Abstract
Monocytes as antigen-presenting cells play an important role in host defence. There are several cytokines affecting monocyte function. We demonstrate that both adult and cord blood monocytes constitutively express hepatocyte growth factor (HGF) receptor, MET. HGF significantly down-regulated MET expression of adult blood monocytes, compared with cord blood monocytes, when cultured either in RPMI-1640 containing 10% FBS or serum-free medium. Surface levels of MET correlated with c-met mRNA levels both in adult and cord blood when cultured. MET expression was down-regulated by treating with actinomycin D or cycloheximide. HGF stimulated DNA synthesis of adult monocytes, but not cord blood. HGF enhanced antigen-presenting capacity of adult blood monocytes but not cord blood monocytes. HGF up-regulated HLA class I expression in adult monocytes but not in cord blood monocytes. The current results suggest that the failure of cord blood monocytes to respond to HGF may be responsible, in large part, for their functional immaturity.
Keywords: antigen presentation, cord blood, hepatocyte growth factor, monocyte, transplantation
Introduction
Antigen-presenting cells, including monocytes, may play an important role in host defence and transplantation by priming of naive T-lymphocytes [1]. Several functional abnormalities of cord blood monocytes have been reported, including decreased HLA-DR expression, reduced chemotaxis, phagocytosis, cytokine and prostaglandin release [2,3]. Clinical results suggest a lower incidence of graft-versus-host disease (GVHD) in children receiving cord blood transplantation [4].
Hepatocyte growth factor (HGF) plays a role in regulation of cell motility, cell growth, morphogenesis and angiogenesis of a wide variety of cell types in addition to hepatocytes [5,6]. All functions attributed to HGF are mediated by the c-met tyrosine kinase, the high affinity HGF receptor [5]. HGF is produced by hepatocytes and macrophages; HGF may increase during infection [7].
To elucidate the potential role of HGF in monocyte functions, we analysed the expression c-met protein (MET) and the effect of HGF using cord blood and adult peripheral blood monocytes. Our results demonstrate that internalization of MET does occur following receptor–ligand interaction in cord blood monocytes, but signal transduction might be immature in cord blood monocytes.
Materials and methods
Cell preparation and culture
Cord blood was collected with informed consent. Adult peripheral blood was obtained from healthy volunteers. Mononuclear cells were separated by Histopaque (Sigma, St Louis, MO, USA), washed twice in PBS and resuspended in PBS plus 0·6% ACD-A and 2% FBS (PBS-2), as described [8]. Negative selection of monocytes was performed (Stemcell Technologies Inc, Vancouver, USA). StemSep antibody cocktail for human monocytes (CD2, CD3, CD19, CD56, glycophorin) and antihuman CD16 tetramer were used. Monocytes were washed twice with PBS-2, and then resuspended in TCM-10 (RPMI-1640 supplemented with 10% FBS, 5 × 10−5 2-mercaptoethanol, 10 mm HEPES). Cell preparations were tested for viability (> 99%) by trypan blue exclusion and for purity (> 95% CD14+ monocytes) by flow cytometric analysis. Recombinant human hepatocyte growth factor/scatter factor (HGF; Becton Dickinson, Bedford, MA, USA) was used in this study. Other cytokines that are associated with GVHD and infections were used at the indicated concentrations: IFN-γ (400 U/ml; Genzyme, Cambridge, UK), IL-4 (10 ng/ml; Pepro Tech EC Ltd, Rocky Hill, NJ, USA), IL-10 (10 ng/ml; Pepro Tech EC Ltd) and GM-CSF (50 ng/ml; Kirin Brewery Co. Ltd, Gunma, Japan) [7,9–13]. Highly purified monocytes were cultured with graded concentrations of HGF (0·1, 1, 10 and 20 ng/ml) at 37°C for 48 h (cord blood) or 24 h (adult blood), or cultured with 10 ng/ml HGF for 12 h to day 6 for kinetic studies. Cycloheximide (CHX; 10 µg/ml) and actinomycin D (10 µg/ml) (Sigma, St Louis, MO, USA) were added to cell cultures at indicated times. Monocytes were detached by rubber policeman. After washings, cells were cultured either in TCM-10 or HB101 serum-free medium (Irvine Scientific, Santa Ana, CA, USA).
