Abstract
In general, exogenous proteins are processed by antigen-presenting cells in the endosomes for major histocompatibility complex (MHC) class II presentation to CD4+ T cells, while proteins synthesized endogenously are processed in the cytoplasm for MHC class I presentation to CD8+ T cells. However, it is recognized that exogenous proteins can be processed for MHC class I presentation also, and evidence in favour of alternatives to the conventional MHC class I processing and presentation pathway is accumulating. Here, we show that exogenous recombinant influenza A virus nucleoprotein (rNP) is processed for MHC class I presentation to CD8+ cytotoxic T lymphocytes (CTL) by EBV-transformed, B-lymphoblastoid cell lines (B-LCL). Processing of rNP for HLA-B27-associated presentation seemed to follow the conventional MHC class I pathway predominantly, as presentation was diminished in the presence of lactacystin and brefeldin A, but was less sensitive to chloroquine and NH4Cl. HLA-B27-associated presentation was also observed using cells lacking a functional transporter associated with antigen processing, suggesting that alternative pathways may be exploited for processing of rNP.
Keywords: influenza, nucleoprotein, antigen processing, HLA-B27, B-lymphoblastoid cells
INTRODUCTION
In virus-infected cells, antigenic peptides are liberated in the cytoplasm from endogenously-synthesized proteins and are presented to CD8+ cytotoxic T lymphocytes (CTL) by major histocompatibility complex (MHC) class I molecules. These proteins are degraded by a multi-enzyme complex, the proteasome, which generates peptides 9–12 amino acids long that are subsequently transported to the endoplasmic reticulum (ER) by the transporter associated with antigen processing (TAP). In the ER, the peptides associate with MHC class I molecules, and the resulting complex traverses the Golgi apparatus to be presented at the surface of the cell for recognition by specific CTL [1–5].
Although proteins synthesized in the cytoplasm of infected cells are the main substrate for the conventional (endogenous) MHC class I processing and presentation pathway, it has become evident that peptides liberated from exogenous proteins can be presented in a MHC class I-restricted fashion also, and evidence in favour of alternatives to the conventional MHC class I pathway is accumulating [6–8]. Indeed, it has been shown that macrophages, as well as other antigen-presenting cells (APC), can process a vast diversity of exogenous antigens, including particulate antigens such as bacteria, bead-coupled antigens, cell debris, inactivated viruses, and antigens associated with, or incorporated into, virosomes, liposomes or immune-stimulating complexes (iscoms) for MHC class I presentation [8]. Recently, it was shown that MHC class I presentation of exogenous antigens by APC may be necessary for the induction of CTL responses [9,10].
Several mechanisms for the processing of exogenous proteins and MHC class I presentation have been proposed. The results of several studies suggest a model in which endocytosed exogenous proteins gain access to the cytoplasm either by leakage from endosomal compartments, or by a yet unknown transport mechanism, and subsequently follow the conventional MHC class I pathway [11–15]. Results of other studies suggest an alternative mechanism in which endocytosed exogenous proteins are degraded in an endosomal compartment and subsequently loaded onto MHC class I molecules [16–20]. This could take place either extracellularly following regurgitation, or in the endosomal compartment itself following internalization of MHC class I molecules from the cell surface.
Processing of exogenous proteins for MHC class I presentation has been demonstrated almost exclusively for macrophages and dendritic cells [8]. B-lymphocytes usually process exogenous antigen for MHC class II presentation by means of internalization of antigen bound to surface immunoglobulin (receptor-mediated endocytosis). It has been demonstrated that B-lymphocytes can present exogenous antigen in association with MHC class I molecules also, but only if the antigen matches the surface immunoglobulin [21,22].
Here, we describe MHC class I presentation of exogenous recombinant influenza A virus nucleoprotein (rNP) by EBV-transformed, B-lymphoblastoid cell lines (B-LCL). MHC class I processing and presentation of rNP-derived peptides were studied using CTL clones specific for the HLA-A3-restricted NP epitope ILRGSVAHK265–273 and the HLA-B27-restricted NP epitope SRYWAIRTR383–391.
MATERIALS AND METHODS
Production of rNP
rNP of influenza virus A/HongKong/2/68; H3N2 (A/HK/2/68), A/Netherlands/18/94; H3N2 (A/Neth/18/94), and B/Harbin/7/94 (B/Har/7/94) was produced as described previously [23]. In brief, the nucleoprotein (NP)-encoding genes were cloned at the 3′ end of the maltose-binding protein (MBP)-encoding gene using the bacterial expression vector pMalC (New England Biolabs, Beverly, MA, USA) to yield fusion proteins consisting of MBP at the N-terminus and NP at the C-terminus. Recombinant MBP alone (rMBP) was generated from the empty vector as a control. The resulting recombinant proteins were purified by affinity chromatography using amylose resin columns (New England Biolabs).
Isolation and analysis of NP-specific CTL clones
A HLA-B27-restricted CTL clone, designated NP/B27, with specificity for the influenza A virus NP epitope SRYWAIRTR383–391, was isolated as previously described [24]. An HLA-A3-restricted CTL clone, designated NP/A3, specific for the influenza A virus NP epitope ILRGSVAHK265–273, was kindly provided by Dr W. Biddison, NIH, Bethesda, MD. Phenotype, specificity and HLA restriction of both CTL clones were confirmed as described [24].
