Abstract
The cytokine requirements to differentiate CD34+ progenitor cells from different origins either cord blood (CB) or peripheral blood (PB) into dendritic cells (DC) are known to be different. In addition to DC, macrophages and neutrophils are generated. On the other hand, phorbol esters such as PMA induce primary human CD34+ bone marrow (BM) progenitor cells to differentiate into functional DC and no other lineages are generated. In addition, FCS is used as culture supplement in most of the protocols described which contains additional foreign antigens potentially skewing the resulting immune response. Therefore, we evaluated the ability to differentiate CB- and PB-CD34+ progenitor cells into DC with PMA and under serum-free conditions. In this study, we delineate the maturation of cultured human blood DC by analysis of expression co-stimulatory molecule B7–2 (CD86). Human mature DC with typical morphology and surface antigen phenotype (CD1a−, CD83+ and CD86+) were obtained from CB- and PB-CD34+ progenitor cells after 1 week of culture in serum-free medium upon stimulation with PMA alone. The same result was obtained from ex vivo-expanded BM-CD34+ cells. CD86+ yield was increased by PMA compared to cytokine cocktails (28·0% ± 7·0 versus 15·3% ± 5·6 for CB and 44·6% ± 7·5 versus 28·1% ± 7·5 for PB, respectively). CD86 was most up‐regulated in the presence of the calcium ionophore ionomycin. However, the number of viable cells after differentiation was decreased by PMA plus ionomycin (P < 0·05) or plus TNF-alpha (P > 0·05) as compared with that in PMA alone. We conclude that PMA is a potent activator to differentiate human CD34+ cells into mature DC in serum-free medium. This may be used for in vitro studies of primed or genetically modified DC against infectious and tumour-associated antigens.
Keywords: CD34+ cells, co-stimulatory molecules, dendritic cells, PMA, serum-free medium
Introduction
Dendritic cells (DC) are found at trace levels in tissues or in circulation and are inducing many lymphocyte functions [1]. During the past decade, DC have been identified as the most potent antigen-presenting cells of the immune system [2,3]. The superior ability of DC to present antigens to T cells has led to the development of DC-based strategies for the purpose of enhancing the immune response against tumours and infectious agents [1,4]. However, the application of DC in immunotherapy has been hampered by the low number of these cells and the difficulty of isolating them [5]. The identification of culture conditions enabling the generation of DC in vitro are useful to study their biology and to prepare large amounts of antigen-presenting cells (APCs) for immunotherapeutic purposes [6].
It is now possible to generate a large number of DC from either CD34+ progenitor cells [7–9] or from peripheral blood monocytes [10,11]. CD34-derived dendritic cells (CD34-DC) have a preferential capacity to activate CD8+ T cells [6]. Introducing genes into DC by retroviral transduction of CD34+ progenitor cells is now an established method to potentiate a specific immune response against tumour antigens [12]. DC can be generated in vitro from CD34+ progenitors in cord blood (CB) [9,13], bone marrow (BM) [8,14] and peripheral blood (PB) after cytokine mobilization [15]. However, the cytokine requirements to differentiate CB- and PB-CD34+ progenitor cells into DC are known to be different [16] and GM-CSF plus TNF-alpha ±SCF not only generate DC but also macrophages and neutrophils [9,14,17]. In addition, FCS used as culture supplement in most of the protocols described contains additional foreign antigens leading to the potential skewing of the resulting immune response. Recently, serum-free culture conditions have been described for the generation of cord blood-derived dendritic cells (CB-DC) in the presence of SCF, GM-CSF, TNF-alpha and TGF-beta1 [18]. In addition, DC can be generated from mobilized peripheral blood CD34+ cells in the absence of bovine products using ex-vivo 10 culture medium containing 10% autologous serum, rhGM-CSF, rhTNF-alpha and rhIL-4 [19].
Mature DC are typically characterized by expression of MHC class I, MHC class II, the co-stimulatory molecules CD80 (B7–1) and CD86 (B7–2) and the dendritic cell lineage marker CD83 [20]. CD86 is the most important ligand to induce CD28-mediated co‐stimulation for CD8+ T cell activation [21].
Phorbol ester (PMA) is a stable analogue of 2,3-diacylglycerol that activates the classical (alpha, beta1, beta2 and gamma) and new (delta, epsilon, eta, theta and mu) isoforms of protein kinase C (PKC) [22]. PMA-mediated signalling induced the expression of the Re1B transcription factor, suggesting a pathway by which genetic events involved in DC differentiation are initiated [23]. Recently, it was reported that PMA-induced PKC activation specifically leads to differentiation of bone marrow CD34+ progenitor cells to mature DC and no other lineages are generated [23,24].
Here we evaluated the ability to differentiate CB- and PB-CD34+ progenitor cells into DC under serum-free conditions in the presence of PMA. In addition, we delineate the maturation of cultured human blood DC by analysis of expression co‐stimulatory molecule B7–2 (CD86).
Materials and methods
Recombinant human cytokines and growth factors
Flt-3 ligand (Flt-3L), granulocyte-macrophage colony-stimulating factor (GM-CSF) and stem cell factor (SCF) were obtained from Immunex Corp (Seattle, WA, USA), Peprotech (UK) and Amgen (Thousand Oaks, CA, USA), respectively, and interleukin-3 (IL-3), interleukin-4 (IL-4), transforming growth factor beta 1 (TGF-beta1) and tumour necrosis factor-alpha (TNF-alpha) were purchased from Tebu GmbH (Frankfurt/M., Germany).