Flow cytometric analysis
Cells (1 × 105) were incubated with unconjugated mouse anti-Human c-met monoclonal antibody (MoAb) (IgG1κ) to detect c-met protein (MET) (Upstate Biotechnology Inc, Lake Placid, NY, USA). Isotype-matched control FITC-IgG1κ (Pharmingen, San Diego, CA, USA) was used. After washings, 5000 cells were analysed on a FACScan flow cytometer (Becton Dickinson). The mean fluorescence intensity (MFI) was used to compare the statistical significance. Other cell surface antigens were also analysed. Monoclonal antibodies used in the study included FITC-conjugated HLA-ABC (Serotec, Oxford, UK), FITC-HLA-DR, FITC-CD4, PE-conjugated CD13, FITC-CD14, FITC-CD15, PE-CD33, PE-CD49d, PE-CD54 (Becton Dickinson) and CD40, FITC-CD80, PE-CD86 (Pharmingen). Isotype-matched MoAb, IgG1 and IgG2a (Becton Dickinson) and IgG1κ (Pharmingen) were used as an appropriate control.
Detection of c-met mRNA by reverse transcription-polymerase chain reaction (RT-PCR) and Southern blot analysis
C-met-specific primer was synthesized (Amersham Pharmacia Biotech, Tokyo, Japan): forward primer, 5′-TGG GAA TCT GCC TGC GAA-3í; reverse primer, 5′-CCA GAG GAC GAC GCC AAA-3′ [14]. The β-actin primer sequences were as follows: forward primer, 5′-ACT ACC TCA TGA AGA TCC TCA-3′; reverse primer: 5′-CAG GAG GAG CAA TGA TCT TGA-3′. Total RNA was isolated and c-DNA was synthesized as described previously [15]. The PCR was run for 40 cycles on a Program Temp Control System PC-700 (Astec Co. Ltd, Fukuoka, Japan). Ten µl of the expected PCR products of 395 bp (c-met) and 440 bp (β-actin) were analysed on 2% agarose gels containing 0·5 µg/ml ethidium bromide. DNA in the gels was denaturated, neutralized and transferred to nylon membranes. Probes were labelled with a DNA labelling kit (Waco Pure Chemical Industries Ltd, Osaka, Japan). The probe used for the analysis of c-met contained two fragments (2·0 kb and 2·3 kb) of the promoter region of c-met after EcoR1 digestion [16]. After hybridization, the membranes were washed, dried and autoradiographed on Kodak X-ray film [17]. X-ray films were scanned.
Mixed lymphocyte culture (MLC)
A constant number of 1 × 105 allogeneic responder cells were incubated with graded numbers of 30 Gy-irradiated, freshly isolated monocytes or with CD14+ monocytes cultured for the indicated time periods. Experiments were performed in triplicate in round-bottomed 96-well plates in TCM-10. Cells cultured in the presence of cytokines were washed three times before being added into wells. Radioactivity was monitored by measuring [3H]-thymidine incorporation on day 5 of culture. The use of an allogeneic MLC to assess the stimulatory activity of monocytes was previously described [10].
Statistical analysis
Statistical analysis was performed using unpaired Fisher's t-test. P < 0·05 was considered significant.
Results
Detection of MET expression on monocytes by flow cytometry
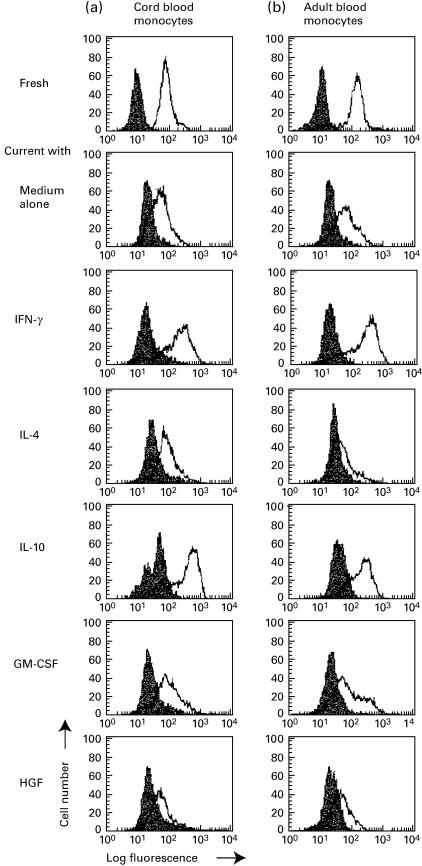
We found that CD14+ monocytes in cord blood expressed similar levels of MET compared with that of monocytes in adult blood (Fig. 1). After cultivation of highly purified monocytes with cytokines, MET expression was determined. HGF down-regulated MET of cord blood monocytes more significantly when compared with adult blood (see Fig. 1 and Table 1). IFN-γ and IL-10 enhanced MET in both cord blood and adult blood. IL-4 did not modulate cord blood, but down-regulated adult blood. GM-CSF had no effect. Relative expression of fluorescence intensity is shown in Table 1. Actual MET mean fluorescence intensity of cord and adult blood monocytes were 90±7 and 1067±13 (fresh), 126±59 and 141±13 (medium control), 342±92 and 276±51 (IFN-γ), 127±37 and 62±7 (IL-4), 376±80 and 264±69 (IL-10), 105±22 and 122±28 (GM-CSF), and 71±6 and 113±21 (HGF), respectively. Similar results were obtained when cells were cultured either in TCM-10 or HB101 serum-free medium.