CTL assays
B-LCL cells (1 × 106) of an HLA-A3- and -B27-positive donor were incubated with rNP derived from influenza virus A/HK/2/68, A/Neth/18/94 or B/Har/7/94, or rMBP (0–500 µm), or were incubated with the peptide ILRGSVAHK or SRYWAIRTR (0–10 µm) in 1 ml RPMI 1640 medium containing l-glutamin (2 mm), penicillin (100 IU/ml), streptomycin (100 µg/ml) and 10% FBS (culture medium) at 37°C and 5% CO2. In addition, untreated B-LCL cells were infected with influenza virus (Resvir-9; a H3N2 reassortant virus obtained from the influenza viruses A/Nanchang/933/95; H3N2 and A/Puerto Rico/8/34; H1N1) at a multiplicity of infection (m.o.i.) of 0·1. Infections were performed for 1 h in 1 ml RPMI 1640 medium without FBS, washed with culture medium and further cultured in 1 ml of this medium. Also, 1 × 106 BM28·7 and BM36·1 cells, both transfectant cell lines expressing HLA-B27 with the BM36·1 cell line differing from BM28·7 in a 2 bp deletion in TAP2 leading to a non-functional TAP1/TAP2 complex [25], were incubated with rNP derived from influenza virus A/HK/2/68 (0–50 µm), the peptide SRYWAIRTR (0–10 nm) or infected with influenza virus as described above. The BM28·7 and BM36·1 cell lines were cultured in the presence of 300 µg/ml geneticin. In selected experiments, cells were cultured in the continuous presence of lactacystin (3 µm) or brefeldin A (50 µm), which was added to the cells simultaneously with exogenous rNP or 1 h after infection with virus. After an incubation of 1 h (peptide-pulsed cells) or 16 h (rNP-incubated or influenza virus-infected cells), target cells were washed with culture medium to remove excess peptide, antigen, lactacystin and brefeldin A, and labelled for 1 h with 75 µCi Na2[51 Cr]O4. After washing three times with culture medium, 104 target cells were incubated with 105 cells of the NP/A3 or NP/B27 CTL clone (E:T ratio = 10) in a total volume of 150 µl, for 4 h at 37°C. Since the BM28·7 and BM36·1 cell lines are HLA-A3 negative, only NP/B27 CTL were used as effector cells in experiments with these cell lines. Target cells were also incubated in culture medium (spontaneous release) or in 10% Triton X-100 (maximum release). Supernatant fluids were harvested and radioactivity was measured by gamma counting. The percentage specific lysis was calculated as: 100 × [experimental release – spontaneous release]/[maximum release – spontaneous release]. CTL assays were performed with 10 replicates per target per experiment. Experiments were performed at least twice. Statistical analysis was performed using Student's t-tests.
Lymphocyte stimulation test (LST)
B-LCL cells (1 × 106) were incubated with rNP derived from influenza virus A/HK/2/68 (0·5–1 mm), or infected with influenza virus as described above, and cultured in the presence or absence of lactacystin (3 µm), brefeldin A (50 µm), chloroquine (50 µm) or NH4Cl (20 mm). Also, 1 × 106 BM28·7 and BM36·1 cells were incubated with rNP derived from influenza virus A/HK/2/68 (1 mm) and cultured in the presence or absence of these inhibitors. Culture conditions were as described above. After an incubation of 16 h, cells were washed twice with PBS to remove antigen and inhibitors, fixed with paraformaldehyde (1·5%), and resuspended in RPMI 1640 medium containing l-glutamin (2 mm), penicillin (100 IU/ml), streptomycin (100 µg/ml), 2-mercapto-ethanol (5 × 10−5 m) and 10% human pool serum; 5 × 104 of these stimulator cells were incubated with 5 × 104 cells of the NP/A3 or NP/B27 CTL clone in a total volume of 200 µl at 37°C and 5% CO2. After 3 days, 10 µl[3H]-labelled thymidine (50 µCi/ml) was added and after an incubation of 16 h, cells were harvested and thymidine incorporation was measured using a β-plate reader (LKB Wallac, Turku, Finland). The stimulation index was calculated as: radioactivity sample/radioactivity negative control (medium). LST were performed in triplicate.
Radio immuno-precipitation assay (RIPA)
B-LCL cells (6 × 106) were washed in PBS and resuspended in 3 ml RPMI 1640 medium, supplemented with 5% dialysed FBS but without l-methionin and l-cystin, and incubated at 37°C and 5% CO2. After 30 min, 300 µCi of a mixture of 35S-labelled l-methionin and l-cystin were added and incubated for 15 min. Cells were pelleted and resuspended in 2 ml ice-cold RIPA buffer (10 mm Tris pH 7·8, 150 mm NaCl, 600 mm KCl, 4 mm EDTA, 2% Triton X-100, 2·5 mm iodocetamide and protease inhibitor (Complete, Boehringer Mannheim, Germany). After incubation on ice for 10 min, the lysate was centrifuged and the supernatant fluid used for immuno-precipitation using Protein A beads. A 1% aliquot of the supernatant fluid was pre-cleared with 10 µl control mouse ascites fluid, followed by incubation of 5 µl normal mouse serum, both for 1 h at 4°C. This pre-cleared supernatant fluid was used for specific precipitation with monoclonal antibodies directed against HLA-A3 or -B27 (Biotest). Beads were pelleted and the supernatant fluid was subjected to a second round of precipitation. The pellets of both precipitations were resuspended in SDS-PAGE sample buffer. Samples were boiled for 3 min and run on a 15% polyacrylamide gel. After electrophoresis, gels were fixed, incubated with amplify solution (Amersham Pharmacia, Little Chalfont, UK), dried under vacuum at 80°C and exposed to X-ray film.