Culture media
The following culture media were used throughout experiments: complete serum-containing media IMDM (Iscove's modified Dulbecco's medium) and RPMI-1640 (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, and complete serum-free media CellGro® SCGM and CellGro® DC (CellGenix, Freiburg, Germany) supplemented with 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin.
CD34+ cell isolation
Cord blood samples from normal full-term deliveries, human bone marrow from healthy donors and G-CSF mobilized peripheral blood were used in this study. Low-density mononuclear cells (LD-MNCs) were separated on Ficoll-Paque gradient (specific gravity 1·077 g/ml, Seromed, Berlin, Germany). CD34+ cells were separated by magnetic cell sorting (CD34 isolation kit, MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer's instructions. After two cycles of magnetic separation, the purity of CD34+ cells measured by flow cytometric analysis using non-cross-blocking antibody (HPCA-2 FITC, Becton Dickinson GmbH, Heidelberg, Germany) was about 98%.
Expansion of BM-CD34+ progenitor cells
Ex vivo expansion of CD34+ progenitor cells facilitates the identification of culture conditions useful to generate DC to study their biology and their retroviral transduction for immunotherapeutic purposes [25]. To test the ability to expand CD34+ cells in serum-free medium, the increase in cell counts of BM-CD34+ (5 × 104 cells/ml) cultured in complete serum-containing medium IMDM or RPMI-1640 was compared with that cultured in complete serum-free medium CellGro® SCGM or CellGro® DC containing one of the two following cytokines cocktails: Flt-3L (150 ng/ml), IL-3 (100 ng/ml), SCF (50 ng/ml) and TGF-beta1 (0·5 ng/ml) or Flt-3L (300 ng/ml), IL-3 (100 ng/ml) and SCF (100 ng/ml). Cultures were incubated for 7 days at 37°C in a humidified 5% CO2-in-air atmosphere.
Generation of human CD34+-derived dendritic cells with cytokines and in serum-free medium
CB-CD34+ cells (5 × 104 cells/ml) seeded into 24-well plates (Greiner GmbH, Frickenhausen, Germany) were cultured in complete serum-free medium (CellGro® DC) containing the following cytokines that specifically reported for CB [18]: Flt-3L and GM-CSF (100 ng/ml each), SCF (20 ng/ml), TGF-beta1 (0·5 ng/ml) and TNF-alpha (10 ng/ml) for 14 days at 37°C/5% CO2. The culture medium was exchanged at weekly intervals. The density of cells did not exceed 105 cells/ml.
Also PB-CD34+ cells were cultured under the above conditions but with the following different cytokines cocktails reported specifically for PB [16]: Flt-3L (150 ng/ml), IL-3 (100 ng/ml), SCF (50 ng/ml) and TGF-beta1 (0·5 ng/ml) for the first week, Flt-3L (150 ng/ml), GM-CSF (100 ng/ml), IL-4 and SCF (50 ng/ml each) and TGF-beta1 (0·5 ng/ml) for the second week and Flt-3L (150 ng/ml), GM-CSF (100 ng/ml), IL-4 and SCF (50 ng/ml each), TGF-beta1 (0·5 ng/ml) and TNF-alpha (2 ng/ml) for the third and fourth weeks. At day 26, 20 ng/ml TNF-alpha was added to induce maturation.
Generation of human CD34+-derived dendritic cells with PMA and in serum-free medium
CB- or PB-CD34+ cells at a concentration of 5 × 104 cells/ml were cultured in 24-well plates containing complete serum-free medium (CellGro® DC). Cultures were stimulated with 15 ng/ml PMA (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany) and incubated for 7 days at 37°C/5% CO2. PB-CD34+ cells were also stimulated with PMA plus 10 ng/ml TNF-alpha or plus 100 ng/ml ionomycin (Calbiochem-Novabiochem, Schwalbach, Germany). Expanded BM-CD34+ cells in complete CellGro® SCGM containing Flt-3L (300 ng/ml), IL-3 (100 ng/ml) and SCF (100 ng/ml) were also stimulated with PMA.
Flow cytometric analysis and monoclonal antibodies (MoAbs)
Adherent and loosely adherent cells after indicated periods of time were harvested with 1 mm EDTA in cold PBS, washed twice, and resuspended in staining buffer (PBS plus 0·2% BSA). Phenotypic analysis of cells was performed by flow cytometer (FACScalibur with CellQuest software, Becton Dickinson) using the following MoAbs: CD1a (clone BL6) from Immunotech GmbH (Hamburg, Germany), CD14 (clone MφP9) and HLA-DR (clone L243) both from Becton Dickinson and CD83 (clone HB15e) and CD86 (clone IT2·2), both from PharMingen GmbH (Hamburg, Germany). Non-specific binding was blocked by the addition of FcR blocking reagent (Human IgG, MACS, Miltenyi Biotec). Appropriate conjugated isotype-matched antibodies were used as controls. To exclude dead cells, 7-amino-actinomycin D (7-AAD, PharMingen) was added to each sample. Ten thousand cells from each sample were analysed on flow cytometer. Data presentation was performed using the WinMDI version 2·8 program.