Fig. 1.
Expression of MET/HGF receptor on (a) cord and (b) adult blood monocytes. MET expression of fresh monocytes or monocytes cultured with indicated cytokines was evaluated by flow cytometer. Bold lines represent MET, shaded lines represent isotype-matched control antibody. The results are representative of three separate experiments with reproducible results (see Table 1).
Table 1.
MET expression on cord and adult blood monocytes†
| Cultured with | |||||||
|---|---|---|---|---|---|---|---|
| Fresh | Medium | IFN-γ | IL-4 | IL-10 | GM-CSF | HGF | |
| Cord blood | 9·1 ± 3·3 | 2·5 ± 1·2 | 8·4 ± 2·2*b | 6·2 ± 1·8* | 9·5 ± 3·1* | 3·4 ± 1.5 | 1·2 ± 0·8* |
| Adult blood | 8·6 ± 2·1 | 2·9 ± 1·3 | 9·1 ± 2·5* | 2·1 ± 1·5 | 6·1 ± 2·2* | 4·1 ± 2.1 | 2·1 ± 0.6* |
MET expression of fresh and cultured monocytes was evaluated by flow cytometer. HGF down-regulated MET in both cord and adult blood. Other cytokines had various effects on MET expression. Results are expressed as RFI (MFI test antibody/MFI control antibody). Data are given as mean ± SD of three independent experiments.
P < 0·05, compared with medium control.
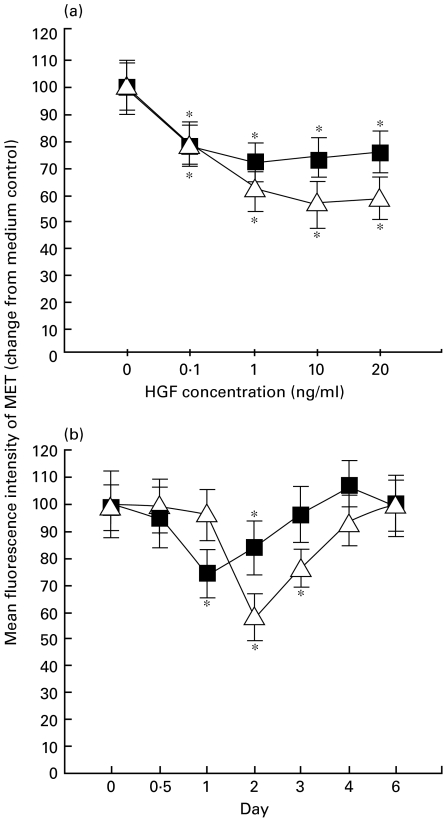
Kinetic studies of MET expression
Monocytes were incubated in the presence of HGF at graded concentrations, ranging from 0·1 to 20 ng/ml. The incubation time was 24 h for adult blood monocytes and 48 h for cord blood monocytes based on the kinetic study (see below). The results are shown in Fig. 2a. The addition of HGF to the culture resulted in a significant decrease of MET expression in monocytes of both cord and adult blood. Maximal inhibition was seen in the presence of 10 ng/ml of HGF for cord blood and 1 ng/ml for adult blood. In a kinetic study, monocytes were cultured with 10 ng/ml HGF on day 0, then MET expression was examined from 12 h to day 6. Significant inhibition on MET expression of monocytes was observed on day 2 for cord blood when compared with day 0 value (Fig. 2b). HGF also significantly inhibited MET expression on adult blood monocyte in a time-dependent fashion with maximal inhibition observed after a 24-h incubation period (Fig. 2b). The expression of MET recovered to the original level at day 4 for cord blood and day 3 for adult blood when compared with medium control. When HGF was washed out on day 2, MET expression returned to the original level on day 4 (data not shown).