RESULTS
MHC class I presentation of rNP
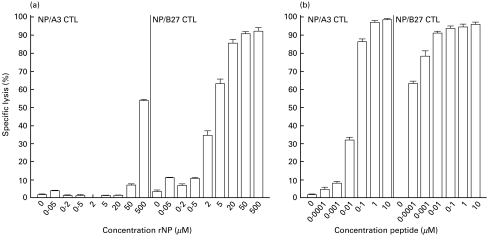
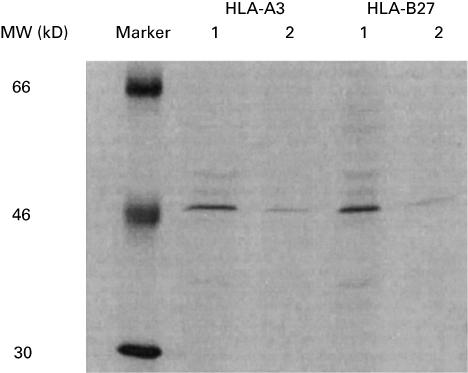
B-LCL cells of an HLA-A3- and -B27-positive donor were incubated with various concentrations of rNP derived from influenza virus A/HK/2/68 and used as target cells in CTL assays (Fig. 1a). B-LCL cells incubated with as little as 2 µm rNP were recognized by CTL specific for the HLA-B27-restricted NP epitope SRYWAIRTR383–391 (NP/B27 CTL). The percentage of specific lysis by these CTL increased with increasing concentrations of rNP. The B-LCL cells were also recognized and lysed by CTL specific for the HLA-A3-restricted NP epitope ILRGSVAHK265–273 (NP/A3 CTL), but only after incubation with rNP at a concentration of 50 µm or higher. The recognition of the rNP-derived peptides ILRGSVAHK265–273 and SRYWAIRTR383–391 by NP/A3 and NP/B27 CTL, respectively, was also studied with B-LCL from other donors, which were either HLA-A3-, HLA-B27-, or HLA-A3- and -B27-positive, but with mismatching other HLA-A and -B alleles. With these B-LCL, comparable results were obtained (data not shown). Using peptide-pulsed cells, it was found that at saturating concentrations of peptide, the B-LCL cells were lysed equally well by NP/A3 and NP/B27 CTL, whereas at low concentrations of peptide (below 0·01 µm), only NP/B27 CTL specifically lysed the B-LCL cells (Fig. 1b). Thus, approximately 100-fold less rNP or peptide was required for efficient lysis by NP/B27 CTL compared with NP/A3 CTL. Furthermore, in lymphocyte stimulation tests (LST), proliferation of NP/B27 CTL was observed after stimulation with B-LCL cells incubated with rNP (Fig. 4b), while NP/A3 CTL failed to respond (data not shown). In order to address the observed differences in recognition by NP/A3 and NP/B27 CTL further, the expression levels of HLA-A3 and HLA-B27 molecules in the B-LCL cells were determined by a radio immuno-precipitation assay (RIPA) using saturating amounts of monoclonal antibodies directed against HLA-A3 and -B27. Comparable numbers of HLA-A3 and -B27 molecules were detected after a first precipitation, with a limited but comparable number of HLA-A3 and -B27 molecules detectable in a subsequent precipitation (Fig. 2). Scanning of the bands revealed no significant differences in intensity, indicating that the total expression levels of HLA-A3 and HLA-B27 in the B-LCL cells used were comparable. However, it cannot be excluded that the surface expression levels of the respective MHC class I molecules may have differed.
Fig. 1.
MHC class I presentation of rNP. B-LCL cells of an HLA-A3- and -B27-positive donor were incubated with various concentrations of rNP derived from influenza virus A/HK/2/68 (a) or various concentrations of the HLA-A3-restricted peptide ILRGSVAHK (b, left panel) or the HLA-B27-restricted peptide SRYWAIRTR (b, right panel), and used as target cells in CTL assays with NP/A3 and NP/B27 CTL as effector cells. Experiments were performed with 10 replicates per target per experiment. Mean percentages of specific lysis at an E:T ratio of 10:1 are shown.
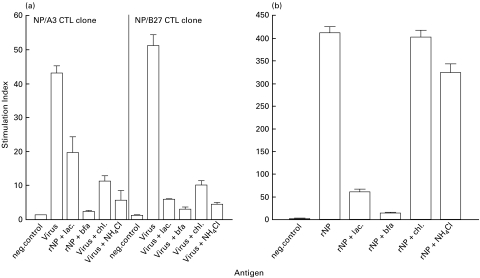
Fig. 4.
Effect of inhibitors of the conventional MHC class I pathway and endosomal antigen processing on MHC class I presentation of rNP. B-LCL cells of a HLA-A3- and -B27-positive donor were infected with influenza virus (m.o.i. = 0·1) in the presence or absence of lactacystin, brefeldin A, chloroquine or NH4Cl and used as stimulator cells in LST with NP/A3 and NP/B27 CTL (a). B-LCL cells were also incubated with 1 mm rNP derived from influenza virus A/HK/2/68 in the presence or absence of these inhibitors and used as stimulator cells in LST with NP/B27 CTL (b). LST were performed in triplicate. Mean stimulation indices are shown.
