epitopes. We demonstrate that the dominant epitopes clustered in two separate areas of tTG, one spanning from the N-terminus to the catalytic region and the other comprising the two C-terminal domains. The identification of defined epitope boundaries suggests that theses tTG regions may harbour amino acid sequences of high immunogenicity which could be crucially involved in the induction of autoimmunity in CD.
Tissue TG is an unique member of transglutaminases in that it exhibit two distinct enzyme activities: a TGase activity involved in crosslinking of extracellular matrix proteins, cell adhesion and wound healing, and a guanosine triphosphate/adenosine triphosphate (GTP/ATP) hydrolysis activity associated with signal transduction in apoptosis and cell cylce [7,15]. This bifunctional role of tTG is related to different domains of the protein. tTG is formed by a N-terminal β-sandwich domain (aa 1–139), a central catalytic core region (140–454) and two β-barrel regions (spanning between aa 479–585 and aa 586–687) [15,16]. The TGase activity requires the putative calcium binding region between aa 446–453 and the active site located at Cys-227 whereas the GTP/ATP binding domains reside in the N-terminal 185 aa residues [17,18]. On the basis of these data we constructed tTG fragments comprising one or two structural and/or functional domains to increase the probability to maintain the three-dimensional structure of truncated proteins. The analysis of overlapping mutants demonstrated that dominant epitopes of tTG autoantibodies reside in the N- and C-terminal half (N2 and C2) of the molecule. In addition, the comparison of immunoreactivity patterns revealed that the majority of sera from patients with CD did not react with linear epitopes. The presence of conformational epitopes is in line with previous studies reporting on the failure to detect tTG-Ab using denatured protein in Western blots [2]. This observation may also provide an explanation why liquid phase antibody assays, which tend to preserve the protein conformation more accurately than solid phase immunoassays, might be more sensitive for the detection of tTG-Ab as compared to ELISA [19]. The appearance of autoantibodies directed to conformational epitopes were also described in other T cell mediated autoimmune diseases including glutamic acid decarboxylase and the tyrosine phosphatase-like protein IA-2 in type 1 diabetes or thyroid peroxidase in Hashimoto's thyroiditis [20–22].
Although the absence of linear epitopes complicated mapping of autoantigenic domains by polyclonal sera, further progressive deletion of immunoreactive fragments was successful to produce truncated tTG polypeptides with preserved antigenic conformation. Using C- and N-terminal deletion mutants of fragment N2, three distinct epitopes were identified. The major epitope, recognized by 67·4% of the sera, was mapped between aa residues 1–281 which was dependent on the presence of aa 228–281 and aa 1–13. The observation that the deletion of the first 13 residues completely abolished antibody reactivity in all tested sera suggest that the near N-terminal region of the β-sandwich domain is of crucial importance for the formation of the N-terminal tTG epitopes. Previous studies reported that the first N-terminal aa residues of tTG are involved in the binding of fibronectin which represents a substrate for TGase [23]. On the basis of the present data we can not exclude that residues aa 1–13 are directly involved in antibody binding towards mutant N4. However, the complete loss of immunoreactivity in sera positive for distinct antibody specificities (N2-4) suggests that the deletion of residues 1–13 may induce a major conformational change of the molecule. These findings may be interesting for studies on the relation between structure and function in the TG enzyme family.
The dominant binding domain within the C-terminus was found to be located between aa 473–687 corresponding to the two β-barrels in the tTG molecule (69·4% positive). A more detailed mapping of this epitopes was not successful, since deletion of residues aa 473–496 or aa 648–687 completely abolished autoantibody reactivity in all tested sera. Thus, both C-terminal β-barrel domains of tTG seems to be important for the formation of this epitope.