Fig. 2.
Kinetics of MET expression regulated by HGF. (a) Dose dependency of HGF affecting MET expression. Monocytes were cultured for 48 h (cord blood) or 24 h (adult blood) in the presence of HGF (0·1–20 ng/ml). (b) Kinetics of MET expression regulated by HGF. Monocytes (n = 4) were cultured in the presence of 10 ng/ml HGF on day 0 and further cultured for 6 days. MET expression was evaluated by flow cytometer. Mean fluorescence intensity (MFI) of MET expression was normalized by medium control. Asterisks represent P < 0·05 as compared with medium control. ▵, Cord blood; ▪, adult blood.
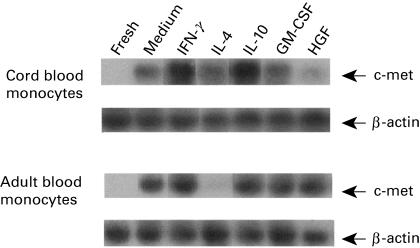
Expression of c-met mRNA in monocytes of cord and adult blood as detected by RT-PCR/Southern blot analysis
Freshly isolated monocytes from cord or adult blood contained no c-met mRNA as detected by RT-PCR/Southern blot analysis (Fig. 3). The expression of c-met mRNA was induced after culturing with medium alone for 48 h. Compared with medium control, HGF down-regulated c-met mRNA expression in cord blood monocytes and was unchanged in adult blood monocytes. IFN-γ and IL-10 up-regulated c-met expression in both cord and adult blood monocytes. IL-4 down-regulated c-met expression of adult blood monocytes to the level of fresh monocytes, but was unchanged in cord blood monocytes. GM-CSF had no effect on c-met expression in both cord and adult blood monocytes.
Fig. 3.
Expression of c-met mRNA in cord and adult blood monocytes detected by RT-PCR/Southern blot analysis. Monocytes were cultured in the presence or absence of various cytokines for 48 h and total RNA was extracted. Then, RT-PCR/Southern blot analysis using c-met probe was performed. Differential responses to cytokines were observed. The result is representative of two separate experiments with similar results.
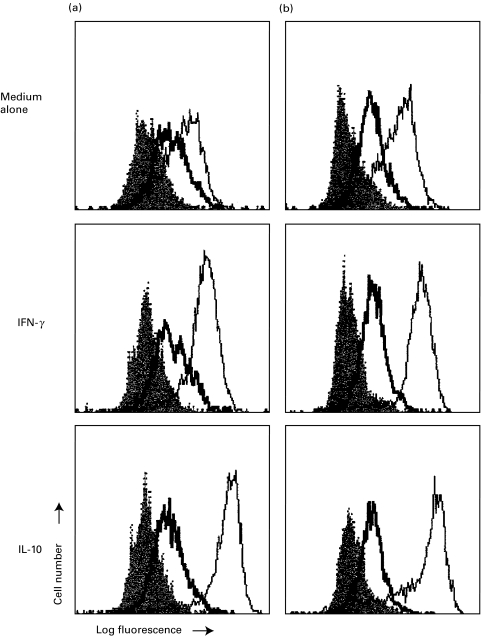
Inhibition of MET expression in cord and adult blood monocytes by actinomycin D
Since up-regulation of MET by IFN-γ and IL-10 occurred (Fig. 1), we examined whether MET expression would be affected by acitnomycin D to modulate mRNA level. Monocytes of cord or adult blood were cultured with actinomycin D for 15 min, and then IFN-γ or IL-10 was added. As shown in Fig. 4, the expressions of MET were inhibited in both cord and adult blood monocytes in these culture conditions. Similar results were obtained when cycloheximide was used instead of actinomycin D (data not shown).
Fig. 4.