Fig. 2.
Analysis of expression levels of HLA-A3 and HLA-B27 in B-LCL cells. B-LCL cells incubated with 35S-labelled cysteine and methionine were lysed and the pre-cleared lysate was subjected to two rounds of specific precipitation using HLA-A3- and HLA-B27-specific monoclonal antibodies. Lanes 1: first precipitation; lanes 2: second precipitation.
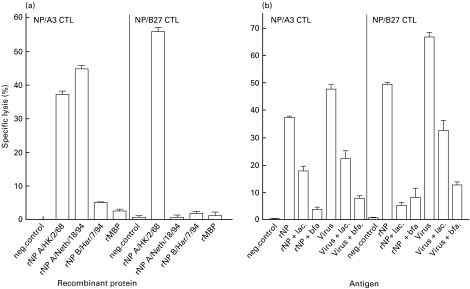
To confirm that the recognition of the B-LCL cells incubated with rNP by NP/A3 and NP/B27 CTL was specific for the epitopes ILRGSVAHK265–273 and SRYWAIRTR383–391, respectively, B-LCL cells were incubated with rNP derived from influenza virus A/HK/2/68, A/Neth/18/94 or B/Har/7/94, or recombinant maltose binding protein (rMBP), the latter two lacking the HLA-A3- and HLA-B27-restricted epitopes and serving as negative controls (Fig. 3a). B-LCL cells were lysed by NP/B27 CTL after incubation with 5 µm rNP derived from influenza virus A/HK/2/68, but not after incubation with the same amount of rMBP or rNP derived from influenza virus B/Har/7/94 or A/Neth/18/94, which contain an R384G mutation in the HLA-B27-restricted epitope SRYWAIRTR383–391. This mutation has been associated with the loss of recognition by CTL specific for this epitope [24]. B-LCL cells incubated with 500 µm rNP (required to measure specific lysis by NP/A3 CTL; see Fig. 1a) derived from influenza virus A/HK/2/68 or A/Neth/18/94 (which both contain the ILRGSVAHK265–273 epitope) were recognized by NP/A3 CTL. Like NP/B27 CTL, NP/A3 CTL did not lyse B-LCL cells incubated with the same amount of rNP derived from influenza virus B/Har/7/94 or rMBP. Part of the HLA-B27-associated presentation was attributed to extracellular processing of rNP, most probably by serum proteases. In LST, NP/B27 CTL could be stimulated to a certain extent with prefixed B-LCL cells that had been incubated with rNP in medium containing 10% FBS, but not when the same cells had been incubated with rNP in medium devoid of FBS (data not shown).
Fig. 3.
MHC class I presentation of rNP derived from several influenza viruses and the effect of lactacystin and brefeldin A. B-LCL cells of an HLA-A3- and -B27-positive donor were incubated with 500 (left panel) or 5 µm (right panel) rNP derived from influenza virus A/HK/2/68, A/Neth/18/94 or B/Har/7/94, with rMBP or left untreated (a), or incubated with 500 (left panel) or 5 µm (right panel) rNP derived from influenza virus A/HK/2/68 or infected with influenza virus (m.o.i. = 0·1) in the presence or absence of lactacystin or brefeldin A (b). Cells were used as target cells in CTL assays with NP/A3 and NP/B27 CTL as effector cells. Experiments were performed with 10 replicates per target per experiment. Mean percentages of specific lysis at an E:T ratio of 10:1 are shown.
Effect of lactacystin and brefeldin A on MHC class I presentation of rNP
In order to study further the route of processing of rNP, B-LCL cells were incubated with rNP derived from influenza virus A/HK/2/68 in the continuous presence of lactacystin, which inhibits proteasome activity, or brefeldin A, which blocks transport from the ER (Fig. 3b). As a control, B-LCL cells infected with influenza virus Resvir-9 (of which the NP contains both the HLA-A3- and HLA-B27-restricted epitopes) were included. Both NP/A3 and NP/B27 CTL lysed virus-infected target cells. As expected, recognition of virus-infected cells by these CTL was sensitive to the action of lactacystin and brefeldin A (P < 0·001). The recognition of B-LCL cells incubated with rNP in the presence of these inhibitors was also reduced (P < 0·001), suggesting that the conventional MHC class I pathway is of importance for processing and presentation of rNP in these cells. The inhibitors did not affect surface expression of MHC class I molecules, as B-LCL cells that had been incubated with peptide in the presence of inhibitors were lysed equally well by CTL as B-LCL cells that had been incubated with peptide without inhibitors (data not shown). The results obtained with the CTL assays were confirmed in an LST. Proliferation of NP/A3 and NP/B27 CTL was reduced after stimulation with influenza virus-infected B-LCL cells which had been cultured in the presence of lactacystin or brefeldin A (Fig. 4a). Likewise, proliferation of NP/B27 CTL was reduced when B-LCL cells had been incubated with rNP derived from influenza virus A/HK/2/68 in the presence of these inhibitors (Fig. 4b). The relatively strong proliferation of NP/B27 CTL upon stimulation with B-LCL cells that had been incubated with rNP, as compared with CTL stimulated by virus-infected B-LCL cells, most likely reflects the different nature of the antigens used.