Although the three-dimensional conformation of tTG is still unknown, alignment with the homologous structured domains of human plasma transglutaminase, known as blood coagulation factor XIII, suggest that the active site is buried in a cleft between the N-terminal β-sandwich region and the two β-barrel domains [24]. The structural requirement of tTG epitopes suggest that the native folded protein may be the immunogen for autoreactive B cells. The accessibility of the N-terminal and C-terminal regions on the surface of the molecule could explain why the autoimmune reaction is predominately directed to these regions. In addition, there is the possibility that the tTG–gliadin crosslinking protects the catalytic domain from the recognition of tTG-reactive B cells. Whether or not the binding of autoantibodies influences the TGase or the GTPase/ATPase activity of tTG remains an interesting question of further studies.
Analysis of the immunoreactivity patterns in individual sera revealed that the majority of patients exhibit autoantibodies directed to more than one epitope. The appearance of autoantibodies to multiple epitopes is a well known finding in other autoimmune diseases such as type 1 diabetes, Addison's disease and autoimmune thyroid disorders [25–27]. This has been explained by the intramolecular spreading of an autoimmune response from a single or few epitope(s) to multiple epitopes during the natural history of the disease. In type 1 diabetes and systemic lupus erythematosus, a temporal spreading of the humoral autoimmune response has been described from immunodominant epitopes in an early, preclinical phase to less immunogenic domains at the manifestation of the disease [28–30]. Thus, our data may be important for the development of an epitope specific diagnostic in CD. Prospective follow-up studies may be required to answer the question whether or not a specific epitope or epitope pattern is associated with the activity of the autoimmune process or the progression from a silent state of the disease to clinically overt CD.
In conclusion, we here define for the first time antigenic epitopes of the target autoantigen tTG. We demonstrate that (i) autoantibodies are predominantly directed to conformational epitopes; (ii) the autoimmune response is frequently directed to multiple epitopes; (iii) dominant epitopes are located in distinct functional domains of tTG which require the presence of critical residues near the start and the end sites of the molecule. These finding may provide the basis to study the interaction between B cells and T cells in CD and characterize the role of distinct tTG autoantibody specificities in the disease process.
Acknowledgments
The study was supported by grants from the Deutsche Zöliakie-Gesellschaft (JS), the Deutsche Forschungsgemeinschaft (Se 725/2–1), the Lilly Foundation International (JS), and the BMBF IZKF-project A1 (BOB).
References
- 1.Mäki M, Collin P. Coeliac disease. Lancet. 1997;349:1755–9. doi: 10.1016/S0140-6736(96)70237-4. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 3.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–8. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich W, Laag E, Schöpper H, Volta U, Ferguson A, Gillet H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317–21. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 5.Seissler J, Boms S, Wohlrab U, Morgenthaler NG, Mothes T, Boehm BO, Scherbaum WA. Antibodies to human recombinant tissue transglutaminase measured by radioligand assay: Evidence for high diagnostic sensitivity for celiac disease. Horm Metab Res. 1999;31:375–9. doi: 10.1055/s-2007-978758. [DOI] [PubMed] [Google Scholar]
- 6.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg CS, Birckbichler P, Rice RH. Transglutaminases multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991;5:3071–7. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 8.Molberg Ø, Mcadam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AVS. In vivo challenge in celiac disease identifies a single transglutaminase-modified peptide as a dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 10.Sollid LM. Autoantibodies in coeliac disease: tissue transglutaminase – guilt by association? Gut. 1997;47:851–2. doi: 10.1136/gut.41.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halttunen T, Mäki M. Serum IgA from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology. 1999;116:566–72. doi: 10.1016/s0016-5085(99)70178-2. [DOI] [PubMed] [Google Scholar]