Actinomycin D inhibited MET expression of (a) cord and (b) adult blood monocytes cultured with IFN-γ or IL-10. Highly purified monocytes were pretreated with actinomycin D for 15 min, then IFN-γ or IL-10 was added and incubated for 48 h. MET expression was evaluated by flow cytometer. Bold line represents actinomycin D, solid line for medium control and shaded line for isotype-matched control antibody. The results are representative of three separate experiments with reproducible results.
Antigen-presenting capacity of monocytes regulated by HGF
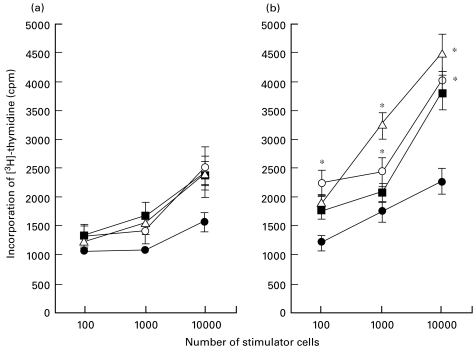
As shown in Fig. 5, cord blood monocytes, freshly isolated or cultured with medium alone for 48 h, showed lower APC function when compared with adult blood monocytes. After stimulation with HGF for 48 h, APC function of cord blood monocytes showed no changes compared with the culturing with medium alone (P = 0·32). In contrast, increased APC function were found in adult blood monocytes (P = 0·002). We also evaluated the APC modulated by other cytokines that may affect monocyte function. GM-CSF augmented APC of adult blood (P = 0·03) but not cord blood monocytes (P = 0·21). Other cytokines had no effect. The level of [3H]-thymidine incorporation in monocytes was consistent with other reports [10]. Experiments using different combinations of responders and stimulators resulted in similar reproducible data, suggesting that there was HLA disparity among samples used in this study.
Fig. 5.
Antigen-presenting capacity of cultured monocytes from (a) cord and (b) adult blood. Allogeneic adult peripheral blood mononuclear cells were incubated with freshly isolated monocytes or cultured monocytes. Incorporation of [3H]-thymidine was measured on day 5 of culture. Given are mean values ±SD from five separate experiments. *P < 0·05 as compared with medium control. Treatment: •, fresh; ▪, medium; ○, GM-CSF; ▵, HGF.
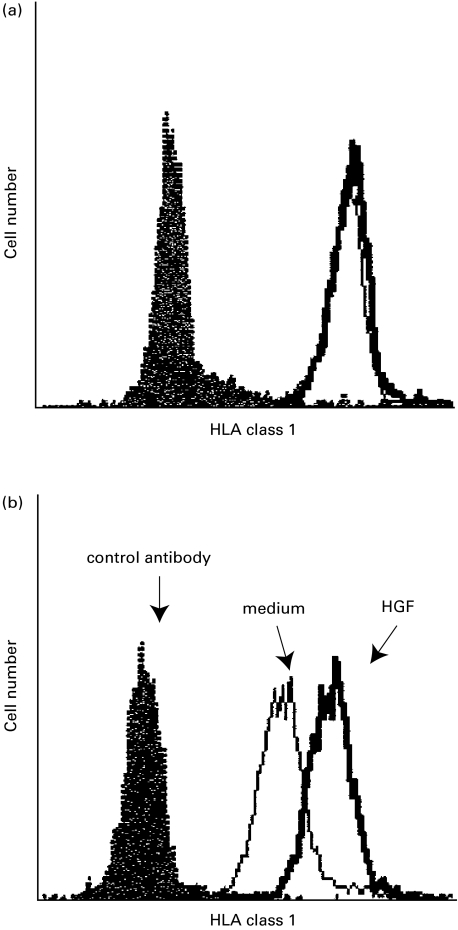
HGF up-regulated HLA class I expression in adult blood monocytes but not in cord blood
HLA, co-stimulatory molecules and adhesion molecules may affect the antigen-presenting capacity of monocytes. Figure 6 shows that HGF up-regulated HLA class I in adult blood monocytes but not in cord blood monocytes. There were no changes in other tested antigens such as HLA-DR, CD4, CD13, CD14, CD15, CD33, CD49d, CD40, CD80 and CD86 (data not shown).
Fig. 6.
HGF upregulated HLA class I in adult blood monocytes (b) but not in cord blood (a). Monocytes (n = 3) were cultured in the presence of HGF, followed by cytometric analysis. Bold line represents HLA class I. solid line for medium control and shaded line for isotype-matched control antibody.