Effect of chloroquine and NH4Cl on MHC class I presentation of rNP
A possible role for endosomal processing was studied using chloroquine and NH4Cl, which prevent acidification of endosomes and, consequently, proteolysis. The effect of these inhibitors was not tested in CTL assays as the required continuous presence of these agents affected CTL activity. Therefore, paraformaldehyde-fixed B-LCL cells, which had been infected with influenza virus Resvir-9 or incubated with rNP derived from influenza virus A/HK/2/68 in the presence or absence of these inhibitors, were used as stimulator cells in LST. Proliferation of NP/A3 and NP/B27 CTL was reduced after stimulation with infected B-LCL cells that had been cultured in the presence of chloroquine or NH4Cl, as compared with CTL stimulated with untreated infected B-LCL cells (Fig. 4a). This is explained by the fact that a low pH in the endosomes is essential for conformational changes in the haemagglutinin, allowing fusion of the viral membrane with the membrane of endosomes and subsequent release of viral antigens into the cytoplasm. In contrast, no significant difference in proliferation of NP/B27 CTL was observed upon stimulation with B-LCL cells incubated with 1 mm rNP in the presence or absence of these inhibitors (Fig. 4b). When B-LCL cells were incubated with a lower amount of rNP (0·5 mm), only a limited reduction in proliferation of NP/B27 CTL was observed (data not shown).
MHC class I presentation of rNP in the absence of TAP
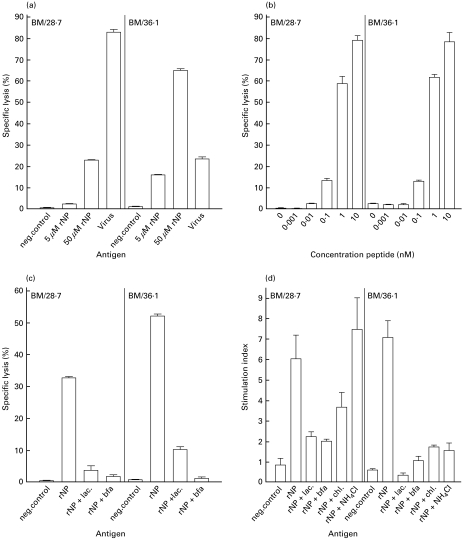
To study the role of TAP in the processing of rNP, BM28·7 and TAP-deficient BM36·1 cells were used. The recognition of virus-infected BM36·1 cells by NP/B27 CTL was significantly reduced (P < 0·001) compared with the recognition of the corresponding TAP-competent BM28·7 cells (Fig. 5a). This difference could not be attributed to differences in the infection rates of the respective cell lines, as both cell lines were infected equally well, as demonstrated by immunofluorescense using an NP-specific monoclonal antibody (data not shown). Surprisingly, the opposite was found after incubation with rNP derived from influenza virus A/HK/2/68. BM36·1 cells incubated with rNP were lysed more efficiently by NP/B27 CTL than BM28·7 cells (P < 0·001). To rule out the possibility that the observed differences between the BM28·7 and BM36·1 cells are the result of differences in the expression of peptide-receptive HLA-B27 molecules, the recognition of these cells by NP/B27 CTL was studied after incubation with limiting amounts of peptide (Fig. 5b). Both cell lines were recognized equally well by the NP/B27 CTL under these conditions, indicating that HLA-B27 was expressed at comparable levels in both cell lines. In contrast to the CTL assay, no differences in proliferation of NP/B27 CTL stimulated with BM28·7 or BM36·1 cells incubated with rNP were observed (Fig. 5d). Proliferation of NP/B27 CTL stimulated either with BM28·7 or BM36·1 cells was less pronounced than that observed for other B-LCL cells (Fig. 4b) and seems to depend on the cell line used.
Fig. 5.
MHC class I presentation of rNP in the absence of TAP and the effect of inhibitors of the conventional MHC class I pathway and endosomal antigen processing. BM28·7 and BM36·1 cells were incubated with 5 or 50 µm rNP derived from influenza virus A/HK/2/68 or infected with influenza virus (m.o.i. = 0·1) (a), incubated with various concentrations of the HLA-B27-restricted peptide SRYWAIRTR (b), or incubated with 50 µm rNP derived from influenza virus A/HK/2/68 in the presence of lactacystin or brefeldin A (c). Cells were used as target cells in CTL assays with the NP/B27 CTL as effector cells. Experiments were performed with 10 replicates per target per experiment. Mean percentages of specific lysis at an E:T ratio of 10:1 are shown. BM28·7 and BM36·1 cells incubated with 1 mm rNP derived from influenza virus A/HK/2/68 in the presence or absence of lactacystin, brefeldin A, chloroquine or NH4Cl were used as stimulator cells in LST with the NP/B27 CTL (d). LST were performed in triplicate. Mean stimulation indices are shown.
The effect of the MHC class I pathway inhibitors, lactacystin and brefeldin A, as well as inhibitors of endosomal processing (chloroquine and NH4Cl), was also studied for BM28·7 and BM36·1 cells incubated with rNP derived from influenza virus A/HK/2/68 (Fig. 5c, d). Specific lysis by NP/B27 CTL was reduced when cells had been incubated with rNP in the presence of either lactacystin or brefeldin A (Fig. 5c). Similarly, proliferation of NP/B27 CTL was reduced under these circumstances (Fig. 5d). Although proliferation of NP/B27 CTL stimulated with BM28·7 cells which had been incubated with rNP in the presence of chloroquine was reduced, no reduction was observed when BM28·7 cells had been incubated with rNP in the presence of NH4Cl. In contrast, proliferation of NP/B27 CTL was severely reduced when TAP-deficient BM36·1 cells had been incubated with rNP in the presence of chloroquine or NH4Cl (Fig. 5d).