Cell counts
Viable cell counts were performed using a Neubauer counting chamber (Hawksely, UK) with at least 200 cells being counted per sample. Cell viability was assessed using trypan blue exclusion (Sigma-Aldrich Chemie GmbH).
CD86+ yield was calculated as the ratio of the output number of CD86+DC relative to the input number of CD34+ progenitor cells after indicated periods of time using the following equation:
 |
T cell proliferation assay
PB-autologous T cells were purified from MNCs by negative selection using the Pan T Cell Isolation Kit (MACS, Miltenyi Biotec) following the manufacturer's instructions. PB-autologous monocytes (Mo) were obtained by plastic adherence of MNCs. After 2 h non-adherent cells were removed by 3× washing with PBS.
To test the ability of PMA-generated DC to process and present superantigen and soluble whole antigen, gamma-irradiated (3000 rad 137Cs) Mo or DC from day 7 PMA-treated PB-CD34+ cell cultures were plated in triplicate wells of 96-well flat-bottom plates (Nunc, Denmark) at 2 × 104 cells/well. Purified autologous T cells (1 × 105 cells/ml) in complete RPMI-1640 medium were added to the Mo- or DC-containing wells without or with 3 μg/ml staphylococcal enterotoxin B (SEB, Sigma-Aldrich Chemie GmbH) or 10 μg/ml preservative-free tetanus toxoid (TT, Calbiochem-Novabiochem). Also autologous T cells were plated in triplicate wells of 96-well flat-bottom plates with media alone (no stimulus) or with media containing SEB or TT. Cultures were incubated for 7 days at 37°C in a humidified 5% CO2-in-air atmosphere.
T cell proliferation was assessed after 0·5 micro-Ci/well [3H]-thymidine (Amersham, Braunschweig, Germany) had been added for the final 18 h of culture. Cells were harvested using a 96-well cell harvester, and [3H]-thymidine incorporation was measured using a beta scintillation counter.
Statistics
Data are presented as mean values ±s.d. For comparison of two groups, unpaired t-tests and the Mann–Whitney test (U-test) were used. For comparison of three groups, the Kruskal–Wallis test was used. P-values of <0·05, < 0·01 and <0·001 were considered statistically significant, highly significant and very highly significant, respectively.
Results
Expansion of BM-CD34+ cells in serum-free media compared with serum-containing media and with different cytokine cocktails
In order to determine the useful method for ex vivo expansion of CD34+ progenitors, we compared BM-CD34+ cells cultured in complete serum-containing medium IMDM or RPMI-1640 with cells cultured in complete serum-free medium CellGro® SCGM or CellGro® DC containing either Flt-3L, 150 ng/ml; IL-3, 100 ng/ml; SCF, 50 ng/ml and TGF-beta1, 0·5 ng/ml (cocktail 1) or Flt-3L, 300 ng/ml; IL-3, 100 ng/ml and SCF, 100 ng/ml (cocktail 2).
Table 1 shows that there is no statistical difference (P = 0·344, n = 12) in expansion between serum-containing media and serum-free media, the best (4·36 ± 0·91, n = 6) and the worse (3·28 ± 0·51, n = 6) expansion were in CellGro® SCGM and CellGro® DC, respectively. The expansion using cytokines cocktail 2 is significantly better (P = 0·001, n = 12) than that using cytokines cocktail 1.
Table 1.
Expansion of BM-CD34+ in serum-free medium compared with serum-containing media and with different cytokine cocktails. BM-CD34+ cells (5 × 104 cells/ml) cultured in complete serum-containing media IMDM or RPMI-1640 or complete serum-free medium CellGro® SCGM or CellGro® DC containing either first cytokines cocktail (Flt-3L, 150 ng/ml; IL-3, 100 ng/ml; SCF, 50 ng/ml and TGF-beta1, 0·5 ng/ml) or second cytokines cocktail (Flt-3L, 300 ng/ml; IL-3, 100 ng/ml and SCF, 100 ng/ml). Cultures were incubated for 7 days
| Expansion (fold) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Complete serum-containing media | Complete serum-free media | |||||||
| IMDM | RPMI-1640 | CellGro® SCGM | CellGro® DC | |||||
| Experiment | 1st cytokines cocktail | 2nd cytokines cocktail | 1st cytokines cocktail | 2nd cytokines cocktail | 1st cytokines cocktail | 2nd cytokines cocktail | 1st cytokines cocktail | 2nd cytokines cocktail |
| 1 | 3·16 | 4·35 | 3·00 | 4·36 | 2·90 | 4·70 | 2·40 | 3·30 |
| 2 | 3·55 | 4·80 | 3·70 | 4·50 | 3·90 | 5·50 | 3·10 | 3·39 |
| 3 | 4·19 | 5·11 | 3·77 | 5·21 | 4·21 | 4·94 | 3·60 | 3·90 |
| Mean ±s.d.a | 3·63 ± 0·52 | 4·75 ± 0·38 | 3·49 ± 0·43 | 4·69 ± 0·46 | 3·67 ± 0·68 | 5·05 ± 0·41 | 3·03 ± 0·60 | 3·53 ± 0·32 |
Unpaired t-tests.