Discussion
In this study, we have investigated the functional significance of MET/HGF receptor on cord and adult blood monocytes and have found that the differential responsiveness of two types of monocytes to HGF. HGF plays a crucial role as a haematopoietic regulator in the proliferation and differentiation of haematopoietic progenitors in humans [18]. HGF/MET signalling may play an additional role during inflammation [12].
We have demonstrated that monocytes, either adult or cord blood, constitutively express MET/HGF receptor on their surface (Fig. 1). We tested the effect of various monocyte-related cytokines on MET expression of monocytes. When monocytes were cultured in the presence of these cytokines there was a significant change of MET expression (Fig. 1 and Table 1). Adult and cord blood monocytes responded to them differently. These results suggest that the response of cord blood monocytes to HGF is hyporeactive when compared with adult blood. We also investigated c-met gene expression by RT-PCR/Southern blot (Fig. 3). Compared with medium control, HGF down-regulated c-met mRNA expression in cord blood monocytes and unchanged in adult blood monocytes. Other cytokines also had differential effects on cord and adult blood monocytes (Fig. 3). We also examined the effect of actinomycin D on surface expression of MET. We found that actinomycin D inhibited MET expression in both cord and adult blood monocytes (Fig. 4). The results demonstrated that, when they were cultured, surface levels of MET correlated with c-met mRNA levels in adult and cord blood. Chen et al. reported in human adult monocytes that untreated monocytes, when stimulated with IFN-γ, TNF-α and IL-6, do not express c-met mRNA (Northern blot) and protein (Western blot) [11]. Beilman et al. reported that c-met expression was found neither in freshly isolated human monocytes nor in cells cultured for 5 days, but could be induced by LPS or PHA stimulation [12]. Since both Chen et al. and Beilman et al. failed to detect c-met mRNA in fresh adult monocytes, we chose highly sensitive RT-PCR/Southern blot analysis. However, using the current method we also could not detect c-met mRNA in fresh adult monocytes in spite of the surface expression of MET. Some conflicts between their data and ours may be explained partly by the difference between materials (adult blood versus cord blood) and methods (Western blot analysis versus flow cytometry for MET expression; Northern blot analysis versus RT-PCR/Southern blot analysis for c-met).
Then, we investigated the functional difference of HGF/MET signalling in two types of monocytes. We tested the alloantigen-presenting capacity of monocytes cultured in the presence of cytokines (Fig. 5). The MLC data demonstrated that the MLC-stimulating capacity of the monocytes by culturing in the presence of HGF was not significantly changed in cord blood monocytes, whereas HGF significantly augmented allo-stimulating activity of adult blood monocytes. The results demonstrate that adult blood monocytes exhibit enhanced APC upon HGF stimulation, whereas cord blood monocytes showed none of the response. Furthermore, we examined the function-associated molecules which may have an impact on APC, e.g. HLA class I and class II, co-stimulatory molecules and adhesion molecules on cord and adult blood monocytes following HGF stimulation. HGF up-regulated HLA class I antigen in adult blood monocytes, but not in cord blood monocytes (Fig. 6). Although we cannot fully explain our results, other MHC class II molecules (HLA-DPB1, HLA-DQA1, or HLA-DS) and other function-associated molecules, which we did not include in this study, may be associated with the MLC data. Based on these in vitro studies, the findings presented here may suggest that internalization of MET do occur following receptor–ligand interaction in cord blood monocytes, but signal transduction thereafter might be immature in cord blood monocytes as compared to adult blood monocytes.
The clinical relevance of our data is yet to be determined. HGF may be increased in systemic inflammatory response syndrome [7], Staphylococcus aureus infection [19] or Epstein–Barr virus infection [20]. Since stem cell transplantation is often complicated with infections and exacerbation of GVHD may be observed, HGF may be increased locally or systemically in these clinical settings. It may be possible that HGF may activate monocytes during inflammation and modulate specific monocytic/macrophage functions at the site of the inflammatory reaction. Moreover, macrophages themselves produce HGF [21]. Based on these reports and our current in vitro results, it is tempting to speculate that HGF may be produced by monocytes/macrophages at the site of GVHD and released HGF in turn activate them through autocrine mechanism, resulting in exacerbation of GVHD. The differential responsiveness of these two types of monocytes may, in part, explain the degree of GVHD that develops following allogeneic stem cell transplantation.