DISCUSSION
Although exogenous proteins are usually processed for MHC class II presentation to CD4+ Th cells, it has been shown that some APC (e.g. macrophages and dendritic cells) are capable of processing these proteins for MHC class I presentation to CD8+ CTL as well [8]. With the exception of proteins that are delivered into the cytosol artificially (e.g. proteins associated with iscoms), or proteins that are internalized by receptor-mediated endocytosis, MHC class I presentation of exogenous protein by B-lymphocytes has not thus far been demonstrated [11,21,22,26–31]. Here, we show MHC class I presentation of the HLA-A3-restricted peptide ILRGSVAHK265–273 and the HLA-B27-restricted peptide SRYWAIRTR383–391, derived from exogenous rNP by B-LCL.
The NP/B27 CTL recognized B-LCL cells which had been incubated with rNP at a concentration of 2 µm, while approximately 100 times higher concentrations of rNP were required to sensitize B-LCL cells for lysis by NP/A3 CTL. Recently, it has been demonstrated that HLA-B27-restricted peptides have higher affinity for TAP than HLA-A3-restricted peptides [32] and, using peptides, we have shown that B-LCL cells could be sensitized for lysis by NP/B27 CTL at peptide concentrations much lower than needed for measuring HLA-A3-associated presentation. Peptides with higher affinities for their MHC class I molecule and/or TAP may be presented more efficiently than peptides possessing low affinity. The efficient presentation by HLA-B27 molecules may also be related to some unique features of these MHC class I molecules, including their ability to bind peptides without associating with TAP or tapasin, and their ability to bind peptides longer than nonamers and to form peptide-binding homodimers [33–35]. While these seem likely explanations, it cannot be totally excluded that possible differences in the CTL clones or surface expression levels of MHC class I molecules may have contributed to the observed difference in HLA-A3- and HLA-B27-associated presentation.
The mode of processing of exogenous proteins for MHC class I-restricted presentation is not fully clear. It has been suggested that some proteins can be processed extracellularly by serum proteases [36]. In contrast to the HLA-A2-restricted epitope of the influenza virus matrix protein [31] and the HLA-A3-restricted epitope of rNP, the presentation of the HLA-B27-restricted epitope of rNP was possible, to a certain extent, by paraformaldehyde-fixed B-LCL cells in the presence of FCS, but not without FCS, indicating that extracellular processing of rNP by serum proteases contributed to the generation of presentable peptides (data not shown). However, MHC class I presentation of the HLA-A3- and HLA-B27-restricted peptides derived from exogenous rNP, like presentation of these peptides derived from de novo synthesized NP by virus-infected cells, was found to depend on proteasome activity and to be sensitive to brefeldin A. This suggests that rNP gained access to the cytoplasm, following endocytosis and subsequent leakage from endosomes, and that processing and presentation followed the conventional MHC class I pathway. It is unlikely that endocytsosis was receptor-mediated since the B-LCL cells did not express NP or MBP-specific immunoglobulin (data not shown). In addition, it can be excluded that the rNP preparations contained presentable peptides, as the presentation of synthetic peptides was not inhibited by lactacystin or brefeldin A. Previously, we have shown that influenza virus M1 protein fused to MBP only sensitized B-LCL cells for recognition by CTL specific for the M1 protein, HLA-A2-restricted epitope GILGFVFTL58–66 when the protein was associated with iscoms [31]. In addition, B-LCL cells incubated with high doses (1 mm) of this recombinant M1 protein (without iscoms) were not recognized by specific CTL (unpublished data). These results rule out a direct role of MBP in entry of rNP into the MHC class I pathway.
HLA-B27-associated presentation by B-LCL cells was shown to be insensitive to chloroquine and NH4Cl at a high concentration of rNP, while at a lower concentration, limited sensitivity to these agents was observed. This suggests that, in addition to the conventional MHC class I pathway, endosomal processing may only be exploited by these cells to a limited extent. It is possible that in the endosomal compartments of B-LCL cells, peptides were generated from rNP by proteolysis and subsequently loaded onto peptide-receptive, internalized, MHC class I molecules, which are known to recycle through endosomal compartments [37–39]. While this mechanism may have contributed to the observed MHC class I presentation of rNP, it is most probably not the major route.
Surprisingly, HLA-B27-associated presentation of rNP also occurred in the TAP-deficient cell line BM36·1. Since, in cells lacking a functional TAP, the conventional MHC class I pathway is hampered, as demonstrated by the fact that recognition of influenza virus-infected BM36·1 cells by NP/B27 CTL was reduced, processing and presentation of rNP in these cells must have followed alternative routes which appeared to be highly efficient. TAP-independent MHC class I-restricted presentation has been described for peptides liberated from N-terminal signal sequences of proteins that enter the ER via translocation [40–47]. In addition, it has been suggested that entry of proteins or peptides into the ER of TAP-deficient cells may take place by (unknown) mechanisms other than translocation [46]. As the influenza virus NP does not contain a signal sequence and as it was cloned C-terminal of the MBP, translocation-mediated entry into the ER is unlikely. MHC class I presentation of rNP by BM36·1 cells, but not BM28·7 cells, was found to be severely inhibited by chloroquine and NH4Cl, indicating that the endosomal route is involved in the processing of rNP in these cells. These results are in agreement with a study showing that processing and presentation of an ovalbumin epitope (aa 257–264), contained within an ovalbumin fusion protein, was sensitive to chloroquine in TAP-deficient macrophages, but not in normal macrophages [48].