These findings demonstrate that the best conditions for expansion of CD34+ cells are provided in serum-free medium (CellGro® SCGM) containing Flt-3L (300 ng/ml), IL-3 (100 ng/ml) and SCF (100 ng/ml).
Phorbol ester induces differentiation of ex vivo expanded BM-CD34+ haematopoietic progenitors to DC in serum-free medium
Generation of mature DC from CD34+ progenitors using different cytokine cocktails has been studied extensively. Recently, it has been suggested that direct activation of PKC by PMA is sufficient to trigger DC differentiation out of BM-CD34+ and no other lineages are generated [23].
To test if morphological and phenotypic changes induced by PMA were affected by pretreatment of BM-CD34+ cells with cytokines cocktail that induced a large expansion under serum-free conditions, the cells were expanded for 7 days in CellGro® SCGM containing Flt-3L (300 ng/ml), IL-3 (100 ng/ml) and SCF (100 ng/ml) and then differentiated in CellGro® DC containing 15 ng/ml PMA until day 14.
We found that the DC differentiation induced by PMA under serum-free conditions was not affected morphologically by pretreatment of BM-CD34+ cells with the above cytokines cocktail that induced CD34+ cell expansion connected with myeloid, but not with DC differentiation, as described previously [23]. In addition, the cells were positive for HLA-DR, CD83 and CD86 and negative for CD1a and CD14 (data not shown). Table 2 demonstrates that at day 14, 2·94 ± 0·42-fold increase of the cell number was obtained.
Table 2.
Viable cell counts of BM-CD34+ cells after expansion and differentiation for 1 week with PMA under serum-free conditions. The data enclosed in brackets represent the fold increase in cell number
| Cell number | ||
|---|---|---|
| Experiment | After expansion (day 7) | After differentiation (day 14) |
| 1 | 2·35 × 105 (4·70) | 1·48 × 105 (2·96) |
| 2 | 2·80 × 105 (5·60) | 1·80 × 105 (3·60) |
| 3 | 2·24 × 105 (4·48) | 1·28 × 105 (2·56) |
| 4 | 2·05 × 105 (4·10) | 1·29 × 105 (2·57) |
| 5 | 2·59 × 105 (5·18) | 1·50 × 105 (3·00) |
| Mean ±s.d. | (4·81 ± 0·59) | (2·94 ± 0·42) |
Because the used cytokines facilitate murine onco-retroviral transduction [25], our method may be used preferentially for introducing genes into DC by retroviral vectors.
Phorbol ester induces differentiation of CB-and PB-CD34+ haematopoietic progenitors to DC under serum-free conditions
The ability of PMA to differentiate CB-and PB-CD34+ progenitor cells to DC using serum-free conditions has been examined.
By day 7, about 40–60% of CB- and PB-CD34+ progenitor cells cultured in serum-free medium (CellGro® DC) with 15 ng/ml PMA became large and loosely adherent and a subset developed long dendrites and hair-like cytoplasmic projections (Fig. 1a), morphology reported to be characteristic of DC [20]. Morphological changes were present within 1 day of stimulation and completed within 3 days. They were stable over at least 2 weeks in culture.
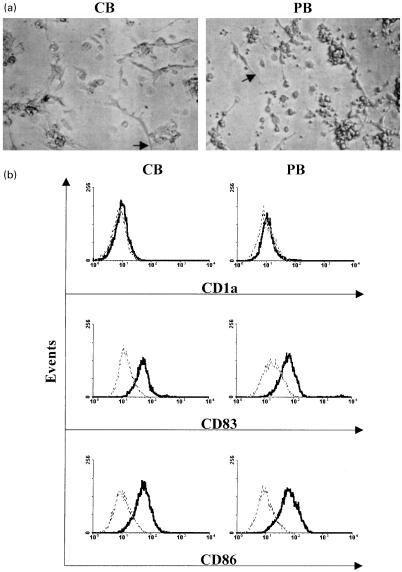
Fig. 1.
Morphology and phenotype of PMA-derived DC from human CB- and PB-CD34+ progenitor cells. (a) Typical morphology of CB- and PB-CD34+ cells cultured in serum-free medium (CellGro® DC) upon stimulation with PMA alone for 7 days (arrows indicate elongated dendrites). (b) Surface antigen phenotype characterization of day 7 PMA-treated CB- and PB-CD34+ cell cultures in serum-free medium. Total cells in cultures (adherent and non-adherent) were analysed by flow cytometric analysis. Isotype-matched controls are indicated by dotted lines. Results are representative of three different experiments.
Expression of the co-stimulatory molecule CD86 (B7–2), and the DC lineage marker CD83 typically characterize mature DC. Phenotypic analysis at day 7 after PMA-induction of CB- and PB-CD34+ progenitor cells (adherent and non-adherent) demonstrated that all cells were positive for both CD83 and CD86 and negative for CD1a (Fig. 1b). In addition these cells were CD1a− at day 3 of differentiation (data not shown).
These data are in accordance with the finding that PMA induces DC differentiation without causing cell proliferation and the generation of cellular intermediates [23].
CD86+ yield of PMA-derived DC from CB- and PB-CD34+ progenitors compared with that of cytokines-derived DC under serum-free conditions
We delineated the maturation of cultured human blood DC by surface analysis of the co-stimulatory molecule B7–2 (CD86), because it is the most important ligand to induce CD28-mediated co-stimulation for CD8+ T cell activation [21].