Acknowledgments
We gratefully thank Prof. T. Nakamura at Osaka University for supplying cDNA of c-met proto-oncogene and Kirin Brewery Co. Ltd for providing GM-CSF. This study was supported in part by Grants-in-Aid from The Ministry of Health and Welfare, Japan, The Ministry of Education, Science, Sports and Culture, Japan, Lions Clubs International (District 334-B), and Japan China Medical Association.
References
- 1.Bhardwaj N, Bender A, Gonzalez N, et al. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Invest. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marodi L, Leijh PC, van Furth R. Characteristics and functional capacities of human cord blood granulocytes and monocytes. Pediatr Res. 1984;18:1127–31. doi: 10.1203/00006450-198411000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Weatherstone KB, Rich EA. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989;25:342–6. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, Nishizawa T, Hagiya M, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–3. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 6.Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochim Biophys Acta. 1993;1155:357–71. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 7.Sakon M, Kita Y, Yoshida T, et al. Plasma hepatocyte growth factor levels are increased in systemic inflammatory response syndrome. Surg Today. 1996;26:236–41. doi: 10.1007/BF00311581. [DOI] [PubMed] [Google Scholar]
- 8.Umemoto M, Azuma E, Hirayama M, et al. Cytokine-enhanced mixed lymphocyte reaction (MLR) in cord blood. Clin Exp Immunol. 1998;112:459–63. doi: 10.1046/j.1365-2249.1998.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara JL. Paradigm shift for graft-versus-host disease. Bone Marrow Transplant. 1994;14:183–4. [PubMed] [Google Scholar]
- 10.Pickl WF, Majdic O, Kohl P, et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–9. [PubMed] [Google Scholar]
- 11.Chen Q, DeFrances MC, Zarnegar R. Induction of met proto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ. 1996;7:821–32. [PubMed] [Google Scholar]
- 12.Beilmann M, Odenthal M, Jung W, et al. Neoexpression of the c-met/hepatocyte growth factor-scatter factor receptor gene in activated monocytes. Blood. 1997;90:4450–8. [PubMed] [Google Scholar]
- 13.Allavena P, Piemonti L, Longoni D, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Park M, Dean M, Kaul K, et al. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA. 1987;84:6379–83. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka M, Hayashida Y, Sakaguchi K, et al. Growth hormone-independent expression of insulin-like growth factor I messenger ribonucleic acid in extrahepatic tissues of the chicken. Endocrinology. 1996;137:30–4. doi: 10.1210/endo.137.1.8536628. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi O, Mizuno K, Vande Woude GF, et al. Expression of c-met proto-oncogene in COS cells induces the signal transducing high-affinity receptor for hepatocyte growth factor. FEBS Lett. 1992;301:282–6. doi: 10.1016/0014-5793(92)80257-h. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi K, Tanaka M, Ohkubo T, et al. Induction of brain prolactin receptor long-form mRNA expression and maternal behavior in pup-contacted male rats: promotion by prolactin administration and suppression by female contact. Neuroendocrinology. 1996;63:559–68. doi: 10.1159/000127085. [DOI] [PubMed] [Google Scholar]
- 18.Goff JP, Shields DS, Petersen BE, et al. Synergistic effects of hepatocyte growth factor on human cord blood CD34+ progenitor cells are the result of c-met receptor expression. Stem Cells. 1996;14:592–602. doi: 10.1002/stem.140592. [DOI] [PubMed] [Google Scholar]
- 19.Baroni A, Perfetto B, Ruocco E, et al. Lipoteichoic acid and protein-A from Staphylococcus aureus stimulate release of hepatocyte growth factor (HGF) by human dermal fibroblasts. Arch Dermatol Res. 1998;290:211–4. doi: 10.1007/s004030050292. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Gohda E, Matsunaga T, et al. Production of hepatocyte growth factor by human haematopoietic cell lines. Cytokine. 1994;6:285–94. doi: 10.1016/1043-4666(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 21.Yamada Y, Saito S, Morikawa H. Hepatocyte growth factor in human breast milk. Am J Reprod Immunol. 1998;40:112–20. doi: 10.1111/j.1600-0897.1998.tb00399.x. [DOI] [PubMed] [Google Scholar]