The observed MHC class I presentation of rNP by BM36·1 cells was also found to be sensitive to brefeldin A, indicating that antigenic peptides were loaded onto MHC class I molecules prior to their transport to the cell surface. In addition to inhibition of transport of MHC class I molecules from the ER to the cell surface, brefeldin A is known to inhibit transport of MHC class II molecules from the endosomes to the cell surface [49]. It may be speculated that brefeldin A could also affect transport of internalized MHC class I molecules, loaded with peptide in the endosomes, back to the cell surface. Alternatively, brefeldin A treatment could have resulted in a reduced availability of MHC class I molecules for recycling in endosomes.
Interestingly, the processing of rNP in BM36·1 cells was sensitive to lactacystin, implying that, at least in part, the peptides that were presented to the NP/B27 CTL were liberated by proteasome activity. Since BM36·1 cells are unable to transport these peptides in the ER by TAP, they were probably loaded onto MHC class I molecules through alternative, yet unkown routes which may involve endosomes, the ER or a post ER compartment.
Collectively, the data presented show MHC class I presentation of exogenous rNP by B-LCL cells. While in normal cells the conventional MHC class I pathway seems to predominate, in TAP-deficient cells alternative pathways, including endosomal processing, may operate for HLA-B27-associated presentation. The results of this study also show that care should be taken in drawing general conclusions with respect to the steps involved in processing and presentation of exogenous proteins.
Acknowledgments
Part of this work was supported by the Foundation for Respiratory Virus Infections, notably Influenza (SRVI). We acknowledge W. Biddison, NIH, Bethesda, Md, for providing us with the NP/A3 CTL clone. The authors wish to thank Ger van der Water for continuous support.
REFERENCES
- 1.Monaco JJ. A molecular model of MHC class-I-restricted antigen processing. Immunol Today. 1992;13:173–8. doi: 10.1016/0167-5699(92)90122-N. [DOI] [PubMed] [Google Scholar]
- 2.Neefjes JJ, Momburg F. Cell biology of antigen presentation. Curr Opin Immunol. 1993;5:27–34. doi: 10.1016/0952-7915(93)90077-6. [DOI] [PubMed] [Google Scholar]
- 3.Heemels MT, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–91. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 4.Hahn YS, Yang B, Braciale TJ. Regulation of antigen processing and presentation to class I MHC restricted CD8+ T lymphocytes. Immunol Rev. 1996;151:31–49. doi: 10.1111/j.1600-065x.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 5.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–96. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 6.Reimann J, Böhm W, Schirmbeck R. Alternative processing pathways for MHC class I-restricted epitope presentation to CD8+ cytotoxic T lymphocytes. Biol Chem Hoppe-Seyler. 1994;375:731–6. doi: 10.1515/bchm3.1994.375.11.731. [DOI] [PubMed] [Google Scholar]
- 7.Reimann J, Schirmbeck R. Alternative pathways for processing exogenous and endogenous antigens that can generate peptide for MHC class I-restricted presentation. Immunol Rev. 1999;172:131–52. doi: 10.1111/j.1600-065x.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 8.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher TNM. Accessory to murder. Nature. 1999;398:26–7. doi: 10.1038/17927. [DOI] [PubMed] [Google Scholar]
- 10.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 11.Rock KL, Rothstein L, Gamble S, Fleischacker C. Characterization of antigen-presenting cells that present exogenous antigens in association with class I MHC molecules. J Immunol. 1993;150:438–46. [PubMed] [Google Scholar]
- 12.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 13.Reis e Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigens by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–51. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 15.Brossart P, Bevan MJ. Presentation of exogenous proteins on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–33. [PubMed] [Google Scholar]
- 17.Bachmann MF, Oxenius A, Pircher H, et al. TAP1-independent loading of class I molecules by exogenous viral proteins. Eur J Immunol. 1995;25:1739–43. doi: 10.1002/eji.1830250637. [DOI] [PubMed] [Google Scholar]
- 18.Schirmbeck R, Melber K, Reimann J. Hepatitis B virus small surface antigen particles are processed in a novel endosomal pathway for major histocompatibility complex class I-restricted epitope presentation. Eur J Immunol. 1995;25:1063–70. doi: 10.1002/eji.1830250431. [DOI] [PubMed] [Google Scholar]
- 19.Song R, Harding CV. Roles of proteasomes, transporter for antigen presentation (TAP), and β2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J Immunol. 1996;156:4182–90. [PubMed] [Google Scholar]
- 20.Liu T, Chambers B, Diehl AD, Van Kaer L, Jondal M, Ljunggren H. TAP peptide transporter-independent presentation of heat-killed sendai virus antigen on MHC class I molecules by splenic antigen-presenting cells. J Immunol. 1997;159:5364–71. [PubMed] [Google Scholar]