To study the efficacy of PMA to generate mature DC in serum-free medium, we compared CD86+ yield of PMA-derived DC with cytokine-derived DC using the same serum-free culture conditions. CD86+ yield was calculated as the ratio of the output number of CD86+DC relative to the input number of CD34+ progenitor cells with the following conditions, in case of a shift, as found in PMA-induced DC, all cells have been considered to be positive. In contrast, CD86+ and CD86− cells in cytokine-induced DC have been separable because not all cells shifted towards a positive CD86-expression. In this case only positive cells have been considered as induced DC.
It is well known that the cytokine requirements to differentiate CB- and PB-CD34+ progenitor cells into DC are different. Therefore, we used CD34+ progenitors from CB and PB in parallel experiments.
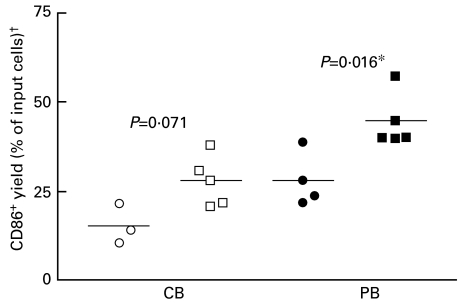
CD86+ yield was 28·0% ± 7·0 and 44·6% ± 7·5 for CB- and PB-derived dendritic cells upon stimulation with PMA for 1 week in serum-free medium (CellGro® DC), respectively (Fig. 2). In contrast, the yield was 15·3% ± 5·6 and 28·1% ± 7·5 for CB- and PB-derived DC stimulated with different cytokines cocktails specifically for CB and PB differentiation, respectively, and for indicated periods of time in the same serum-free medium as mentioned in Materials and methods.
Fig. 2.
CD86+ yield of CB- and PB-derived dendritic cells stimulated with PMA for 1 week or with different cytokines cocktails specifically for CB and PB in serum-free medium (CellGro® DC) and for indicated periods of time as mentioned in Materials and methods. †Number of viable CD86+ DC at the end of experiment × 100/the input number of CD34+ progenitor cells (5 × 104). *Significant, P < 0·05, Mann–Whitney test (U-test). ○, Cytokines specifically for CB; □, PMAKB; •, cytokines specifically for PB; ▪, PMA/PB.
These data suggest that under serum-free conditions CD86+ yield was increased by PMA compared to cytokine cocktails.
Effect of addition of the TNF-alpha or ionomycin to the serum-free culture condition containing PMA on the output number of viable cells and CD86+ expression of PB-derived dendritic cells
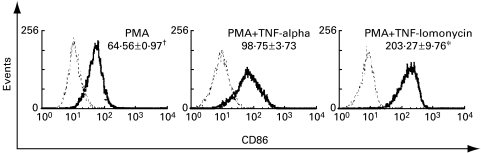
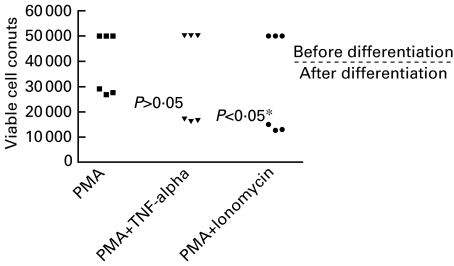
PB-CD34+ cells were also stimulated with PMA plus 10 ng/ml TNF-alpha or plus 100 ng/ml ionomycin in complete serum-free medium (CellGro® DC) for 7 days. While the up-regulation of CD86+ cells was more pronounced with addition of TNF-alpha or ionomycin (Fig. 3), the output numbers of viable cells after differentiation were decreased by PMA plus TNF-alpha (P > 0·05) or ionomycin (P < 0·05) compared with that in PMA alone (Fig. 4). This observation is in accordance with the finding that the percentage of apoptotic cells of stimulated KG1 myeloid cell line with PMA plus TNF-alpha or ionomycin was increased compared with that stimulated with PMA alone [24].
Fig. 3.
CD86+ expression of PB-derived dendritic cells after 1 week stimulation with serum-free medium (CellGro® DC) containing PMA alone or plus TNF-alpha or ionomycin. Isotype-matched controls are indicated by dotted lines. Results are representative of three different experiments. †Mean fluorescence. *Significance, P < 0·05, Kruskal–Wallis test.
Fig. 4.
Viable cell counts of PB-derived dendritic cells after 1 week stimulation with serum-free medium (CellGro® DC) containing PMA alone or plus TNF-alpha or ionomycin. *Significant, P < 0·05, Kruskal–Wallis test.