- 12.Walker-Smith JA, Guandalini S, Schmitz J, Shmerling DH, Visakorpi JK. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–11. [Google Scholar]
- 13.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modelling. Electrophoresis. 1997;18:2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 15.Melino G, Piacentini M. Tissue transglutaminase in cell death: a downstream or a multifunctional upstream effector? FEBS Lett. 1998;430:59–63. doi: 10.1016/s0014-5793(98)00521-3. [DOI] [PubMed] [Google Scholar]
- 16.Casadio R, Polverini E, Mariani P, et al. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur J Biochem. 1999;262:672–9. doi: 10.1046/j.1432-1327.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 17.Gentile V, Saydak M, Chiocca EA, Akande O, Birckbichler PJ, Lee KN, Stein JP, Davies PJ. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991;266:478–83. [PubMed] [Google Scholar]
- 18.Lai TS, Slaughter TF, Koropchak CM, Haroon ZA, Greenberg CS. C-terminal deletion of human tissue transglutaminase enhances magnesium-dependent GTP/ATPase activity. J Biol Chem. 1996;271:31191–5. doi: 10.1074/jbc.271.49.31191. [DOI] [PubMed] [Google Scholar]
- 19.Bazzigaluppi E, Lampasona V, Barera G, Venerando A, Bianchi C, Chiumello G, Bonifacio E, Bosi E. Comparison of tissue transglutaminase-specific antibody assays with established antibody measurements for coeliac disease. J Autoimmun. 1999;12:51–6. doi: 10.1006/jaut.1998.0253. [DOI] [PubMed] [Google Scholar]
- 20.Finke R, Seto P, Rapoport B. Evidence for the highly conformational nature of the epitope (s) on human thyroid peroxidase that are recognized by sera from patients with Hashimoto's thyroiditis. J Clin Endocrin Metab. 1990;71:53–9. doi: 10.1210/jcem-71-1-53. [DOI] [PubMed] [Google Scholar]
- 21.Syren K, Lindsay L, Stoehrer B, Jury K, Luhder F, Baekkeskov S, Richter W. Immune reactivity of diabetes-associated human monoclonal autoantibodies defines multiple epitopes and detects two domain boundaries in glutamate decarboxylase. J Immunol. 1996;157:5208–14. [PubMed] [Google Scholar]
- 22.Xie H, Zhang B, Matsumoto Y, Li Q, Notkins AL, Lan MS. Autoantibodies to IA-2 and IA-2 beta in insulin-dependent diabetes mellitus recognize conformational epitopes: location of the 37- and 40-kDa fragments determined. J Immunol. 1997;159:3662–7. [PubMed] [Google Scholar]
- 23.Jeong JM, Murthy SN, Radek JT, Lorand L. The fibronectin-binding domain of transglutaminase. J Biol Chem. 1995;270:5654–8. doi: 10.1074/jbc.270.10.5654. [DOI] [PubMed] [Google Scholar]
- 24.Yee VC, Pedersen LC, Le TI, Bishop PD, Stenkamp RE, Teller DC. Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII. Proc Natl Acad Sci USA. 1994;91:7296–300. doi: 10.1073/pnas.91.15.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song YH, Connor EL, Muir A, She JX, Zorovich B, Derovanesian D, Maclaren N. Autoantibody epitope mapping of the 21-hydroxylase antigen in autoimmune Addison's disease. J Clin Endocrinol Metab. 1994;78:1108–12. doi: 10.1210/jcem.78.5.7513715. [DOI] [PubMed] [Google Scholar]
- 26.Lampasona V, Bearzatto M, Genovese S, Bosi E, Ferrari M, Bonifacio E. Autoantibodies in insulin-dependent diabetes recognize distinct cytoplasmic domains of the protein tyrosine phosphatase-like IA-2 autoantigen. J Immunol. 1996;157:2707–11. [PubMed] [Google Scholar]
- 27.Zhang B, Lan MS, Notkins AL. Autoantibodies to IA-2 in IDDM. location of major antigenic determinants. Diabetes. 1997;46:40–3. doi: 10.2337/diab.46.1.40. [DOI] [PubMed] [Google Scholar]
- 28.James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med. 1995;181:453–61. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naserke HE, Ziegler AG, Lampasona V, Bonifacio E. Early development and spreading of autoantibodies to epitopes of IA-2 and their association with progression to type 1 diabetes. J Immunol. 1998;161:6963–9. [PubMed] [Google Scholar]
- 30.Söhnlein P, Müller M, Syren K, et al. Epitope spreading and a varying but not disease-specific GAD65 antibody response in type 1 diabetes. Diabetologia. 2000;43:210–7. doi: 10.1007/s001250050031. [DOI] [PubMed] [Google Scholar]