- 21.Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature. 1990;345:258–60. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 22.Ke Y, Kapp JA. Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J Exp Med. 1996;184:1179–84. doi: 10.1084/jem.184.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voeten JTM, Groen J, van Alphen D, et al. Use of recombinant nucleoproteins in enzyme-linked immunosorbent assays for detection of virus-specific immunoglobulin A (IgA) and IgG antibodies in influenza virus A- or B-infected patients. J Clin Microbiol. 1998;36:3527–31. doi: 10.1128/jcm.36.12.3527-3531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voeten JTM, Bestebroer TM, Nieuwkoop NJ, Fouchier RAM, Osterhaus ADME, Rimmelzwaan GF. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J Virol. 2000;74:6800–7. doi: 10.1128/jvi.74.15.6800-6807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly A, Powis SH, Kerr LA, et al. Assembly and function of the two ABC transporter proteins encoded in the human histocompatibility complex. Nature. 1992;355:641–4. doi: 10.1038/355641a0. [DOI] [PubMed] [Google Scholar]
- 26.Rock KL, Gamble S, Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990;249:918–21. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- 27.van Binnendijk RS, van Baalen CA, Poelen MCM, et al. Measles virus transmembrane fusion protein synthesized de novo or presented in immunostimulating complexes is endogenously processed for HLA class I- and class II-restricted cytotoxic T cell recognition. J Exp Med. 1992;176:119–28. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942–6. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee R, Tartour E, van der Bruggen P, et al. Major histocompatibility complex class I presentation of exogenous soluble tumor antigen fused to the B-fragment of shiga toxin. Eur J Immunol. 1998;28:2726–37. doi: 10.1002/(SICI)1521-4141(199809)28:09<2726::AID-IMMU2726>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Bansal P, Mukherjee P, Basu S, George A, Bal V, Rath S. MHC class I-restricted presentation of maleylated protein binding to scavenger receptors. J Immunol. 1999;162:4430–7. [PubMed] [Google Scholar]
- 31.Voeten JTM, Rimmelzwaan GF, Nieuwkoop NJ, Lövgren-Bengtsson K, Osterhaus ADME. Introduction of the haemagglutinin transmembrane region in the influenza virus matrix protein facilitates its incorporation into ISCOM and activation of specific CD8+ cytotoxic T lymphocytes. Vaccine. 2000;19:514–22. doi: 10.1016/s0264-410x(00)00179-1. [DOI] [PubMed] [Google Scholar]
- 32.Daniel A, Brusic V, Caillat-Zucman S, et al. Relationship between peptide selectivities of human transporters associated with antigen processing and HLA class I molecules. J Immunol. 1998;161:617–24. [PubMed] [Google Scholar]
- 33.Peh CA, Burrows SR, Barnden M, et al. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8:531–42. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- 34.Urban RG, Chicz RM, Lane WS, et al. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc Natl Acad Sci USA. 1994;91:1534–8. doi: 10.1073/pnas.91.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen RL, O'Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel β2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–8. [PubMed] [Google Scholar]
- 36.Eberl G, Renggli J, Men Y, Roggero MA, Lopez JA, Corradin G. Extracellular processing and presentation of a 69-mer synthetic polypeptide to MHC class I-restricted T cells. Mol Immunol. 1999;36:103–12. doi: 10.1016/s0161-5890(99)00023-1. [DOI] [PubMed] [Google Scholar]
- 37.Reid PA, Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990;346:655–7. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- 38.Grommé M, Uytdehaag FGCM, Janssen H, et al. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci USA. 1999;96:10326–31. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu I, Davis DM, Strominger JL. Trafficking of spontaneously endocytosed MHC proteins. Proc Natl Acad Sci USA. 1999;96:13944–9. doi: 10.1073/pnas.96.24.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J Exp Med. 1991;174:489–92. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson RA, Michel H, Sakaguchi K, et al. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–6. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 42.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-presenting mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–6. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 43.Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–7. [PubMed] [Google Scholar]
- 44.Guegen M, Biddison WE, Long EO. T cell recognition of an HLA-A2-restricted epitope derived from a cleaved signal sequence. J Exp Med. 1994;180:1989–94. doi: 10.1084/jem.180.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliot T, Willis A, Cerundolo V, Townsend A. Processing of major histocompatibility class I-restricted antigens in the endoplasmic reticulum. J Exp Med. 1995;181:1481–91. doi: 10.1084/jem.181.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snyder HL, Bacik I, Bennink JR, et al. Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing. J Exp Med. 1997;186:1087–98. doi: 10.1084/jem.186.7.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yewdell JW, Snyder HL, Bacik I, et al. TAP-independent delivery of antigenic peptides to the endoplasmic reticulum: therapeutic potential and insights into TAP-dependent antigen processing. J Immunother. 1998;21:127–31. doi: 10.1097/00002371-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Wick MJ, Pfeifer JD. Major histocompatibility complex class I presentation of ovalbumin peptide 257–264 from exogenous sources: protein context influences the degree of TAP-independent presentation. Eur J Immunol. 1996;26:2790–9. doi: 10.1002/eji.1830261135. [DOI] [PubMed] [Google Scholar]
- 49.Pond L, Watts C. Characterization of transport of newly assembled, T cell-stimulatory MHC class II-peptide complexes from MHC class II compartments to the cell surface. J Immunol. 1997;159:543–53. [PubMed] [Google Scholar]