PMA-generated DC from CD34+ progenitor cells are functional APCs
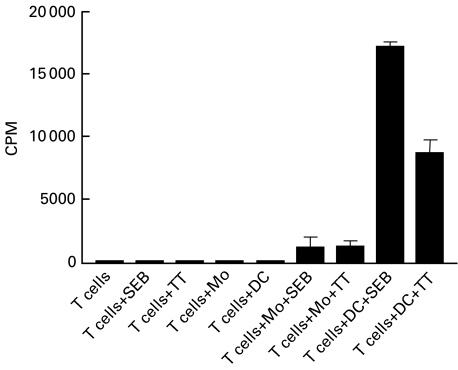
The functional hallmark of DC is their ability to activate T cells [11,26,27]. While T cell proliferation was induced by autologous PMA-generated DC in the presence of SEB or TT, PMA-generated DC cannot induce the proliferation of autologous T cells in the absence of antigen (Fig. 5). This lack of proliferation, in the absence of antigen, makes it unlikely that T cell proliferation is due to phorbol ester carryover. In addition, SEB and TT did not induce T cell activation in the absence of PMA-derived DC. SEB-mediated T cell activation is not MHC-restricted and does not require antigen processing but TT-mediated T cell proliferation is MHC restricted and require both antigen processing and presentation. In Fig. 5 only minimal proliferation can be demonstrated for T cells with added autologous monocytes in the presence of SEB or TT. In contrast, after adding autologous CD34+‐derived DC, a marked proliferation could be induced.
Fig. 5.
Autologous T cell proliferation induced by PMA-generated DC, from PB-CD34+ cells, pulsed with staphylococcal enterotoxin B (SEB) or preservative-free tetanus toxoid (TT). Monocytes or day 7 gamma-irradiated DC (2 × 104 cells) were added to purified autologous T cells (1 × 105 cells) in the absence or presence of 3 μg/ml SEB or 10 μg/ml TT. As negative controls, autologous T cells were plated in triplicate wells of 96-well flat-bottom plates with media alone (no stimulus) or with media containing SEB or TT. Data are presented as the mean counts per minute ± s.d. of triplicate cultures.
Discussion
DC are attractive cellular adjuvants for vaccination strategies due to their professional antigen-presenting capacity [28,29]. Currently, most in vitro culture systems for the production of these DC include serum. However, this is undesirable because serum contains growth factors that vary between individuals and could affect DC development [30]. Unless the patient's own serum is used, serum adjuvants such as FCS will lead to a skewing of the immune response by foreign antigens. In addition, serum preparations contain a series of cytokines not yet identified which are possibly involved in DC differentiation. In order to study the requirements to differentiate a CD34+ cell into DC, we provide data that DC can differentiate out of CD34+ cells from different origins in the presence of PMA without addition of serum.
Human mature DC with typical morphology (large and loosely adherent, and a subset developed long dendrites and hair-like cytoplasmic projections) and surface antigen phenotype (CD1a−, CD83+ and CD86+) were obtained from CB- and PB-CD34+ progenitor cells after 1 week of culture in serum-free medium upon stimulation with PMA alone. Morphological changes began within 1 day of stimulation, were fully manifested within 3 days and were stable over at least 2 weeks in culture. The same result was obtained from ex vivo-expanded BM-CD34+ cells. CD86+ yield under serum-free conditions was increased by PMA compared to cytokine cocktails, 28·0% ± 7·0 versus 15·3% ± 5·6 for CB (P = 0·071) and 44·6% ± 7·5 versus 28·1% ± 7·5 for PB (P = 0·016), respectively (Fig. 2).
As described previously, there are two stages of DC differentiation from CD34+ haematopoietic progenitor cells (HPC) using cytokines [31]. The first stage involves differentiation of multipotential HPC to immature CD1a+DC, cells with high capacity for antigen uptake but relatively poor ability to activate T cells. This intermediate DC adhered to plastic surfaces, expressed Birbeck granules and were negative for CD80, CD83 and CD86 co-stimulatory molecules. This differentiation is induced in vitro within 2 weeks by GM-CSF and TNF-alpha ± other cytokines [30]. The second stage involves maturation of immature CD1a+DC to mature DC, cells that have decreased antigen uptake capability but are much more potent in activating T cells. This maturation involved increased expression of CD80, CD83, CD86, CMRF-44, HLA-A, -B, -C and -DR as well as down-regulation of CD1a and CD11b. Activated DC are characterized by the lack of adherence to plastic surfaces and the absence of Birbeck granules [30]. In vitro maturation/activation can be induced by TNF-alpha ± other cytokines [20] or CD40 receptor cross-linking [32]. Spontaneous maturation of intermediates into fully activated DC expressing CD83 and co-stimulatory molecules occurred asynchronously over the ensuing 2–3 weeks. In vivo this second stage is triggered by live bacteria, components (LPS and DNA), viral infection and inflammatory cytokines [1].
Because cytokine receptor stimulation activates complex signalling cascades that initiate multiple responses, differentiation to other lineages is almost simultaneously induced. Thus cytokine application not only generates DC but also macrophages and neutrophils [9,14,17].
In contrast to cytokines or CD40 ligand, PMA alone induces DC differentiation only, without causing cell proliferation and the generation of cellular intermediates [23]. In this regard, PMA-induced DC differentiation out of CD34+ cells and more closely resembles cytokine-driven DC differentiation out of monocytes, in which there is also no proliferation, and 20–90% of input cells are lost during culture [10,11].
Our observation that about 40–60% of cells are lost during culture depending upon the source of CD34+ progenitors is supported by the observation that PMA had a negative effect on CD34+ survival that did not differentiate to DC [23]. The ability to differentiate into DC in response to PMA is limited to CD34+ cells, as PMA caused macrophage differentiation in CD34−CD15+ cells and cell death in CD14+ monocytes [23]. In addition, the CD34+ myeloid cell line KG1 differentiates into dendritic-like cells in response to PMA (with or without the calcium ionophore ionomycin or TNF-alpha). Comparison of KG1 to the PMA-unresponsive subline KG1a reveals differences in expression of TNF receptors (1 and 2), PKC isoforms (alpha, beta1, beta2 and mu) and RelB, suggesting that these components/pathways are important for DC differentiation [24].
All the receptor-mediated stimuli (GM-CSF, IL-4, TNF-alpha and CD40 cross-linking) that induce DC differentiation can also activate PKC as part of their intracellular signalling pathways [33–35]. Direct activation of PKC with PMA alone is sufficient to induce differentiation of human BM-CD34+ cells into mature and fully functional DC within 7 days only, suggesting a critical and specific role of this pathway for the progenitor lineage committed to DC [23]. This specificity may be facilitating further identification of signalling pathways involved in DC differentiation.
The rarity of CD34+ HPC makes sufficient isolation for larger-scale studies laborious. This problem could be overcome by ex vivo CD34+ expansion. We examined the effects of different cytokine combinations and culture conditions on BM-CD34+ expansion. We found that cells were maximally expanded (5·05 ± 0·41-fold) after 1 week under serum-free conditions (CellGro® SCGM) with Flt-3L, 300 ng/ml; IL-3, 100 ng/ml and SCF, 100 ng/ml. In the case that 50% of the cytokines-containing medium was exchanged after 3 days and the density of cells did not exceed 105 cells/ml, the increase of cells was 18·5-fold (data not shown). The DC differentiation induced by PMA was not affected by pretreatment of the BM-CD34+ cells with the above cytokines cocktail that induced CD34+ cell expansion connected with myeloid but not with DC differentiation. Because this cytokines cocktail facilitates retroviral transduction [25], our method may be used preferentially for introducing genes into DC by onco-retroviral vectors.
We have found that up-regulation of CD86 was most pronounced with addition of the calcium ionophore ionomycin. However, the output numbers of viable cells after differentiation were decreased by PMA plus ionomycin (P < 0·05) or plus TNF-alpha (P > 0·05) compared with that in PMA alone (Fig. 4). The decrease of cell viability by PMA plus ionomycin or TNF-alpha compared to PMA alone may be due to the apoptotic effect of these two stimuli. TNF-alpha and ionomycin appear to be using the same signalling pathway because the combination of PMA, ionomycin and TNF-alpha was no different from PMA plus TNF-alpha or ionomycin [24].
Functionally, PMA-generated DC were capable of stimulating autologous T cell proliferation by processing and presenting whole soluble antigen and superantigen. The absence of autologous T cell proliferation when cultured with DC (no SEB or TT) demonstrates that DC themselves or the drug by which they have been treated do not stimulate responding T lymphocytes.
We conclude that PMA is a potent activator to differentiate human CD34+ into mature DC in serum-free medium. The procedure described here may represent a method for generating pure populations of DC from freshly isolated or ex vivo-expanded CD34+ progenitor cells. This may facilitate an in vitro immune response using primed or genetically modified DC against infectious and tumour-associated antigens.
Acknowledgments
The authors thank Dr J. Schwender, Cytonet GmbH Hannover, for providing the G-CSF mobilized peripheral blood and the delivery room midwives of Neubethesda Hospital, Hannover for providing the cord blood samples. We would also like to thank Dr Andreas Tiede, Department of Haematology and Oncology, Hannover Medical School and Dr rer. nat. Hartmut Herrmann, Institute of Biometry, Hannover Medical School for support with statistical analysis. This work was supported by the Egyptian Administration of Mission and BMBF 01 GE 9906.
References
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 3.Rughetti A, Biffoni M, Sabbatucci M, et al. Transfected human dendritic cells to induce antitumor immunity. Gene Ther. 2000;7:1458–66. doi: 10.1038/sj.gt.3301266. [DOI] [PubMed] [Google Scholar]
- 4.Kirk CJ, Mule JJ. Gene-modified dendritic cells for use in tumor vaccines. Hum Gene Ther. 2000;11:797–806. doi: 10.1089/10430340050015419. [DOI] [PubMed] [Google Scholar]
- 5.Heemskerk MH, Hooijberg E, Ruizendaal JJ, van der Weide MM, Kueter E, Bakker AQ, Schumacher TN, Spits H. Enrichment of an antigen-specific T cell response by retrovirally transduced human dendritic cells. Cell Immunol. 1999;195:10–7. doi: 10.1006/cimm.1999.1520. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo G, Wesa A, Wei WZ, Galy A. Dendritic cells generated either from CD34+ progenitor cells or from monocytes differ in their ability to activate antigen-specific CD8+ T cells. J Immunol. 1999;163:3597–604. [PubMed] [Google Scholar]
- 7.Ratta M, Rondelli D, Fortuna A, et al. Generation and functional characterization of human dendritic cells derived from CD34 cells mobilized into peripheral blood: comparison with bone marrow CD34+ cells. Br J Haematol. 1998;101:756–65. doi: 10.1046/j.1365-2141.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 8.Reid CD, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992;149:2681–8. [PubMed] [Google Scholar]
- 9.Santiago-Schwarz F, Belilos E, Diamond B, Carsons SE. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992;52:274–81. [PubMed] [Google Scholar]
- 10.Kiertscher SM, Roth MD. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J Leukoc Biol. 1996;59:208–18. doi: 10.1002/jlb.59.2.208. [DOI] [PubMed] [Google Scholar]
- 11.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves ME, Royal RE, Lam JS, Rosenberg SA, Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996;56:5672–7. [PubMed] [Google Scholar]
- 13.Sato K, Nagayama H, Takahashi TA. Generation of dendritic cells from fresh and frozen cord blood CD34+ cells. Cryobiology. 1998;37:362–71. doi: 10.1006/cryo.1998.2136. [DOI] [PubMed] [Google Scholar]
- 14.Szabolcs P, Moore MA, Young JW. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995;154:5851–61. [PubMed] [Google Scholar]
- 15.Strunk D, Rappersberger K, Egger C, et al. Generation of human dendritic cells/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–302. [PubMed] [Google Scholar]
- 16.Garbe A, Köhler G, Schulz G, Lindemann A. Serum-free culture conditions for the in vitro generation of dendritic cells from peripheral blood CD34 positive progenitor cells of human adults [Abstract no. 1519, American Society of Hematology, 40th annual meeting] Blood. 1998;92:369a. [Google Scholar]
- 17.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 18.Bello-Fernandez C, Matyash M, Strobl H, et al. Efficient retrovirus-mediated gene transfer of dendritic cells generated from CD34+ cord blood cells under serum-free conditions. Hum Gene Ther. 1997;8:1651–8. doi: 10.1089/hum.1997.8.14-1651. [DOI] [PubMed] [Google Scholar]
- 19.Ardeshna KM, Corney CP, Ings SJ, Watts MJ, Linch DC, Devereux S. A clinically applicable method for the ex vivo generation of antigen-presenting cells from CD34+ progenitors. Vox Sang. 2000;79:46–52. doi: 10.1159/000031205. [DOI] [PubMed] [Google Scholar]
- 20.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 21.Van Gool SW, Vermeiren J, Rafiq K, Lorr K, de Boer M, Ceuppens JL. Blocking CD40–CD154 and CD80/CD86–CD28 interactions during primary allogeneic stimulation results in T cell anergy and high IL-10 production. Eur J Immunol. 1999;29:2367–75. doi: 10.1002/(SICI)1521-4141(199908)29:08<2367::AID-IMMU2367>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–96. [PubMed] [Google Scholar]
- 23.Davis TA, Saini AA, Blair PJ, et al. Phorbol esters induce differentiation of human CD34+ hemopoietic progenitors to dendritic cells: evidence for protein kinase C-mediated signaling. J Immunol. 1998;160:3689–97. [PubMed] [Google Scholar]
- 24.St. Louis DC, Woodcock JB, Fransozo G, et al. Evidence for distinct intracellular signaling pathways in CD34+ progenitor to dendritic cell differentiation from a human cell line model. J Immunol. 1999;162:3237–48. [PubMed] [Google Scholar]
- 25.Glimm H, Flugge K, Mobest D, et al. Efficient serum-free retroviral gene transfer into primitive human hematopoietic progenitor cells by a defined, high-titer, nonconcentrated vector-containing medium. Hum Gene Ther. 1998;9:771–8. doi: 10.1089/hum.1998.9.6-771. [DOI] [PubMed] [Google Scholar]
- 26.Caux C, Saeland S, Favre C, Duvert V, Mannoni P, Banchereau J. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+ hematopoietic progenitor cells. Blood. 1990;75:2292–8. [PubMed] [Google Scholar]
- 27.Rosenzwajg M, Canque B, Gluckman JC. Human dendritic cell differentiation pathway from CD34+ hematopoietic precursor cells. Blood. 1996;87:535–44. [PubMed] [Google Scholar]
- 28.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–8. [PubMed] [Google Scholar]
- 29.Klein C, Bueler H, Mulligan RC. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med. 2000;191:1699–708. doi: 10.1084/jem.191.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luft T, Pang KC, Thomas E, et al. A serum-free culture model for studying the differentiation of human dendritic cells from adult CD34+ progenitor cells. Exp Hematol. 1998;26:489–500. [PubMed] [Google Scholar]
- 31.Winzler C, Rovere P, Rescigno M, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–28. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores-Romo L, Bjorck P, Duvert V, van Kooten C, Saeland S, Banchereau J. CD40 ligation on human cord blood CD34+ hematopoietic progenitors induces their proliferation and differentiation into functional dendritic cells. J Exp Med. 1997;185:341–9. doi: 10.1084/jem.185.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arruda S, Ho JL. IL-4 receptor signal transduction in human monocytes is associated with protein kinase C translocation. J Immunol. 1992;149:1258–64. [PubMed] [Google Scholar]
- 34.Ren CL, Morio T, Fu SM, Geha RS. Signal transduction via CD40 involves activation of lyn kinase and phosphatidylinositol-3-kinase, and phosphorylation of phospholipase C gamma 2. J Exp Med. 1994;179:673–80. doi: 10.1084/jem.179.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mufson RA. The role of serine/threonine phosphorylation in hematopoietic cytokine receptor signal transduction. FASEB J. 1997;11:37–44. doi: 10.1096/fasebj.11.1.9034164. [DOI] [PubMed] [Google Scholar]